Abstract
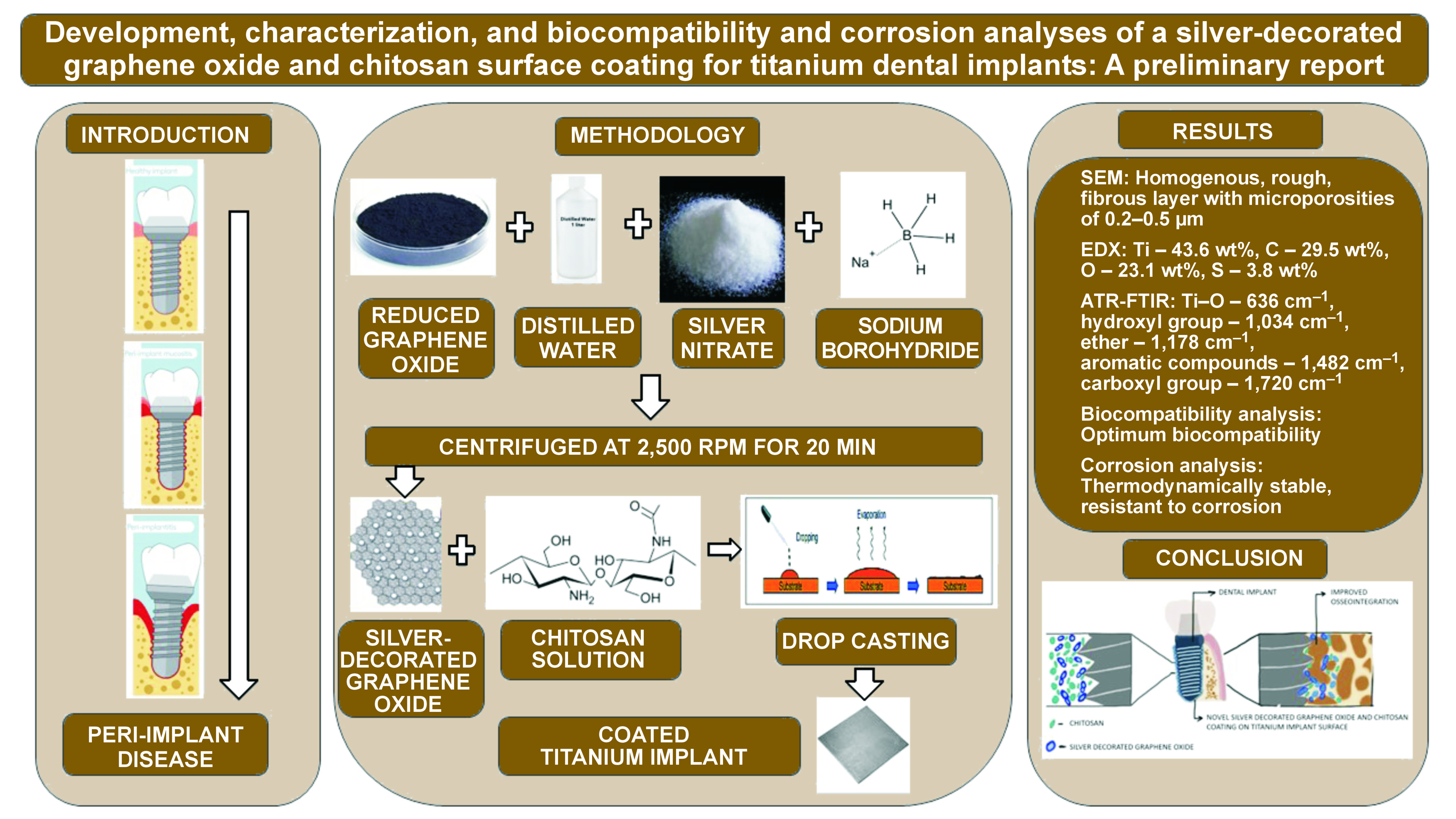
Background. Dental implants are increasingly favored as a therapeutic replacement option for edentulism. Titanium (Ti), due to its excellent biocompatibility and unique osseointegration properties, is commonly used in dental implants. Various surface modifications have been explored to improve osseointegration outcomes. Graphene oxide (GO) is a promising material with various applications. Chitosan, found in the exoskeleton of crustaceans and in marine algae, has several biomedical applications. Silver (Ag) is another promising antibacterial agent that increases permeability and damages the bacterial cell membrane upon binding.
Objectives. The present study applied a novel implant surface coating of Ag-decorated GO and chitosan on Ti implants to promote bone formation. We further analyzed the physiochemical and antibacterial properties of this surface coating.
Material and methods. A solution was prepared by mixing 3 mL of 1% chitosan solution with 10 mg of Ag-GO nanoparticles (NPs). Titanium metal was heated to 70–80°C on a hotplate and the solution was applied onto Ti to obtain an adhesive surface coating. The coated implant was further analyzed for surface properties, using scanning electron microscopy (SEM), the energy dispersive X-ray (EDX) analysis, the attenuated total reflectance-Fourier transform infrared (ATR-FTIR) technique, and the biocompatibility and corrosion analyses.
Results. The SEM analysis revealed a homogenously spread, rough, fibrillar and porous layer of coating on the metal surface. The EDX and ATR-FTIR analyses confirmed the successful coating of the implant surface with Ag-decorated GO and chitosan layers. The cell culture assay demonstrated excellent biocompatibility of the surface coating. The corrosion analysis showed improved corrosion resistance of the developed implant surface coating.
Conclusions. The various analyses of the coating showed ideal properties for improved cell attachment, differentiation and proliferation while maintaining an antimicrobial environment on the implant surface.
Keywords: chitosan, graphene, quality of life, silver, titanium
Introduction
Dental implants are becoming increasingly popular as a therapeutic replacement option for edentulism worldwide.1, 2 Titanium (Ti) is the preferred material due to its excellent biocompatibility and unique property of osseointegration with the surrounding bone. Various risk factors, such as smoking, diabetes, poor plaque control, peri-implant pathogenic microbiota, and iatrogenic factors, significantly affect the success of implants.3 A meta-analysis found that the prevalence of peri-implantitis was 18.5% at the implant level and 12.8% at the patient level.4 Despite personalized risk reduction strategies, a portion of the population is still affected by peri-implant diseases. This may be attributed to the underlying genetic makeup of individuals, which may cause differential tissue expression and a relative increase in implant placement globally. However, implant surface modifications have shown positive outcomes with improved osseointegration and long-term success rates.5
Graphene oxide (GO) is a promising material with various applications in electronics, biosensing, imaging, drug delivery, cancer treatment, tissue engineering, nanotherapeutics, and implantology. It is a single monomolecular layer of graphite with various oxygen-containing functional groups, such as carboxyl, hydroxyl and epoxide ones. Studies have explored the antibacterial potential of GO, finding that synthesized GO has sharp edges that act as nano-knives and disturb the membrane integrity of microbes, leading to cell death.6
Chitosan, a natural biopolymeric polysaccharide found in the exoskeleton of crustaceans and in marine algae, has several biomedical applications. Studies have demonstrated the antimicrobial, antioxidant, anti-inflammatory, and osteogenic properties of chitosan.7, 8, 9, 10, 11, 12 The cationic nature of chitosan is responsible for many of its biological functions, including antimicrobial, hemostatic, wound-healing, and controlled drug release activity.
Silver (Ag) is another promising antibacterial agent that increases permeability and damages the bacterial cell membrane upon binding. Inside the cell, Ag reacts with enzymes, producing reactive oxygen species (ROS) that impair DNA replication and cause cell death.13 The peri-implant space harbors unique and complex microbiota, and peri-implantitis and peri-implant mucositis have been associated with such microbial species as Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Prevotella intermedia, bacteroides, and Filifactor sp. Incorporating antimicrobial agents into implant surface coatings enables controlling microbial species.13
Studies have also shown the osteoinductive properties of Ag nanoparticles (NPs) in stem cells through increasing the expression of alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), bone morphogenetic protein 2 (BMP2), collagen type 1 alpha 1 (COL1A1), osteocalcin (OCN), and osteopontin (OPN).14
The present study applied an Ag-decorated GO and chitosan coating to Ti implants, analyzing its physicochemical properties, biocompatibility and corrosion resistance.
Material and methods
The study was conducted at the Department of Biomaterials, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences (SIMATS), Chennai, India, using analytical-grade chemicals purchased from Sigma Aldrich, Merck Group, Darmstadt, Germany. Commercially pure Ti grade 2 plates with dimensions of 20 mm × 15 mm × 2 mm were purchased from Ti Anode Fabricators, Chennai, India.
Development of the implant surface coating
The development of an Ag-decorated GO and chitosan adhesive surface coating involved dispersing 40 mg of reduced graphene in 100 mL of distilled water, adding 1 mmol of silver nitrate solution and 2 mL of 10 mmol sodium borohydride until a color change from black to grey occurred. The study used 1 mmol of silver nitrate to reduce it to AgNPs, aiming for a biocompatible concentration. The quantity of sodium borohydride was chosen based on molecular calculations. The reduction process continued until the color changed to metallic silver, indicating the complete reduction of metal Ag. The solution was centrifuged at 2,500 rpm for 20 min to obtain a pellet, which was dried to yield 10 mg of Ag-decorated GO NPs. A total of 3 mL of 1% chitosan solution of marine origin was mixed with 10 mg of Ag-GO NPs to obtain the final solution for implant surface coating. Titanium metal was heated to 70–80°C on a hotplate and the solution was added to obtain an adhesive surface coating.
Material characterization
The Ti surface coating was examined for surface properties under a field-emission scanning electron microscope (FE-SEM), using an accelerating voltage of 3 kV at a resolution of 10 µm. The energy dispersive X-ray (EDX) analysis was performed to characterize the samples chemically, and vibrational modes were confirmed through attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy (Alpha II; Bruker, Billerica, USA).
Biocompatibility analysis
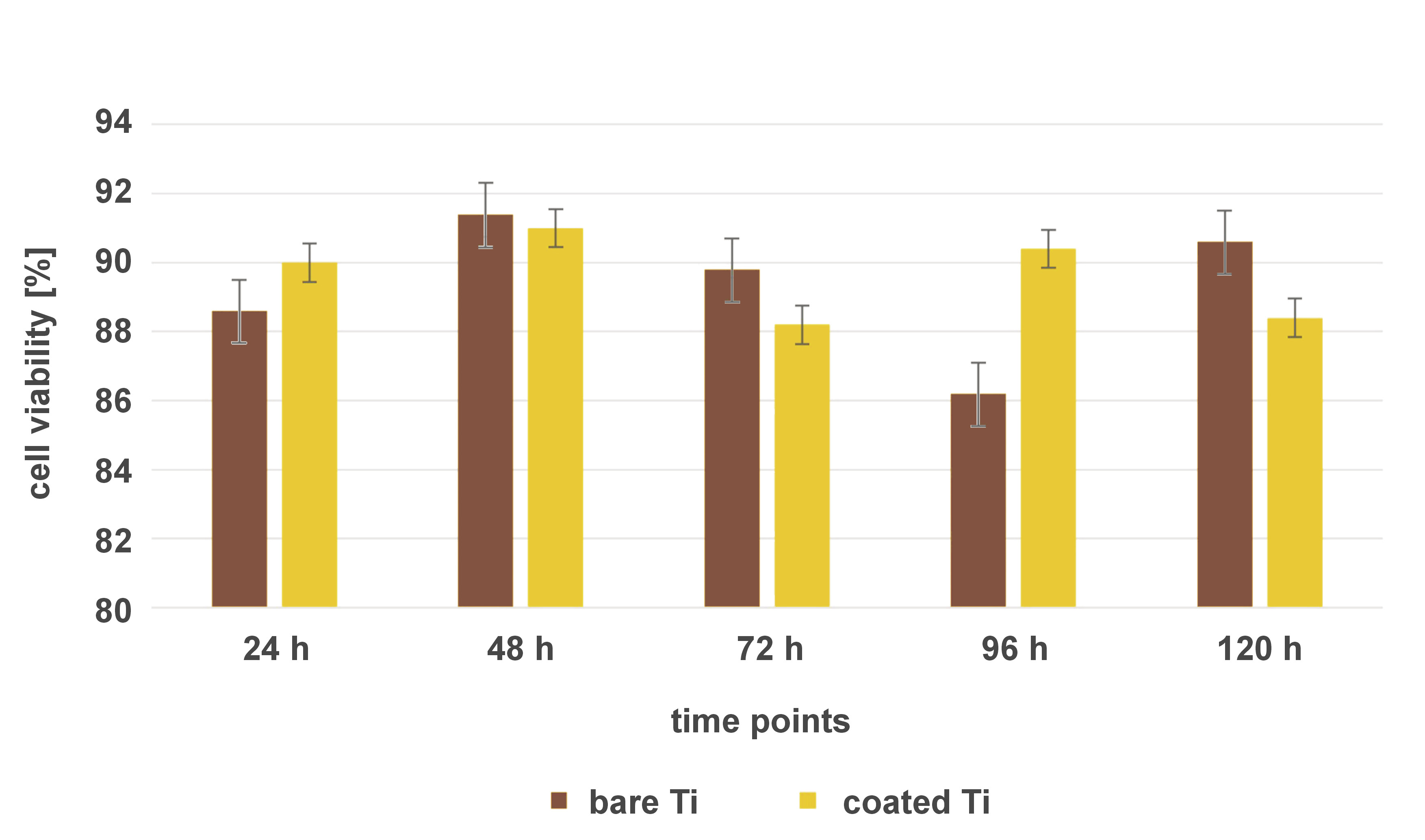
The developed implant surface coating was cultured with human MG-63 osteoblast-like cells (National Centre for Cell Science (NCCS), Pune, India) to analyze biocompatibility, cell morphology and growth pattern. Bare, non-coated Ti implant samples were used as the control. Dulbecco’s Modified Eagle Medium (DMEM) was prepared according to the manufacturer’s instructions. All growth media, supplements and cell stains were purchased from HiMedia Laboratories, Thane, India. The DMEM was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin to support cell growth and prevent contamination. The cryopreserved MG-63 cells were thawed in a water bath at 37°C, following standard protocols. The cells were cultured in the prepared culture medium in T-75 flasks until they reached approx. 80% confluency. They were then seeded onto the surface-coated Ti implant samples at a density of 10,000 cells/cm2 for each independent experiment. The cell-seeded implant samples were placed in cell culture dishes and incubated at 37°C in a humidified atmosphere with 5% CO2. After incubation, the samples were stained with rhodamine B, acridine orange and combination staining methods to analyze cell attachment, spreading and proliferation with the use of a confocal laser scanning microscope (DMi8; Leica Camera, Wetzlar, Germany). The cells were transferred onto glass slides, mounted with DAPI for nuclear counterstaining, and analyzed using the ImageJ software (https://imagej.net/ij). The viability of the MG63 cells on Ti implant surfaces was observed at various time periods (24 h, 48 h, 72 h, 96 h, and 120 h), with the results validated 5 times.
Corrosion analysis
A Ti implant was dipped in a mixture of Ag-decorated GO and chitosan for 45 min under the open circuit potential (OCP). After stabilization, the impedance analysis was performed with an amplitude of ±10 mV. Impedance spectra were recorded over a frequency range from 0.01 Hz to 200 kHz. The polarization study was conducted from −1 V to +0.05 V at a scan rate of 1 mV/s.
Statistical analysis
The data was analyzed using IBM SPSS Statistics for Windows, v. 23.0 (IBM Corp., Armonk, USA), with the unpaired t test assessing the biocompatibility of the coating as compared to a bare Ti surface. A p-value ≤0.05 was considered statistically significant.
Results
The SEM analysis revealed a homogeneous, rough, fibrous layer of the surface coating on the metal surface, with microporosities ranging from 0.2 µm to 0.5 µm. The porosities were well interconnected with each other throughout the thickness of the surface coating. The Ag-decorated GO nanostructures appeared enmeshed within the chitosan fibrous layer. Multiple lamellar layers of the surface coating were noticeable. In the EDX analysis, Ti appeared as the predominant constituent at 43.6 wt%, attributed to the base material of the implant. Carbon (C) constituted 29.5 wt% and oxygen (O) 23.1 wt%, indicating successful coating with GO and chitosan. Trace amounts of sulfur (S) at 3.8% were also attributed to chitosan. The ATR-FTIR analysis showed the absorbance peaks correlating to the presence of various functional groups. The absorbance peak at 636 cm−1 confirmed the presence of the Ti–O functional group. The peaks at 803 cm−1, 915 cm−1, 1,178 cm−1, and 1,314–1,631 cm−1 confirmed the presence of sulfides, amides, ethers, alkenes, alkynes, and aromatic compounds attributed to chitosan. The peaks at 1,178 cm−1, 1,314–1,631 cm−1 and 2,096 cm−1 indicated the presence of ethers, alkenes, alkynes, aromatic compounds, and methylene groups attributed to GO, as shown in Table 1.
The confocal analysis revealed viable cells of osteoblastic lineage that showed homogeneous spread and good adherence to the coated implant surfaces. Multiple layers of cells arranged in a stacking pattern were visible. Staining active cells with well-defined cell organelles and mitochondria was achieved by using rhodamine B. Cells with extending filopodia confirmed the proliferation process. The results indicated viable cells and showed that the developed implant surface coating had no cytotoxic effects. Acridine orange staining showed live cells with viable stained DNA. The results confirmed the absence of cytotoxic effects on the DNA or nuclear material of the cells. Cell multiplication was not affected, as evidenced by cell proliferation. Combination staining further confirmed the homogeneous spread of viable cells with well-marked cell organelles. The viability of MG-63 cells on bare and coated Ti implant surfaces was compared at different time intervals (24 h, 48 h, 72 h, 96 h, and 120 h), using the independent t test, with a p-value ≤0.05 set as statistically significant. No statistically significant difference in cell viability on the coated vs. bare Ti surfaces was observed at any time point (Figure 1). The results showed that the developed surface coating was as biocompatible as the bare titanium surface, and could be safely used for in vivo applications.
In the corrosion analysis, the results showed that the coating had a high corrosion potential (Ecorr) and a low corrosion current density (Icorr) as compared to bare metal. The Nyquist plot showed high charge transfer resistance (RCT), suggesting greater resistance to corrosion. This could be due to the coating preventing corrosive ions from the simulated body fluid from penetrating the metal surface. The Bode impedance value was high at low frequencies, indicating the resistance of the coating. The Bode phase angle was closer to 1, indicating a constant phase element. The corrosion analysis showed that the developed surface coating was more thermodynamically stable with higher impedance than the bare metal surface, which resulted in better corrosion resistance.
Discussion
The results show the successful coating of the implant surface, which aligns with recent studies on chitosan and GO.15, 16, 17, 18 We chose MG-63 cells due to their similar differentiation capabilities to mesenchymal stem cells, making them popular in osteogenesis studies.19, 20, 21 They provide insights into the cell–material interactions and osseointegration issues. Whereas alternative cell sources and lineages, such as normal human dermal fibroblasts, have been explored for wound healing studies, we found MG-63 cells to be more suitable for osseointegration-related research.
Graphene oxide coatings exhibit good biocompatibility, with beneficial osteogenic effects on MSCs, creating a pro-osteogenic environment through the modulation of the Toll-like receptor (TLR) pathway, and the transforming growth factor beta 1 (TGF-β1) and oncostatin M (OSM) genes.22 Studies that analyzed the effect of reduced GO demonstrated its superior ability in the surface adsorption of cells and proteins, promoting osteogenic cell differentiation and proliferation.23 Graphene oxide has also been shown to accelerate osseointegration and tissue regeneration, as evidenced by increased ALP activity and expression of osteogenesis-related genes in both in vitro and in vivo studies.23 This property of GO to improve osteogenesis is attributed to its strong proficiency in terms of surface adsorption. The electron cloud of graphene interacts with the hydrophobic segment of serum proteins, thereby improving cell adhesion to the implant surface.
Moreover, the oxygen functional groups in GO further bolster cell adhesion.24 Graphene oxide-enhanced materials have been shown to improve cell adhesion and osteogenic differentiation by modulating the focal adhesion kinase/p38 (FAK/p38) signaling pathways.25, 26 Additionally, GO has been postulated to exhibit antimicrobial properties through physical and chemical mechanisms.27 Direct contact with the sharp edges of GO leads to cell death, as the ROS generated through charge transfer cause DNA damage and mitochondrial dysfunction, impairing protein-lipid metabolism. Furthermore, due to its electron transfer capability, it can damage membranes, leading to cell death. A recent systematic review supports these findings, stating that GO-functionalized coatings proved to be a promising solution.28 Silver NPs may further enhance the antimicrobial properties of GO. This enhanced activity can be attributed to the nanoscale size of Ag, which increases cell membrane permeability and the production of ROS that impair DNA replication, ultimately leading to cell death.13
Chitosan in a surface coating further contributes to its antimicrobial properties. Chitosan, a natural biomaterial obtained from the shells of crustacean organisms, exhibits a unique polycationic property that has been postulated to be responsible for antimicrobial activity. The binding of chitosan to the negatively charged cell membrane and its attachment to cellular DNA have been shown to increase cell membrane permeability and affect DNA replication, respectively.7 Its chelating property has also been shown to affect cell metabolism, causing cell death.29 Furthermore, studies have demonstrated the osteogenic potential of chitosan.30, 31, 32 It has been proven to increase ALP activity, thereby enhancing calcium (Ca) deposition.
The developed novel implant surface coating with the synergistic combination of Ag-decorated GO and chitosan may contribute to enhanced osteogenic and antimicrobial activity. Cell culture studies prove the optimal biocompatibility of the developed coating. The results of this study suggest that the surface morphology and chemical characteristics of the coating are conducive to improved osteogenesis and antimicrobial action. Based on the preliminary results, it can be concluded that this novel implant surface coating shows promise for further development for clinical applications. In the future, coating the dental implant surface with Ag-decorated GO and chitosan may contribute firstly to improved cell adhesion onto the surface, leading to better cell differentiation. This would ultimately increase the probability of achieving greater secondary stability. Secondly, the antimicrobial properties of the components of the implant coating may help achieve an osteoinductive environment around the implant by preventing the emergence of dysbiotic pathogens that may initiate the disease process. Moreover, the antimicrobial properties of the surface coating may act as an adjunct in the prevention of peri-implant diseases by compensating for any lapses in the maintenance of good oral hygiene practices. These properties of the developed implant surface coating may clinically translate into improved long-term success of dental implants, potentially paving the way for implant placement in anatomically complicated and systemically compromised cases with greater predictability.
Limitations
The preliminary results from the study do not directly demonstrate clinical superiority. Further analysis is needed to optimize the thickness, surface tension and wettability of the implant surface coating to achieve positive clinical outcomes. Research on antimicrobial properties, cell line studies and animal studies are needed to evaluate the therapeutic potential of the coating and its impact on peri-implant diseases. Long-term human control trials are essential to validate the clinical efficacy of the coating and to compare it with the clinically proven alternatives.
Conclusions
The present preliminary report confirmed the successful coating of the implant surface with Ag-decorated GO and chitosan. The various analyses of the coating showed ideal properties for improved cell attachment, differentiation and proliferation while maintaining an antimicrobial environment on the implant surface. The biocompatibility and corrosion resistance of the coating further enhance its value. Further in vitro and in vivo studies should be conducted to explore the potential of this novel coating to achieve improved osseointegration.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.