Abstract
Nocturnal yawning may represent a subtle clinical marker of arousal instability in patients with sleep-disordered breathing (SDB), potentially unveiling latent comorbid insomnia and sleep apnea (COMISA) phenotypes, and expanding the interpretative scope of sleep-related behaviors.
Keywords: obstructive sleep apnea, sleep bruxism, COMISA, yawning
The recent case report by Michałek et al. describes a rare and intriguing co-occurrence of yawning during sleep in a patient with obstructive sleep apnea (OSA) and sleep bruxism (SB).1 The researchers’ hypothesis of a hypoxia-triggered arousal mechanism is compelling and well-grounded in the observed polysomnographic patterns. Nonetheless, it would be interesting to integrate further reflections that may expand and enrich the interpretation of this complex behavioral constellation.
One critical angle that deserves consideration is the possible presence of insomnia-related physiology, particularly within the framework of comorbid insomnia and sleep apnea (COMISA). Although this diagnosis has not been formally addressed, some reported features, such as marked sleep fragmentation, microarousals and low adherence to positive airway pressure (PAP) therapy, are often part of some COMISA phenotypes and may reflect a background of sleep-state hyperarousal, which is frequently observed in those patients.2 Indeed, it has been recently proposed that COMISA encompasses a heterogeneous spectrum, with emerging interest in identifying clinical phenotypes.2
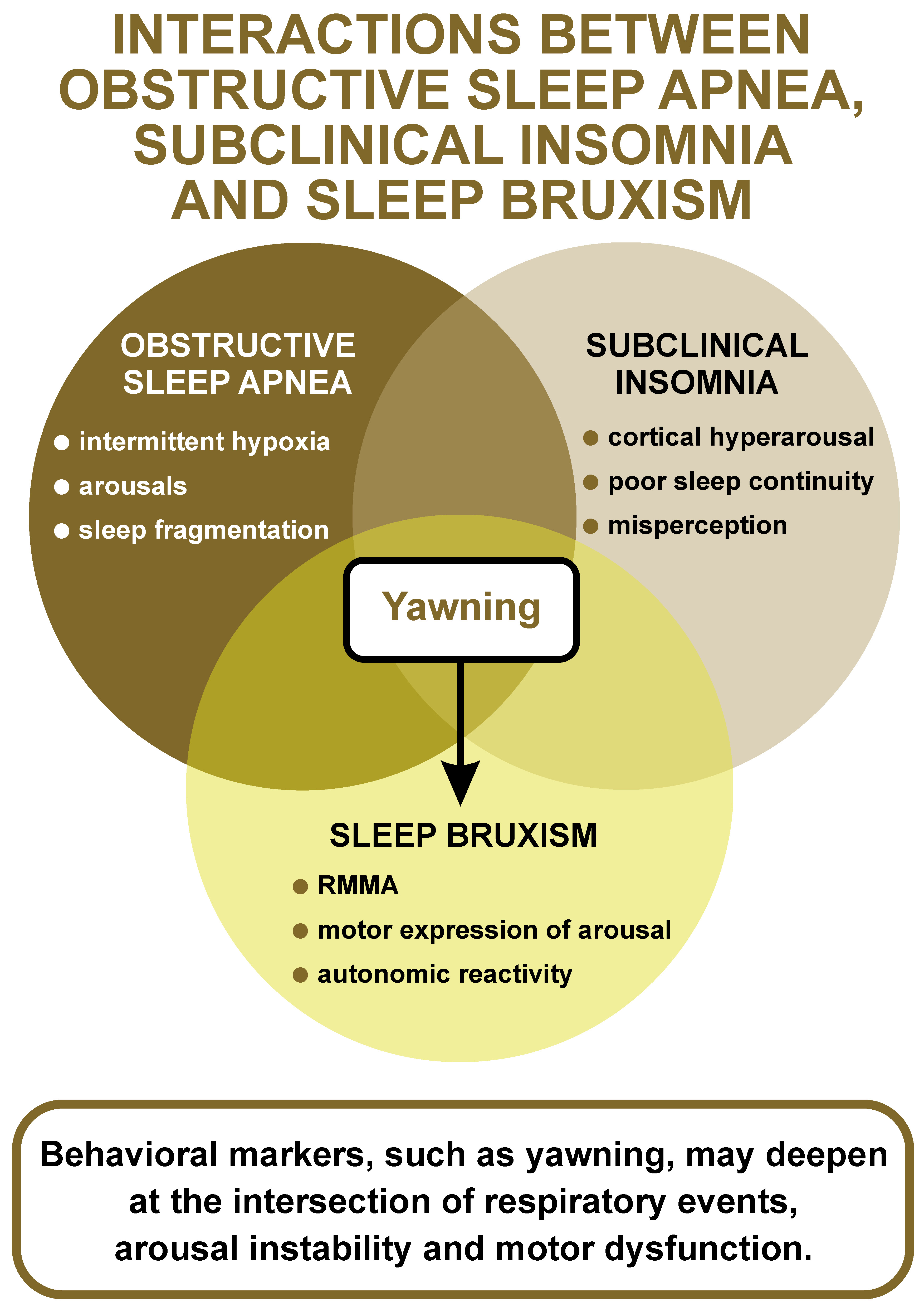
While such phenotypic models are still in the hypothesis-building stage and require stronger empirical validation, the existence of subclinical insomnia manifested through subtle neurophysiological and behavioral mechanisms, even in the absence of subjective complaints, is increasingly recognized as a relevant issue in both clinical practice and sleep research.3 In this context, the observed nocturnal yawning might be not only a direct response to hypoxia, but also a behavioral expression of the underlying arousal instability, which could reflect a latent insomniac phenotype with challenging aspects from the clinical point of view. This possibility highlights an often-overlooked dimension in sleep medicine – the presence of non-overt insomnia traits that nonetheless modulate sleep architecture, treatment adherence and the emergence of sleep-related motor behaviors.4 Moreover, COMISA patients frequently show the dysregulation of sleep–wake transitions, a state that can facilitate the intrusion of transitional behaviors, such as yawning, into otherwise consolidated sleep.5 Also, recent evidence suggests that SB may play a mechanistic and phenotypic role in COMISA, potentially reflecting a hyperarousal-driven motor expression that is intensified in the presence of overlapping insomnia and OSA. In a polysomnographic study by Blaszczyk et al., patients with COMISA presented a significantly higher frequency of rhythmic masticatory muscle activity (RMMA) and bruxism-related arousals as compared to patients with OSA alone.5 These findings support the idea that SB may serve as a physiological link between cortical arousal and autonomic dysregulation, helping to explain the heightened motor instability observed in COMISA cases. Yawning, traditionally associated with sleep onset or state transitions, may in these cases re-emerge inappropriately due to an incomplete inhibition of wake-promoting networks, possibly exacerbated by the repeated respiratory-related arousals of OSA, eventually associated with motor exacerbations. Similar mechanisms were described in clinical reports of unusual yawning patterns associated with SB and OSA.6 This proposal is further strengthened by neurobiological insights. As Blaszczyk et al. noted, yawning, bruxism and sleep fragmentation share overlapping neurochemical pathways, particularly those involving dopaminergic modulation.5 The hypothesis that bruxism and yawning share common subcortical circuitries, particularly within the basal ganglia and hypothalamus, could reflect a broader state of central disinhibition, where motor pattern generators are more likely to get activated in the presence of disturbed arousal regulation.7, 8 These considerations align with recent data on the etiological mechanisms and metabolic consequences of OSA, including inflammation, oxidative stress and insulin resistance.9, 10
From a clinical perspective, raising awareness about such subclinical or latent forms of insomnia embedded in OSA presentations is vital. These forms often go unrecognized due to the absence of explicit complaints, but may substantially influence treatment outcomes, including tolerance to PAP therapy, symptom persistence and behavioral adherence. Recognizing this overlap, particularly through unusual motor or autonomic markers, such as nocturnal yawning, may open new diagnostic and therapeutic pathways.
As illustrated in Figure 1, yawning may emerge at the intersection of OSA, subclinical insomnia and SB, reflecting shared arousal-related mechanisms. Nocturnal yawning might serve as a clinical clue, albeit subtle and unspecific, of latent COMISA physiology, especially in patients who otherwise present a predominantly respiratory sleep disorder phenotype. Such behavioral markers may reflect deeper layers of arousal instability and neurochemical dysregulation that are not easily captured by conventional sleep diagnostics.
In conclusion, the unusual case reported by Michałek et al.1 provides fertile ground for reflection. While the hypoxia–arousal mechanism is essential, the integration of additional domains, namely subclinical insomnia expression, COMISA-related hyperarousal, neurochemical overlap, and behavioral arousal markers, may offer a broader, more integrative understanding of these phenomena. On the other hand, framing yawning as a potential clinical clue, rather than a mere physiological reflex, invites us to revisit the complexity of COMISA phenotypes and rethink how subtle behaviors during sleep can guide us toward more precise diagnostic and therapeutic strategies.