Abstract
Background. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants exhibit different phenotypes and clinical manifestations in comparison to non-mutated viruses. Spike gene target failure (SGTF) is a characteristic feature of the gene in a novel variant that is recognized as highly transmissible. Several studies have demonstrated the virucidal effects of mouthwashes on SARS-CoV-2. Moreover, mouthwashes have proven beneficial for patients undergoing oral and maxillofacial surgery.
Objectives. The present study aimed to analyze the effects of 2 different types of mouthwash (0.2% chlorhexidine gluconate and 1% povidone-iodine) on the cycle threshold (CT) values in coronavirus disease 2019 (COVID-19) patients with and without SGTF.
Material and methods. This single-blind, non-randomized controlled clinical trial comprised 45 patients who were divided into 3 groups based on the intervention method: 0.2% chlorhexidine gluconate mouthwash; 1% povidone-iodine mouthwash; and mineral water (control group). The patients were instructed to gargle with the assigned solution 3 times a day for 5 days. Reverse transcription polymerase chain reaction (RT-PCR) tests were conducted at the time of initial diagnosis and on days 3 and 5. A normality test (Shapiro–Wilk test) was performed. Consequently, the non-parametric Friedman test was used.
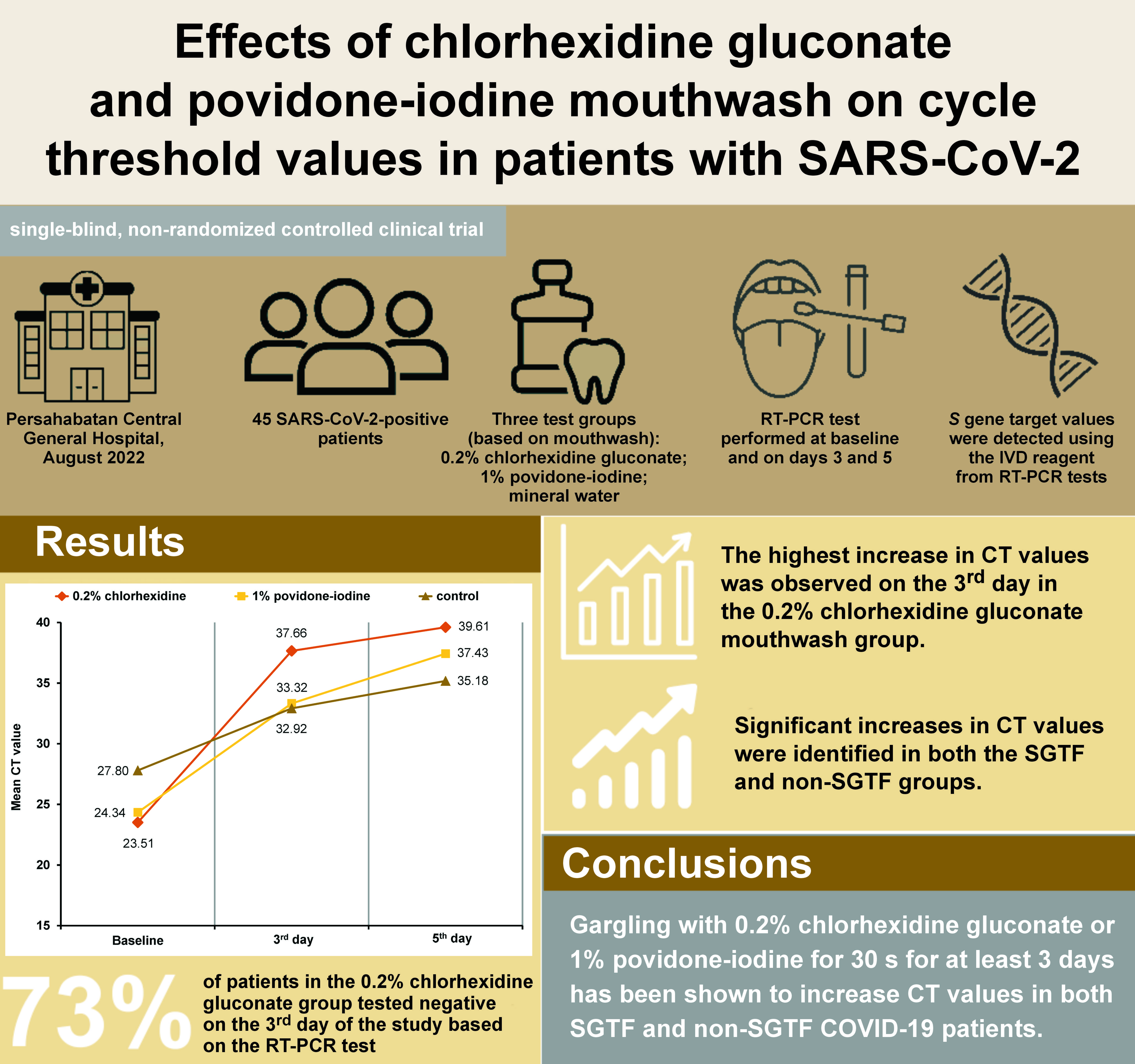
Results. The analysis revealed that the subjects who utilized mouthwashes exhibited higher CT values in comparison to the control group. Furthermore, 73% of patients who used 0.2% chlorhexidine gluconate presented with increased CT values, as indicated by a negative RT-PCR test on the 3rd day.
Conclusions. Gargling with 0.2% chlorhexidine gluconate or 1% povidone-iodine for 30 s for at least 3 days has been demonstrated to increase CT values in both SGTF and non-SGTF COVID-19 patients. Hence, using the mouthwash may be considered for preoperative use in patients undergoing oral and maxillofacial surgery.
Keywords: mouthwash, COVID-19, chlorhexidine, povidone-iodine, CT value
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly infectious virus that primarily affects the respiratory tract. The virus is transmitted via breathing, coughing or sneezing; additionally, it can be disseminated through direct contact with contaminated surfaces and then touching the nose, mouth and eyes.1, 2, 3 Reverse transcription polymerase chain reaction (RT-PCR) is a diagnostic test used for detecting the presence of SARS-CoV-2.4, 5
Different variants of SARS-CoV-2 exhibit different characteristics. The Omicron variant, a novel mutated form of SARS-CoV-2 known as B.1.1.529, has been designated as a variant of concern by the World Health Organization (WHO).6, 7 Omicron demonstrates high transmissibility, spreading more rapidly than other variants.
Several RT-PCR protocols have been used to describe the characteristics of specific variants of SARS-CoV-2. The spike glycoprotein (S) gene is used to detect the SARS-CoV-2 variant.8, 9 Spike gene target failure (SGTF), which refers to a failure to detect this gene, has been observed in patients with the Omicron variant. In contrast, non-SGTF coronavirus disease 2019 (COVID-19) patients often present with other types of SARS-CoV-2 variants.6 The classification of the SARS-CoV-2 variant as SGTF or non-SGTF provides greater specificity and can serve as an initial screening method for the identification of the SARS-CoV-2 variant that has this mutation.
Mouthwash has been used to prevent the transmission of SARS-CoV-2 prior to dental treatment, including oral and maxillofacial surgery. According to Huang and Huang, chlorhexidine gluconate effectively (86.0%) reduced SARS-CoV-2 in the oropharynx.10 The Centers for Disease Control and Prevention (CDC) recommend using a povidone-iodine-based mouthwash before any treatment in the oral cavity.11 In another study, 1% povidone-iodine suppressed the load of the SARS-CoV-2 in the oral cavity.12
However, direct clinical trials on SGTF and non-SGTF COVID-19 patients are limited. The aim of this study was to analyze the effects of 2 types of mouthwash (0.2% chlorhexidine gluconate and 1% povidone-iodine) on the cycle threshold (CT) values in RT-PCR tests in patients with all variants of SARS-CoV-2.
Material and methods
Ethical clearance
The study protocol was reviewed and approved by the Health Ethics Committee of Persahabatan Central General Hospital, Jakarta, Indonesia (protocol No. 73/KEPK-RSUPP/08/2022).
Study design and intervention
The study was performed at Persahabatan Central General Hospital in August 2022. This single-blind, non-randomized controlled clinical trial comprised 45 patients who were divided into 3 intervention groups: a 0.2% chlorhexidine gluconate mouthwash group (n = 15); a 1% povidone-iodine mouthwash group (n = 15); and a mineral water control group (n = 15). The mouthwash was repackaged in 125-mL bottles. Each subject received 2 bottles of mouthwash (250 mL in total).
Patients who met the inclusion criteria and were being treated at the oral and maxillofacial surgery clinic underwent RT-PCR examination. The collection of sample material for RT-PCR was carried out by trained personnel in the microbiology laboratory at Persahabatan Central General Hospital. No specific time for sample collection was stipulated. The patients were instructed to gargle with 15 mL of a mouthwash (30 s in the oral cavity and 30 s in the back of the throat) 3 times a day for 5 days. Subsequent to gargling, the subjects were asked to rinse their mouth with 15 mL of water. Observations were carried out via video call for each gargle. Reverse transcription polymerase chain reaction examinations were performed to obtain CT values at baseline and on days 3 and 5. All sample materials for RT-PCR were taken from the oropharyngeal swabs using a disposable virus sampling tube (Baicare Biotechnology Co., Ltd., Beijing, China). The specimens were then vortexed with an LMS® UZUSIO VTX-3000L vortex mixer (LMS Co., Ltd., Tokyo, Japan) for 20 s and left to stand for 15 min. The IVD Reagent MAD-003941M (Vitro Master Diagnostica®, Madrid, Spain) was mixed with 200 μL of the specimen. The cartridge was loaded into the MagNA Pure 96 instrument (Roche, Basel, Switzerland) for sample extraction. The reaction mixture of the mBioCoV-19 RT-PCR Kit (Bio Farma, Bandung, Indonesia) was used for the detection of open reading frame 1b and RNA-dependent RNA polymerase genes. In brief, 15 μL of the reaction mix were added to each well and subsequently mixed with 5 μL of the extracted specimen. Cycle threshold values were obtained automatically upon the detection of SARS-CoV-2 genetic material with the use of an Exicycler™ 96 (v. 4) (RRID:SCR_022144) Real-Time Quantitative Thermal Block (Bioneer Corporation, Daejeon, South Korea). The use of this reagent in all samples enabled the measurement of CT values and the identification of S gene targets. Samples without the S gene were categorized as SGTF, while those containing the S gene were classified as non-SGTF.
Sample population
Prior to enrollment in the study, the patients were subjected to a screening process that evaluated their eligibility based on a set of predetermined criteria. The inclusion criteria were as follows: SARS-CoV-2-positive patients, as confirmed by RT-PCR results within the previous 3 days; an RT-PCR CT value of ≤30; outpatients with mild or no symptoms; and an age range of 20–50 years. Patients with comorbidities, those with a history of allergy to povidone-iodine mouthwash and chlorhexidine gluconate, pregnant females, and those who were not willing to participate were excluded from the study.
Sample size
The sample size was determined using the G*Power v. 3.1.9.7 software (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower), and the estimated results were 15 patients in each group.
Statistical analysis
The Shapiro–Wilk test was used to check for the normality of data. Then, the non-parametric Friedman test and the post hoc Wilcoxon test were used to evaluate the CT values. The data was analyzed using the IBM SPSS Statistics for Windows software, v. 22.0 (IBM Corp., Armonk, USA). A value of p < 0.05 was considered statistically significant.
Results
Characteristics of patients
As shown in Table 1, 51.1% (n = 23) of the patients were female and the remaining 48.9% (n = 22) were male. The age of the patients ranged from 21 to 48 years, and 40.0% of the patients belonged to the 31–40 age group. Furthermore, 34 patients (75.6%) were identified with SGTF, while the remaining 11 (24.4%) belonged to the non-SGTF group.
CT values
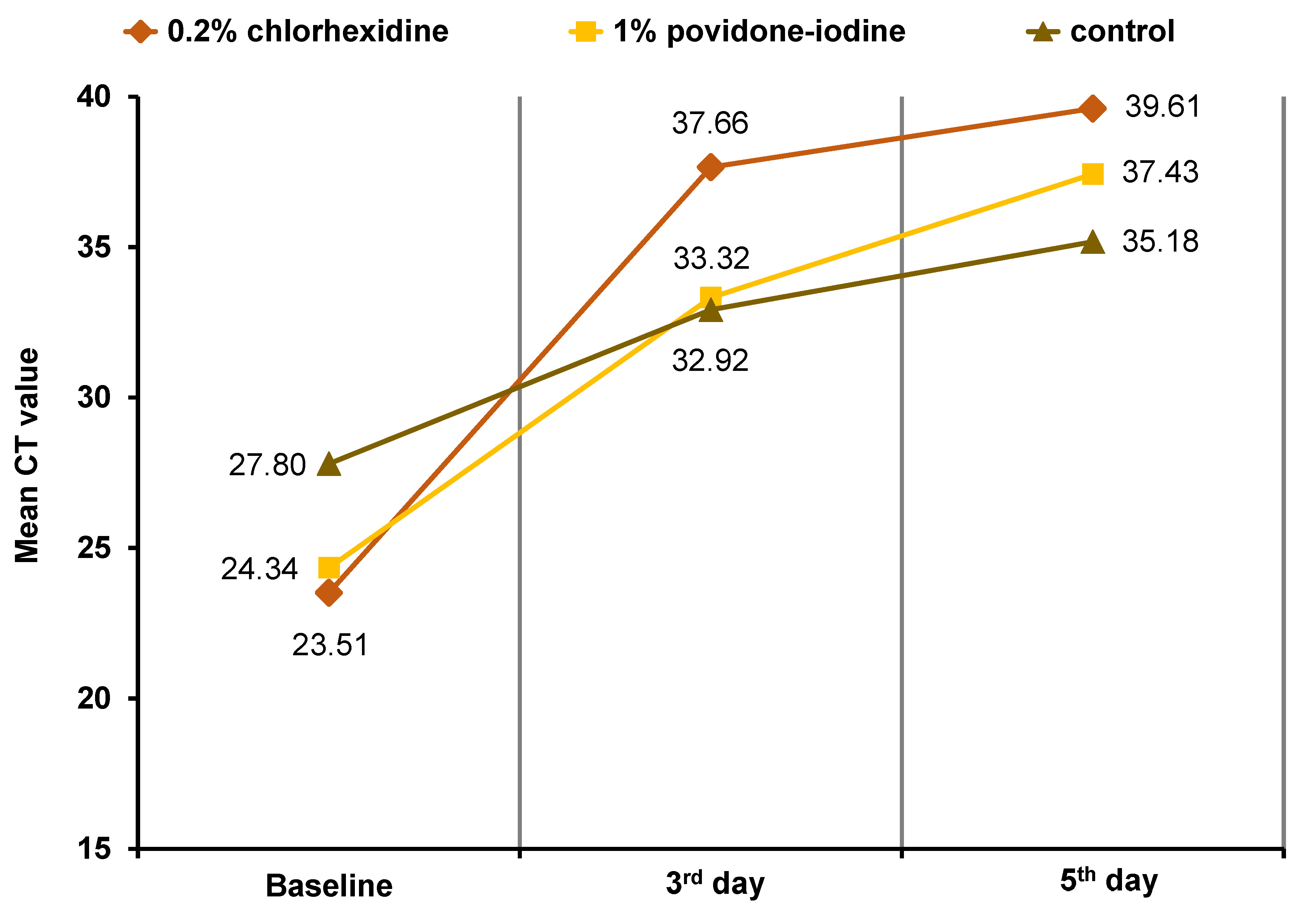
Statistically significant differences in CT values (p < 0.05) were observed in all 3 groups following gargling at baseline and on days 3 and 5 (Table 2). A statistically significant difference was observed based on the intervention time, indicating that CT values increased on a daily basis. The highest average increase in CT values (14.15) was observed from baseline to day 3 in the 0.2% chlorhexidine gluconate mouthwash group. A total of 73% of patients who used 0.2% chlorhexidine gluconate presented with increased CT values, as indicated by a negative RT-PCR test on the 3rd day. The 1% povidone-iodine mouthwash group exhibited an average increase of 4.11 from day 3 to day 5 (Figure 1). Similarly, significant increases in CT values were observed in all 3 groups following gargling in SGTF and non-SGTF COVID-19 patients (Table 3). The CT values in the SGTF and non-SGTF groups exhibited a significant increase on a daily basis until day 5.
Discussion
Severe acute respiratory syndrome coronavirus 2, which caused the global pandemic of COVID-19, has infected more than 627 million people worldwide and more than 6.4 million Indonesians until October 2022.13 At the time of this study, there were 2,087 active cases of the virus in Indonesia. The majority of the patients in the current study were in the 31–40 age group, with an overall age range of 21–48 years. These findings are consistent with those reported by Megasari et al.14 As reported by Karyono et al., 80% of patients infected with SARS-CoV-2 exhibited mild symptoms, and 18% of patients were asymptomatic. Consequently, the patients were unaware of their infection, which resulted in the transmission of the virus to health workers.15
Several studies have been conducted to establish the most effective prevention protocol against SARS-CoV-2. The present study aimed to evaluate the preventive effects of 0.2% chlorhexidine gluconate and 1% povidone-iodine mouthwashes on patients infected with SARS-CoV-2. Many viruses present in the oral cavity and upper respiratory tract can be transmitted through various means, including speech, sneezing or coughing. These pathogens can also be disseminated during medical procedures performed in the oral cavity. Shankar et al. stated that the upper respiratory tract plays the most important role in the transmission of SARS-CoV-2.12 Likewise, Karyono et al. reported the presence of the virus in the oral cavity, particularly the saliva, and further noted that the viral load in saliva at the onset of the infection was higher than that in the oropharynx.15 Gargling with mouthwash has been shown to reduce the number of viruses in the oral cavity and at the back of the throat. The decrease in the number of viruses can be estimated by the CT value in the RT-PCR test, which indicates the concentration of the genetic material of the virus in a specimen.16, 17 Chlorhexidine gluconate and povidone-iodine mouthwashes have antibacterial and antiviral properties; they are commonly used and readily available. In studies by Boyapati et al.18 and Soundarajan and Rajasekar,19 chlorhexidine was considered the gold standard antibacterial mouthwash when compared to other novel types of mouthwash, such as probiotic mouthwash18 and amla seed-mediated graphene oxide-silver (GO-Ag) nanocomposite mouthwash.19
The standard molecular method for the diagnosis of COVID-19 is RT-PCR. The CT value describes the number of amplification cycles required for the target gene to exceed the threshold level during RT-PCR examination. Therefore, CT values are inversely proportional to viral load, thereby serving as an indirect method for calculating the number of copies of viral ribonucleic acid (RNA) present in a sample.16
As illustrated in Figure 1, the study demonstrated an increase in CT values in patients who gargled with the 2 types of mouthwash.20 This finding aligns with the results of previous studies, which demonstrated the effectiveness of chlorhexidine gluconate on SARS-CoV-2.21 Yoon et al. reported a decrease in the SARS-CoV-2 viral load in saliva after gargling with 0.2% chlorhexidine gluconate.22 Other studies have shown that 0.2% chlorhexidine gluconate can reduce the risk of SARS-CoV-2 transmission via aerosols.23, 24 In the present study, the highest increase in CT values was observed in the group that gargled with 0.2% chlorhexidine gluconate within the first 3 days (Table 4) compared to that in the 1% povidone-iodine group (14.15 vs. 8.98, respectively). There was a statistically significant difference in the first 3 days for all groups. The reason for the observed differences in the effectiveness of the 2 types of mouthwash remains unclear.
Table 2 demonstrates statistically significant differences in the mean CT values between the 2 types of mouthwash. The findings indicate that 1% povidone-iodine mouthwash can be used as an alternative to 0.2% chlorhexidine gluconate to effectively increase CT values. The American Dental Association (ADA) and the CDC have recommended using 1% povidone-iodine mouthwash before performing any procedures in the oral cavity, including those pertaining to oral and maxillofacial surgery. Furthermore, gargling with 1% povidone-iodine or 0.2% chlorhexidine gluconate has been shown to reduce the load of SARS-CoV-2 in the upper respiratory tract, thereby increasing the CT value. Gargling instigates a water cycle that mechanically washes away viruses and other infected cells adhered to the cilia in the epithelial mucosa of the oral cavity and throat.25 In the study by Robinot et al., SARS-CoV-2 infection in ciliated epithelial cells resulted in a loss of ciliary motility.26 The cilia in patients infected with SARS-CoV-2 were found damaged and shortened after gargling with 1% povidone-iodine and 0.2% chlorhexidine gluconate.
The control group in this study showed a statistically significant increase in CT values in RT-PCR, consistent with the findings of Satomura et al., who demonstrated that gargling with water at the oropharynx area 3 times a day effectively reduced the incidence of upper respiratory tract infections by 36%.25 Rinsing the upper respiratory tract, which mechanically removes excess mucus, is beneficial for patients infected with SARS-CoV-2. Gargling generates a swirl of water that mechanically removes viruses and virus-infected cells from the oral cavity and back of the throat.21, 22 This action has been shown to decrease viral load and increase CT values in RT-PCR.26
The subjects of this study were divided into 2 groups based on the detection of the S gene from the RT-PCR results. A mutation in the S gene due to a deletion of the H69-V70 amino acids results in the failure of detection of the S gene, or SGTF. This H69-V70 amino acid deletion has been identified in several variants of SARS-CoV-2, including the Omicron variant.6, 8 Significant differences in the increases in CT values were observed after gargling with 0.2% chlorhexidine gluconate and 1% povidone-iodine in both the SGTF and non-SGTF groups (Table 4). These results indicate that these 2 types of mouthwash are effective against all variants of SARS-CoV-2. Hence, gargling can be implemented as a supportive or complementary therapy within the COVID-19 treatment protocol. Beyond its role in mitigating the transmission of SARS-CoV-2, gargling with antimicrobial mouthwash prior to dental and oral surgery procedures has the potential to enhance oral hygiene and reduce gingival and periodontal inflammation. This, in turn, may lead to a reduction in the incidence of complications arising from COVID-19.27 In the context of future studies, other types of antimicrobial mouthwash, such as the probiotic mouthwash, could be evaluated for its efficacy in increasing the CT value of SARS-CoV-2, as reported in the study by Boyapati et al.18 The efficacy of probiotic mouthwash in treating chronic gingivitis has been demonstrated to be comparable.18
Conclusions
Gargling with 0.2% chlorhexidine gluconate or 1% povidone-iodine for 30 s for at least 3 days was shown to reduce the viral load of SARS-CoV-2. An increase in CT values was observed in patients with and without SGTF, indicating that mouthwash is effective against all SARS-CoV-2 variants. Gargling with 0.2% chlorhexidine gluconate or 1% povidone-iodine could be considered as an initial protocol prior to oral and maxillofacial surgical procedures in patients with COVID-19.
Trial registration
This study was registered in the International Standard Randomised Controlled Trial Number (ISRCTN) registry under No. ISRCTN13090248.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Health Ethics Committee of Persahabatan Central General Hospital, Jakarta, Indonesia (protocol No. 73/KEPK-RSUPP/08/2022).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.