Abstract
Background. Effective cleaning protocols are crucial for controlling biofilm formation on oral prostheses and preserving the oral health of patients relying on removable partial dentures (RPDs).
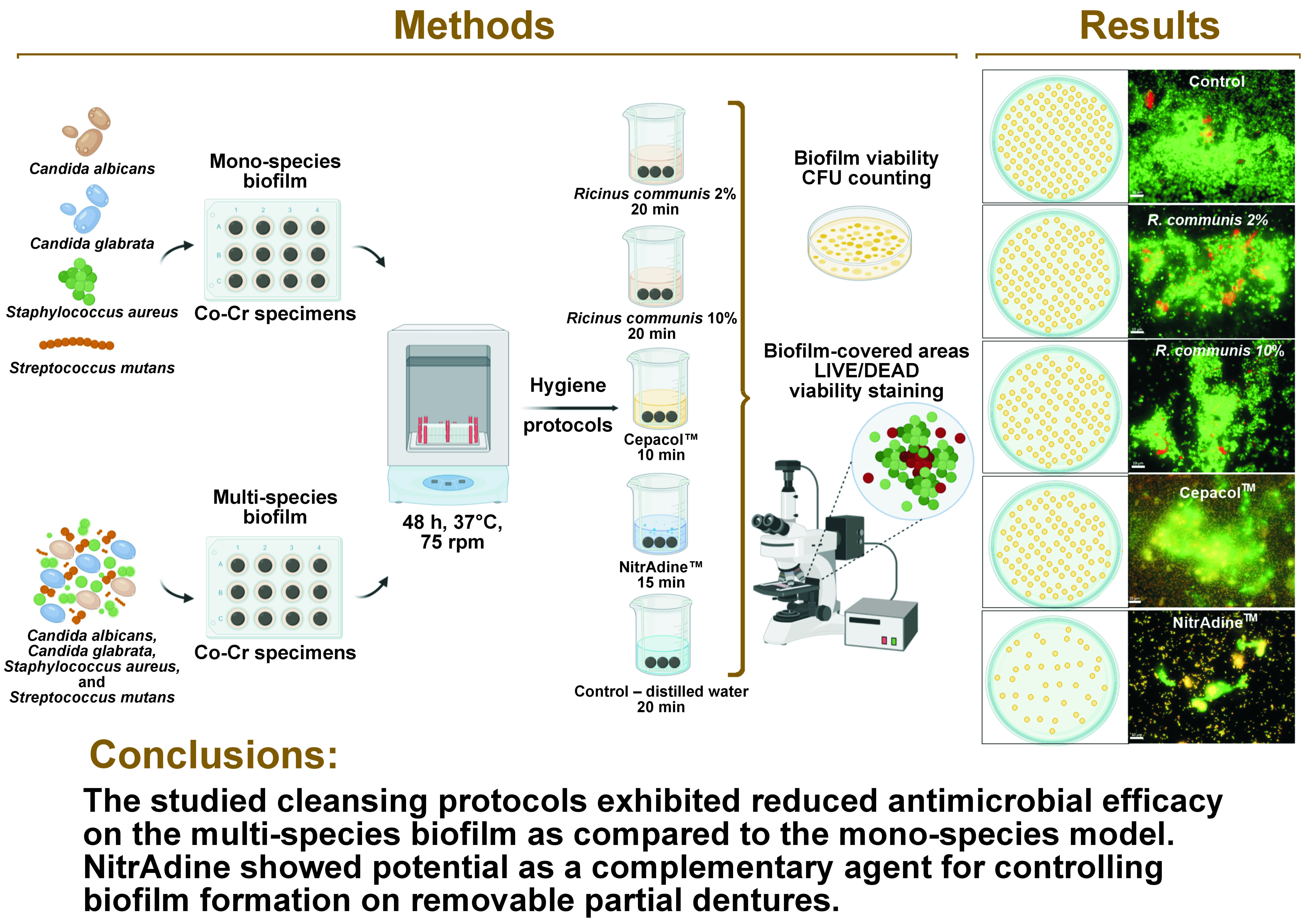
Objectives. The present study aimed to investigate the antibiofilm efficacy of 4 cleansing protocols on a cobalt-chromium (Co-Cr) alloy surface, which is commonly used as the base-metal framework material in dental prosthodontics.
Material and methods. Cobalt-chromium specimens were contaminated with isolated strains of Candida albicans, Candida glabrata, Staphylococcus aureus, and Streptococcus mutans to form mono-species biofilms. For a multi-species biofilm, all strains were grown simultaneously on the surfaces of the specimens. After biofilm maturation, the specimens were immersed in different solutions: Ricinus communis 2%; R. communis 10%; Cepacol™; NitrAdine™; and distilled water (control). After applying the hygiene protocols, the viability of the microorganisms and the amount of residual biofilm were assessed.
Results. Immersion in R. communis-based solutions did not significantly alter the viability of the microorganisms. Cepacol reduced the viability of C. albicans, C. glabrata and S. aureus in the mono-species biofilms, as well as C. glabrata in the multi-species biofilm. NitrAdine demonstrated effectiveness in reducing the viability of C. glabrata and S. mutans in both the mono- and multi-species biofilms. However, its efficacy against S. aureus was only observed in the mono-species pattern. NitrAdine also reduced the area covered by the living biofilm.
Conclusions. The studied cleansing protocols exhibited reduced antimicrobial efficacy on the multi-species biofilm as compared to the mono-species model. NitrAdine showed potential as a complementary agent for controlling biofilm formation on removable partial dentures.
Keywords: removable partial denture, cleansers, antimicrobial action, cobalt-chromium alloy
Introduction
Oral biofilms are composed of bacteria and yeast-like fungi, which adhere and grow on biotic and abiotic surfaces.1 Biofilms, with an inadequate hygiene of prostheses, constitute a source of microorganisms and act as a gate to systemic diseases.2 Although it is known that the control of biofilm formation on oral prostheses is crucial for maintaining general health, there is no consensus regarding a suitable solution for removable partial dentures (RPDs).3, 4, 5
Compatibility with constituent materials is a requisite for an ideal RPD cleanser.4, 5 Additionally, other aspects, such as a low cost, easy manipulation and antibiofilm activity, are desirable.6, 7 There is evidence that diluted sodium hypochlorite is efficient in controlling biofilm formation; however, it is not recommended for to cleaning RPDs, taking into consideration their metal components.3, 8 Mouthwashes are popular in oral care and are frequently used as prosthesis cleansing solutions,9 even though there are no specific guidelines regarding their use. Generally, these formulations include chlorhexidine, chlorine dioxide, cetylpyridinium chloride, and essential oils (e.g., eucalyptol, menthol, thymol, and methyl salicylate).10 Besides mouthwashes, effervescent tablets are also largely used, partly due to their pleasant taste and odor characteristics. They are composed of different active ingredients, such as titanium dioxide, sodium lauryl sulfate and ethylenediaminetetraacetic acid (EDTA).11 Both mouthwashes and effervescent tablets are complex chemical combinations that can damage the dental alloy as a result of ion release in the presence of oxidizing compounds.12 According to previous studies, the NitrAdine™ effervescent tablet acts against oral biofilms13, 14 and may be indicated as an RPD cleanser, as its 5-year use did not induce deleterious effects to the dental alloy.4, 5 Nonetheless, given different characteristics of dental materials, it is fundamental to verify the antibiofilm activity of the cleanser on a metallic surface.
Broadening the knowledge about the antimicrobial properties of natural substances can have an impact on the selection of appropriate products to deal with the resistance of microorganisms.15 Furthermore, it has been suggested that natural products do not have adverse effects inherent to synthetic compounds, and contribute to environmental and economic sustainability.16 The Ricinus communis or castor oil plant belongs to the Euphorbiaceae family and is easily found in tropical zones. The R. communis oil has been used since antiquity, and has been demonstrated in medical and dental research to bring significant benefits.17 Regarding its biological effects, the literature reports its healing, antioxidant, anti-inflammatory,18 and antimicrobial properties.19, 20, 21 In dentistry, previous studies indicated its potential use for prothesis hygiene22, 23 and the improvement of clinical conditions of denture-related stomatitis.24 Even though the scientific literature has pointed out the compatibility of R. communis with the cobalt-chromium (Co-Cr) alloy,5 its antibiofilm effect on a metallic surface has not been investigated.
Considering that inconsistent RPD hygiene can favor the manifestation of opportunistic pathologies,25 new hygiene solutions should be investigated. Given this point, it is important to advertise that the presence of Candida spp. on a denture surface is an etiological factor for denture-related stomatitis.26 In addition, the presence of other species, such as Staphylococcus aureus and Streptococcus mutans, may contribute to the pathogenicity of the biofilm.2, 27 The physical interactions of Candida albicans with various species go beyond simple synergistic and antagonistic associations. These interactions significantly influence the expression of virulence factors, directly impacting colonization and tissue invasion.26 Staphylococcus aureus, S. mutans, C. albicans, and Candida glabrata are common species colonizing the abutment and non-abutment teeth in RPD wearers.28 The presence of respiratory pathogens in the denture biofilm has already been investigated, and prostheses seem to act as a reservoir for S. aureus.29 It is evident that the oral environment in RDP wearers is the habitat a polymicrobial community that interacts and forms a structured biofilm within a short period after clinical rehabilitation. However, the majority of studies refer to hygiene protocols only with regard to mono-species biofilms. Therefore, it is crucial that the antimicrobial analysis of RPD cleansers should explore different biofilm models.
For the aforementioned reasons, the present study analyzed the antimicrobial activity of cleansing solutions (mouthwash Cepacol™, effervescent tablet NitrAdine, and experimental solutions of R. communis (2% and 10%)) against mono- and multi-species biofilms (C. albicans, C. glabrata, S. aureus, and S. mutans) grown on a Co-Cr surface. The null hypothesis of this study was that the viability of the microorganisms and the biofilm-covered areas would be influenced by the cleansing protocols.
Material and methods
Experimental solutions
Castor oil was extracted from seeds, using the cold pressing method (Chemical Institute of São Carlos, University of São Paulo, São Carlos, Brazil). Initially, to formulate the R. communis solution, an esterification reaction with alcohols was performed. Afterward, the ester-containing solution was diluted in distilled water at final concentrations of 2% (RC02) and 10% (RC10) (v/v). The commercial mouthwash Cepacol (Reckitt Benckiser, São Paulo, Brazil) (CPC) was directly applied without dilution. The peroxide-based solution (Ni) was prepared by diluting one NitrAdine effervescent tablet (Bonyf, Vaduz, Liechtenstein) in 150 mL of water at 37°C, as directed by the manufacturer (Table 1).
Specimen manufacturing
A total of 244 Co-Cr disks were manufactured using the lost-wax casting method. Circular wax patterns (Ø 12 × 3 mm) were made using a metal matrix. The wax patterns were covered with the Micro-fine 1700 phosphate coating (Talladium Brazil, Curitiba, Brazil) and casting was performed using the Neutrodyn Easyti electronic machine (F.Lli Manfredi, Turin, Italy) by vacuum electroinduction. The disks were deflated and blasted with 100-micrometer aluminum oxide particles (Aluminum Oxide 100; Asfer Indústria Química, Sao Caetano do Sul, Brazil) at a pressure of 3 bar, using the Microjet III device (EDG, São Carlos, Brazil) for cleaning. After being separated from the feed channel, the opposing surfaces were progressively polished with 220-, 400-, 600-, and 1,200-grit sandpaper (Norton Abrasivos Brasil, Guarulhos, Brazil). The surface roughness of the specimens was standardized in the range of 0.04–0.10 μm.5 The specimens were packaged in envelopes and sterilized with ethylene oxide.
Culture conditions
Four strains from the American Type Culture Collection (ATCC) were used for biofilm development: C. albicans (ATCC 10231); C. glabrata (ATCC 2001); S. aureus (ATCC 25923); and S. mutans (ATCC 25175). The experiment was carried out in 3 biological replications with 3 technical repetitions each, totaling in 9 specimens per group.
Biofilm growth was conducted under aseptic conditions, following the protocol described previously.11 Briefly, the strains kept at −80°C in a glycerol stock were thawed and streaked out on a selected agar culture medium: for C. albicans and C. glabrata – Sabouraud Dextrose Agar (SDA) (HiMedia Laboratories, Mumbai, India); and for S. aureus and S. mutans – Brain Heart Infusion (BHI) broth (HiMedia). The plates were incubated at 37°C for 24 h. Subsequently, a microbial colony was transferred to its respective broth medium and re-incubated at 37°C for 24 h to obtain cells in the exponential growth phase. The cultures were then centrifuged at 4,200 g for 5 min. The resulting pellet was washed twice with phosphate-buffered saline (PBS). Candida spp. counting was performed in the Neubauer chamber (Kasvi, Curitiba, Brazil) due to the variable morphology of the genus. To adjust the cell concentration (108 colony-forming units per milliliter (CFU/mL)), the bacterial suspension was read on a spectrophotometer (Multiskan GO; Thermo Scientific, Waltham, USA) at 625 nm.
For mono-species biofilms, the inoculum was separately prepared in Sabouraud Dextrose Broth (SDB) (HiMedia) (C. albicans and C. glabrata) and BHI Broth (HiMedia) (S. aureus and S. mutans) at a cell concentration of 106 CFU/mL. The specimens were randomly assigned into 12-well cell culture plates (TPP Techno Plastic Products, Trasadingen, Switzerland) and filled with 2 mL of the inoculated culture media. In this model, each specimen was contaminated with only one species.
For a multi-species biofilm, the inoculum was prepared with the mixture of the 4 evaluated microorganisms for the specimens to be simultaneously contaminated with the Candida spp. and bacteria. The inoculum was prepared in BHI Broth at a cell concentration of 107 CFU/mL for bacteria and 106 CFU/mL for Candida spp. As in the case of the mono-species biofilms, the specimens were randomly assigned into 12-well cell culture plates and filled with 2 mL of the inoculated culture medium.
To attest the sterility of the experiment, one additional specimen received a sterile culture medium. The specimens were kept in an incubator (Shaker Incubator CE-320; Cienlab, Campinas, Brazil) at 37°C for 90 min under agitation (75 rpm) for the adhesion period. After this period, the specimens were washed twice with PBS and the same volume of a sterile culture medium was added to the wells. The plates were re-incubated for 48 h. After 24 h, 1 mL of the culture medium was removed, and the same volume of a fresh culture medium was added to the wells. All cultivation steps were performed in a microaerophilic environment.
Hygiene protocols
The specimens were transferred to sterile perforated stainless-steel baskets30 and placed inside containers with 150 mL of a cleanser solution, remaining fully immersed. An adapted stainless-steel wire allowed the baskets to remain suspended and not touch the bottom of the container. Immersion in the R. communis-based solutions and CPC was performed for 20 min and 10 min, respectively. The immersion times were chosen based on the results of previous studies, which demonstrated both antibiofilm effects and the absence of adverse effects for the hygiene solutions.3, 12, 19, 20, 22 Immersion in Ni was performed for 15 min, according to the manufacturer’s instructions. Immersion in distilled water for 20 min was used as a control, and the rationale for the immersion time was based on the longest evaluated period. The specimens of negative control (without contamination) were also immersed in distilled water for 20 min. At the end of the immersion periods, the specimens were rinsed 3 times with sterile PBS to eliminate cleanser residues.
Viability assay
After immersion, the specimens were transferred to a tube containing 10 mL of the Letheen Broth medium (BD Difco™, Sparks, USA). The tubes were sonicated (200W, 40 KHz) (Clean 9CA; Altsonic, Ribeirão Preto, Brazil) for 20 min to detach the remaining microorganisms. The resulting suspension was vortexed for 30 s, and serial dilutions (10−1 to 10−4) were seeded in a selected culture medium: for C. albicans and C. glabrata – CHROMagar Candida Medium (CAC) (BD Difco); for S. aureus – Mannitol Salt Agar (MSA) (HiMedia), supplemented with nystatin (200 U/mL); and for S. mutans – Mitis Salivarius Agar (HiMedia), supplemented with nystatin (200 U/mL) and bacitracin (0.2 U/mL). The plates were incubated at 37°C for 48 h. The incubation of S. mutans was performed in microaerophilic conditions. The number of colonies was registered and expressed in log10CFU/mL.
Biofilm removal capacity
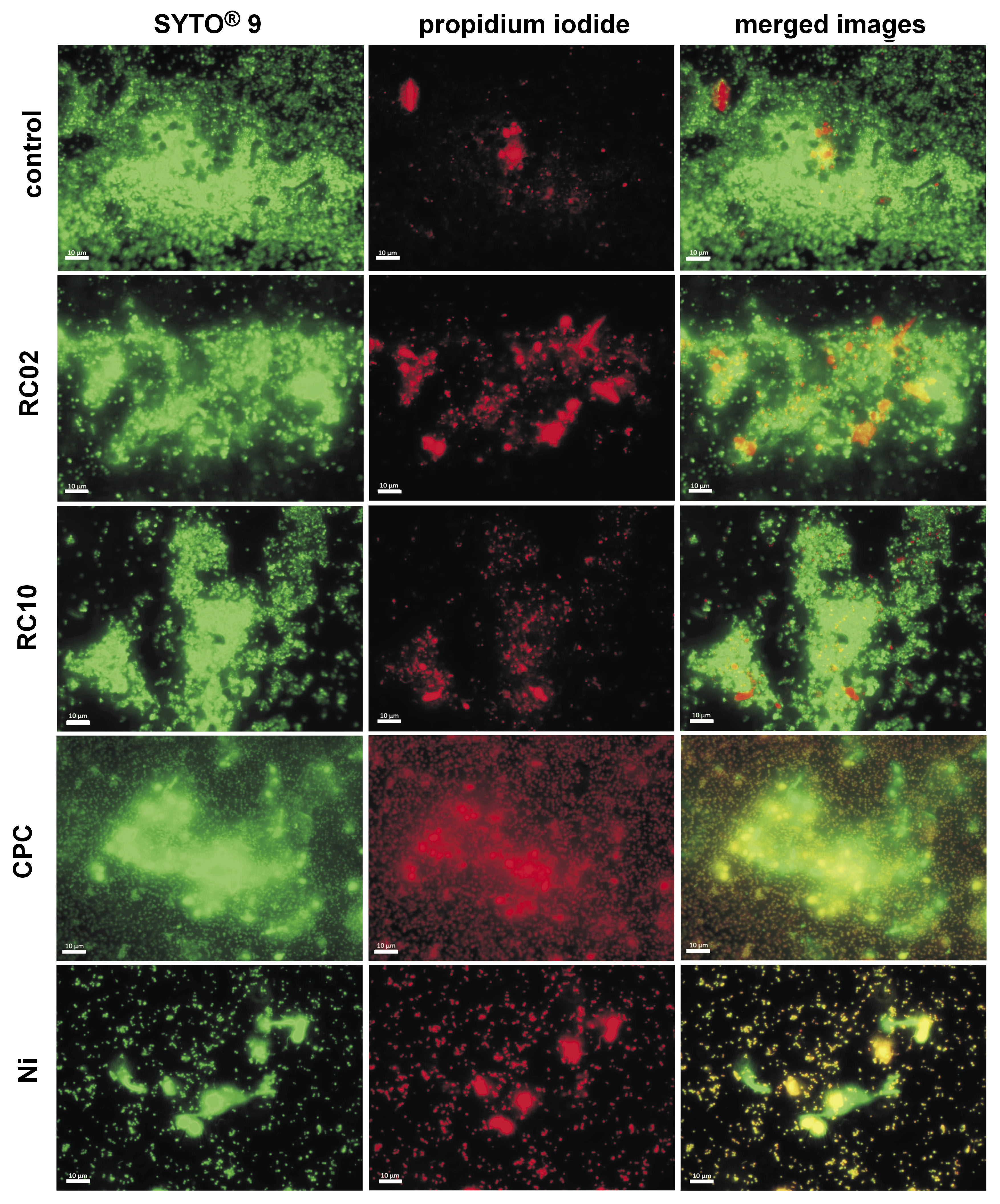
Since elevated resistance to the hygiene protocols was observed in the multi-species biofilm, an evaluation of the biofilm removal capacity was performed in this case. Thus, the specimens with the multi-species biofilm were analyzed by visualizing the amount of live and dead cells on the surfaces of the specimens. After conducting the hygiene protocols, 2 specimens from each group were transferred to a new 12-well plate and stained with 1.5 mL of LIVE/DEAD BacLight™ Kit (Invitrogen Molecular Probes, Eugene, USA), prepared according to the manufacturer’s instructions. Briefly, the working solution was prepared by adding 3 μL of the SYTO® 9 stain and 3 μL of the propidium iodide stain to 1 mL of distilled-sterilized water.
The plates were incubated for 15 min at room temperature, protected from light. The surfaces of the specimens were subsequently washed with PBS and analyzed under an inverted fluorescence microscope (Carl Zeiss, Oberkochen, Germany) with the appropriate filters. Twenty random fields were captured at ×630 magnification to quantify the total area occupied by green and red cells. Images were captured with the ZEN Lite software, v. 2.3 (Carl Zeiss), and the biofilm-covered areas [µm2] were quantified with the AxioVision software, v. 4.8.2 (Carl Zeiss). Since all cells are dyed green, the area was considered as the total biofilm (live and dead cells). Red staining indicated dead cells. The area of the living biofilm was calculated as the difference between the green-stained cell area and the red-stained cell area.14
Statistical analysis
At first, the data was tested to check for normal and homogeneous distribution by the Shapiro–Wilk and Levene tests, respectively. According to distribution, the Kruskal–Wallis test, followed by Dunn’s post-test, or the analysis of variance (ANOVA), followed by Tukey’s post-test were used to compare the results. Statistical analysis was performed using the IBM SPSS for Windows software, v. 21.0 (IBM Corp., Armonk, USA), at a significance level of 0.05.
Results
Viability of microorganisms
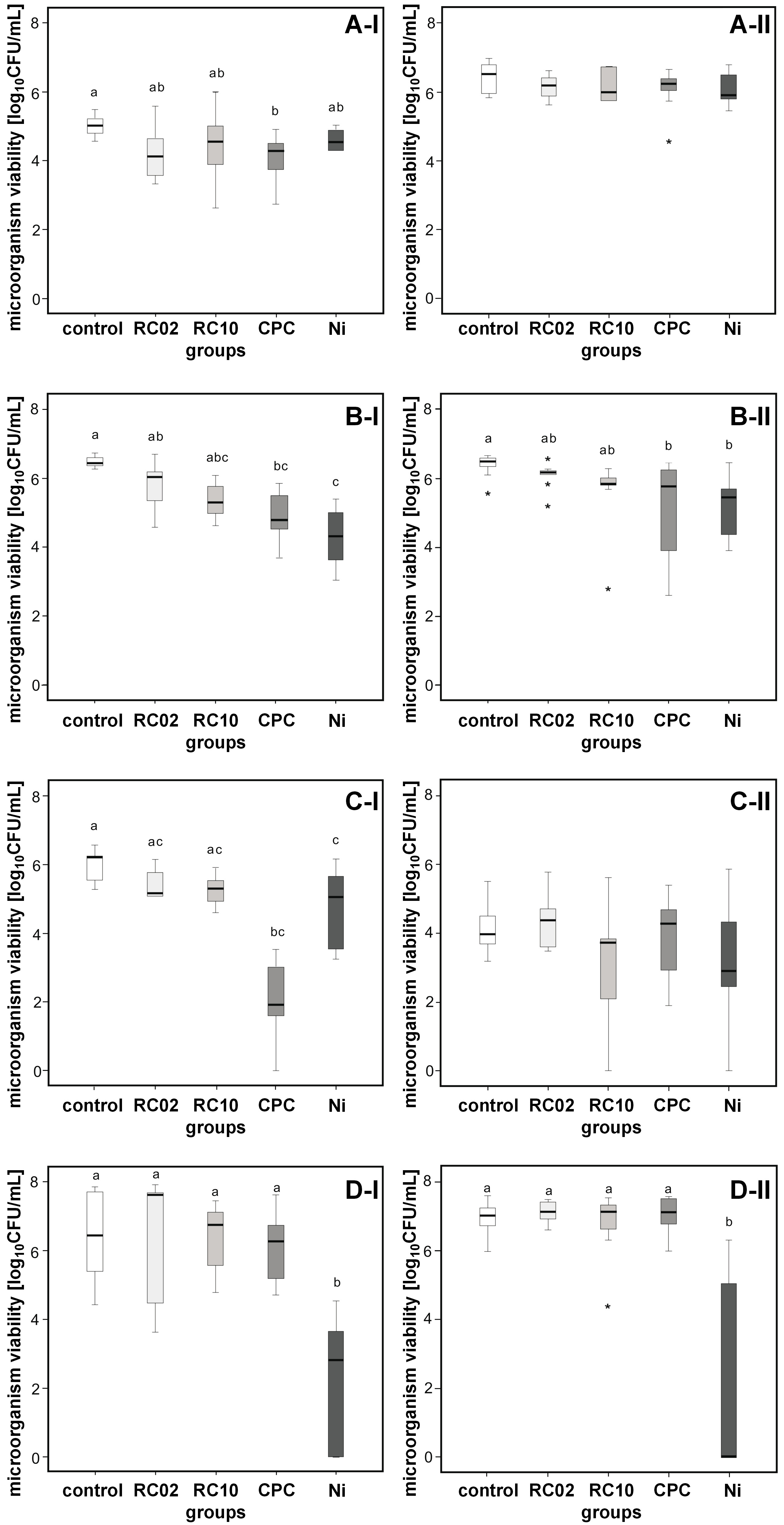
Based on the analysis of the biofilm viability, Ni showed the strongest antimicrobial action. In comparison with the control group, immersion in Ni reduced the microbial load of C. glabrata (2.18 log; p < 0.001), S. aureus (1.37 log; p = 0.012) and S. mutans (4.38 log; p = 0.002) when grown singly. The solution was also effective against C. glabrata (1.20 log; p = 0.006) and S. mutans (4.67 log; p = 0.010) when grown in association with other species. Cepacol promoted reduction in the viability of C. albicans (1.08 log; p = 0.018), C. glabrata (1.82 log; p = 0.001) and S. aureus (4.14 log; p < 0.001) grown in the mono-species biofilms. Regarding the multi-species biofilm, antimicrobial action was observed only against C. glabrata (1.34 log; p = 0.035). The experimental hygiene solutions RC02 and RC10 were not effective in reducing the viability of microorganisms grown in different biofilm patterns (Figure 1 and Table 2).
Growth in association with different species seems to have increased the resistance of C. albicans and S. aureus, since CPC and Ni did not reduce the viability of the microorganisms in the multi-species biofilm, as happened for single biofilms. In contrast, this behavior was not observed for C. glabrata and S. mutans (Table 2).
Biofilm removal capacity
With regard to the biofilm removal capacity, lower rates of live biofilm (green-stained cells) could be observed in comparison with the rates of total biofilm (green- and red-stained cells) (p < 0.001). In agreement with the viability results, Ni promoted a considerable reduction of the living biofilm (p < 0.001). Cepacol, RC2 and RC10 presented moderate efficacy in reducing the amount of the living biofilm. When the total biofilm areas were compared, it was found that Ni resulted in a greater removal of the biofilm than other solutions (Table 3). After immersion in all different hygiene solutions, a large amount of the aggregated dead biofilm (red-stained cells) remained, covering an extensive portion of the surfaces of the specimens (Figure 2). This finding indicates that, although Ni and CPC reduced cell viability (green-stained cells), they could not widely eliminate the biofilm from the surfaces of the specimens.
Discussion
The scientific literature has demonstrated that biofilm development is a remarkable issue in medical device-associated infections.31 This study was carried out using mono- and multi-species biofilms in order to clarify if biofilms developed by single strains have greater susceptibility to hygiene solutions than those developed by multiple strains. The species association seems to have increased the resistance of C. albicans and S. aureus, since CPC and Ni had no effect when the microorganisms grew in a multi-species biofilm model.
Promising biological findings involving R. communis suggest that the ethanolic, methanolic or hexane fractions obtained from its leaves and seeds can be an alternative source of therapeutic substances.17, 18, 21 Previous studies showed that the solutions obtained by the esterification of ricinoleic acid were beneficial for the control of biofilm formation on acrylic resin and silicone surfaces.19, 20, 22, 23 Nonetheless, the scientific literature has brought to light a cascade of controversial results regarding the concentration of the R. communis solution capable of exerting biological effects. The investigated concentrations ranged from 2% to 10%; however, until now, no ideal concentration has been established, leaving researchers struggling with conflicting evidence.8, 19, 20, 22 Therefore, in this study 2 extreme concentrations were evaluated. What should also be taken into account is the fact that RPDs are composed of artificial teeth, acrylic resin and the dental alloy. Since cell adhesion and biofilm formation depend on the composition of the surface,32 one cannot assume that hygiene solutions will have the same effect on all surfaces.
The antibiofilm activity of the R. communis solutions was slightly disappointing. The solutions only promoted a modest reduction of the viability of C. albicans, C. glabrata and S. aureus in both the mono- and multi-species biofilms, yet the reduction was not statistically significant. Andrade et al., investigating the 2% concentration, indicated that the solution had an intermediate antibiofilm action, comparable to the that of an effervescent tablet (Polident).19 The authors concluded that single immersion was insufficient for broadly promoting biofilm removal and suggested that association with mechanical brushing would be suitable for better results.19 Some clinical studies showed biofilm removal capacity, the reduction of the microbial load and the remission of denture-related stomatitis after using R. communis solutions.8, 22, 24
The antimicrobial effect of R. communis is probably linked to its toxicity, which is attributed to the protein ricin. The seeds have ricin at a percentage of up to 5%; the biological function of the protein is inhibiting protein synthesis.33 Worbs et al. indicates that ricin removes adenine from the so-called sarcin-ricin loop of 28S rRNA, thereby preventing the binding of elongation factors and further protein synthesis.33 As reviewed by Yeboah et al., the composition and properties of castor oil vary with respect to the method of extraction, geographical location and the type of cultivar.34 Thus, in view of these statements, we suggest 2 different reasons to explain the insufficient antimicrobial effect of R. communis in this study. First, the discrepancy of results presented by the literature, as well as the absence of antibiofilm activity presented here, might be associated with extraction methods and oil purity. Second, as the antimicrobial effect seems to be attributed to the inhibition of protein synthesis, one single 20-minute application would not alter protein synthesis to the point of presenting reduction in the microbial load.
The mouthwash Cepacol was more effective against the mono-species biofilms. In the multi species biofilm pattern, it only reduced the viability of C. glabrata. These findings are in line with microscopy evaluations. The images obtained from the CPC group showed a modest reduction of the multi-species biofilm-covered areas, suggesting a limited disaggregating capacity. Cepacol has 0.05% of cetylpyridinium chloride as an active ingredient. This is a quaternary ammonium compound that affects cell integrity by interfering with osmoregulation and homeostasis. Diverse in vitro studies report the antibacterial activity of Cepacol against planktonic bacteria.35 The apparent discrepancy between our findings and those of other researchers can be related to the antimicrobial susceptibility of microorganisms in biofilm- and non-biofilm-associated states. Biofilm tolerance to antimicrobial agents is about 100–1,000 times greater as compared to that of the planktonic form.36
NitrAdine presented the best antibiofilm action against the largest number of species evaluated. In both the mono- and multi-species biofilms, Ni reduced the viability of S. mutans and C. glabrata in about 4 log and 1 log, respectively. The S. aureus mono-species biofilm was also reduced in about 1 log after immersion in Ni. The antimicrobial effect of Ni is attributed to sodium lauryl sulfate and sodium bicarbonate that act through injuring the microbial cell membrane.37, 38 In addition, another active ingredient of Ni, citric acid, is associated with the capability of disturbing the microbial metabolism.39 Controversies about the antibiofilm effectiveness of effervescent tablets emerge in the scientific literature. Supporting our findings, Coimbra et al. reported that Ni exhibited satisfactory antibiofilm activity, reducing the microbial load and metabolic activity, and the area covered by the multi-species biofilm composed of C. albicans, S. aureus and Pseudomonas aeruginosa.14 Effective antibiofilm activity of Ni against the S. mutans biofilm in a multi-species biofilm model was demonstrated by Lopes Vasconcelos et al.11
In agreement with the viability reduction observed in CFU counts, the microscopy images indicated a significant reduction of the living biofilm after immersion in Ni. The reaction between sodium bicarbonate and citric acid, the active ingredients of Ni, in the presence of water leads to the liberation of carbon dioxide, promoting the effervescent aspect. It has been postulated that the release of effervescence can induce a mechanical effect that disrupts biofilms, which could explain the superior ability of effervescent tablets in removing microbial deposits.40 Nonetheless, despite significant antimicrobial activity, about 31% of the surfaces of the specimens remained covered by the residual aggregated biofilm after immersion in Ni. This can be interpreted as evident antimicrobial action of Ni, but also as its incapability to completely remove all aggregates. It was suggested by the Council on Dental Materials, Instruments, and Equipment that the release of bubbles from effervescent tablets might promote a mechanical action favoring the detaching of the biofilm from the surface of the prosthesis.40
The current study was limited by the fact that biofilms were grown considering only ATCC samples. It is recognized that hygiene solutions should be tested on clinical samples employing multidrug-resistant strains. In addition, single short-time immersion was applied. In light of future studies, we believe that investigating distinct multi-species biofilms is essential, considering the high diversity of the buccal microbiome. Combinations involving members of both Gram-positive and Gram-negative groups, as well as other Streptococcus spp. or anaerobic rods, could better represent the microbiome of RPDs. Evaluating the clinical effect of RPD immersion in the tested solutions in association with mechanical biofilm removal is another important aspect to be considered in further studies.
Conclusions
Considering the limitations of the study, the findings clearly illustrate that none of the evaluated solutions was able to widely reduce the viability of the microorganisms and the biofilm-covered areas. Although NitrAdine reduced the viability of the largest number of species, it did not alter the microbial load of C. albicans. Cepacol reduced the viability of microorganisms in the mono-species biofilms; however, its action was unsatisfactory in the multi-species biofilms.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.