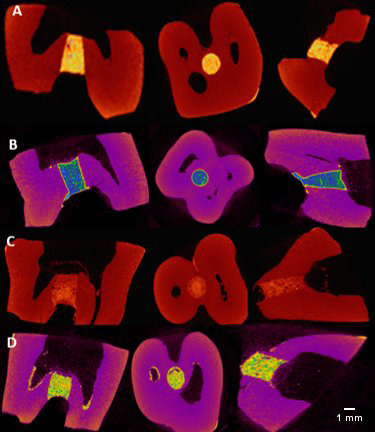Abstract
Background. Porosity is a crucial parameter that affects the solubility, sealing and mechanical strength of a material. It plays a significant role in determining the success of treatment.
Objectives. The present study aimed to evaluate and compare the porosity of different bioceramic-based materials, using micro-computed tomography (micro-CT).
Material and methods. In the study, 76 permanent lower first or second molars that had been extracted for periodontal reasons and were free of calcification, resorption, root cavities, fractures, or cracks, with discrete roots and complete root apex development were selected. In each of the 4 experimental groups, perforations were made in the furcation areas of 19 molars. Mineral trioxide aggregate (MTA) Angelus®, EndoSequence® Root Repair Material (ERRM), Biodentine™, and BioAggregate were placed on the perforated areas of the samples and scanned with a micro-CT to evaluate porosity. The pore volume and the pore percentage with regard to the closed, open and total porosity of these repair materials were calculated individually in each sample.
Results. While no statistically significant differences were found between group I (MTA), group III (Biodentine) and group IV (BioAggregate) when evaluating the total pore percentage (p > 0.05), the parameter in group II (ERRM) was found to be significantly lower as compared to other groups (p > 0.05).
Conclusions. In comparison with the other materials used in this study, the use of ERRM may be more suitable for perforation repair.
Keywords: porosity, endodontics, micro-CT, tissue repair, bioceramic materials
Introduction
Recently, great advances have been made in the biomedical application of ceramics for skeletal repair and reconstruction. Bioceramic materials have been used in both dental implant surgery and endodontic treatment, specifically in the repair of furcation perforations, apexogenesis, apexification, and vital pulp therapy, to induce mineralization.1 Previously, various endodontic and restorative materials, such as amalgam, glass ionomer cement (GIC), zinc oxide–eugenol cement, 2-ethoxybenzoic acid (EBA), super EBA, gutta percha, composite resin, and Cavit™, have been used for perforation repair. However, due to the lack of biocompatibility of these products and less than ideal prognosis, new materials have beeen investigated.2 Mineral trioxide aggregate (MTA) is considered the gold standard for various applications, especially as a repair material.3, 4
Gray ProRoot®, a calcium silicate-based MTA produced by Dentsply Maillefer (Ballaigues, Switzerland), was the only product on the market until white MTA was produced in 2002. Subsequently, new materials have been developed and compared to these 2 gold-standard MTAs. Bioceramic cements have been developed to overcome limitations, such as long hardening duration, difficulty of manipulation, discoloration of dental tissues, low radiopacity, lower compressive strength as compared to dentin, and high costs. These cements have antimicrobial properties, good impermeability and do not contain harmful heavy metals.5
Materials used in endodontics must ensure an impermeable cover. This is one of the most important determinants of successful root canal treatment. The durability of perforation repair is affected by the porosity of the material. The presence of closed and open pores is related to the physical properties of the material, and can have an impact on the long-term prognosis. The porosity of the material depends on its water absorption, solubility, permeability, mechanical resistance, and density. The degree of porosity is a critical factor determining its tightness.6 In previous studies, porosity was evaluated by measuring differences in weight before and after water absorption, using Archimedes’ principle, mercury intrusion porosimetry (MIP) and micro-computed tomography (micro-CT).7, 8, 9, 10 Micro-CT evnables a non-destructive and three-dimensional (3D) assessment of dental tissues and of the quality of the material under in vitro conditions.11 Therefore, in the present study, we evaluated and compared via micro-CT the degree of porosity in the structures of 4 bioceramic-based materials – MTA Angelus®, EndoSequence® Root Repair Material (ERRM), Biodentine™, and BioAggregate – when applied to perforation defects.
Material and methods
Specimen preparation
The study used 76 permanent lower first or second molars extracted for periodontal reasons. The teeth were free of calcification, resorption, root cavities, fractures, or cracks. They had discrete roots with complete root apex development.
The organic debris, soft tissues and calculus were meticulously removed from the teeth using the Cavitron 300 Series Ultrasonic Scaling System (Dentsply Sirona, Bensheim, Germany) and a periodontal curette (Gracey #12–14; HuFriedyGroup, Chicago, USA), without causing any damage to the root surfaces or furcation areas. To ensure disinfection, after being stored in 5% NaCl for 1 week, the teeth were kept in a physiological saline solution at ambient temperature until they were used in the study.12
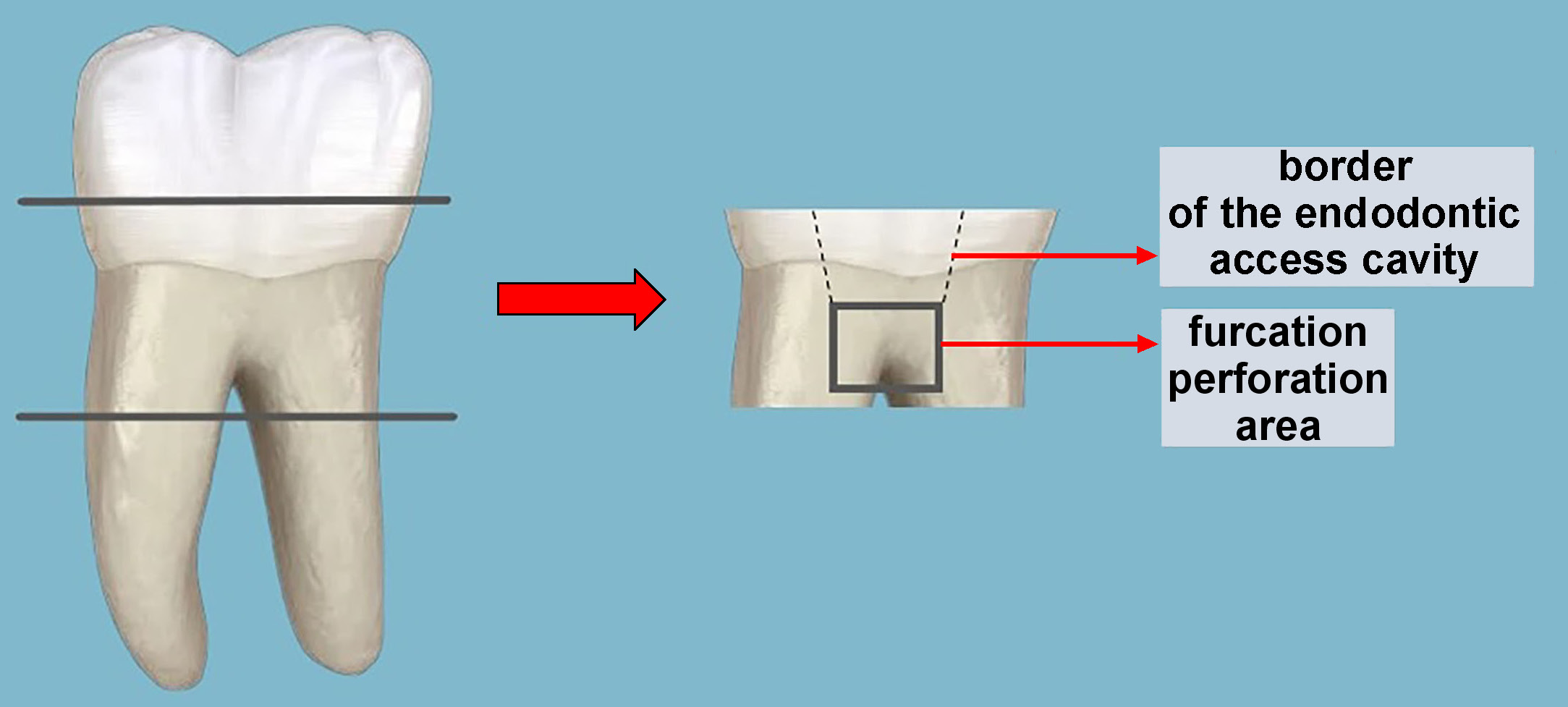
The coronal part of the teeth was removed by making 3-millimeter cuts with diamond disks under water cooling. The apical and middle parts of the roots were then removed by cutting. Standard endodontic access cavities were created with a round diamond drill (Hager & Meisinger, Neuss, Germany) and a fissure diamond drill (Hager & Meisinger) under water cooling. The furcation areas of the obtained teeth were perforated with a 1.6-millimeter fissure drill (Hager & Meisinger). To standardize the dentine–cement thickness in the furcation area, we measured the distance between the base of the pulp chamber and the inter-radicular furcation area using a caliper. Only teeth with a thickness of 2–2.5 mm were included in the study. After washing the perforation area with a physiological saline solution, the cavities were dried with a cotton pellet and the outer surfaces of the teeth were meticulously embedded in silicone (Zetaplus C-Silicone; Zhermack, Badia Polesine, Italy), which had been placed inside a plastic cylinder to imitate the peri-radicular tissues, as in a study by Aggarwal et al.13 The schematic pattern of the tooth/root preparation is presented in Figure 1.
The samples were randomly divided into 4 experimental groups of 19 specimens each (n = 19). The repair materials used were MTA Angelus (group I), ERRM (group II), Biodentine (group III), and BioAggregate (group IV).
The powder component of MTA Angelus (lot No. 45053; Angelus, Londrina, Brazil) contains tricalcium silicate, bismuth oxide, calcium carbonate, and iron oxide, while the liquid part is composed of water. Biodentine (lot No. B05663; Septodont, Saint-Maur-des-Fossés, France) consists of tricalcium silicate, small amounts of dicalcium silicate, calcium carbonate, and zirconium oxide. It has a water-based polymer as a liquid component, with calcium chloride, which accelerates the hardening reaction. BioAggregate (lot No. 1201BA; Innovative Bioceramix, Inc., Vancouver, Canada) consists of tricalcium silicate, dicalcium silicate, monobasic calcium phosphate, calcium hydroxide, hydroxyapatite, silica, and tantalum pentoxide. The liquid component is deionized water.14 EndoSequence Root Repair Material (lot No. BP11001; Brasseler USA, Savannah, USA) contains tricalcium silicate, monobasic calcium phosphate, zirconium oxide, tantalum pentoxide, and filling agents.15
The material mixtures were prepared according to the manufacturers’ instructions and applied to the perforated areas. To ensure hydration, the access cavities were sealed with Cavit G (3M ESPE, Seefeld, Germany) after placing moisturized cotton pellets in them. Subsequently, the samples were incubated for 72 h at 37°C in an environment with 100% humidity.
Cavit G and cotton pellets were removed from the cavities after the hardening reaction. The samples were removed from the cylinder and the silicone material on their surfaces was cleaned in preparation for micro-CT scanning.
Micro-CT scanning
The samples were enumerated and placed in a Falcon® tube, which was secured to the turntable of the micro-CT device (SkyScan 1172; Bruker, Kontich, Belgium) for high-resolution scanning. The X-ray source of the device was set to 100 kVp and 100 mA, and the samples were beamed with a rotation set at 0.40, using a 0.5-millimeter aluminum(Al)/copper (Cu) filter. Each sample was scanned while rotating 360° for 48 min. Each image was captured for 400 ms, and 3 images were taken from each angle and combined into a single image to reduce the noise ratio. Other adjustments and parameters were chosen based on the manufacturer’s recommendations. Raw images were obtained from each sample, using a 10-micrometer pixel scale.
Micro-CT reconstruction and analysis of the samples
The closed pore volume, closed pore percentage, open pore volume, open pore percentage, total pore volume, and total pore percentage of the materials were assessed using micro-CT analysis.
The samples were reconstructed using the NRecon software, v. 1.6.5.2 (Bruker). The parameters for reconstruction were as follows: ring artifact 8; flattening parameter 3; and beam hardening 38%. Contrast adjustments were made according to the instructions provided by the manufacturer. Overall, 1,024 two-dimensional (2D) fractions were obtained from each sample. The raw images were combined using NRecon and fractions in BMP format were obtained for internal structure analysis.
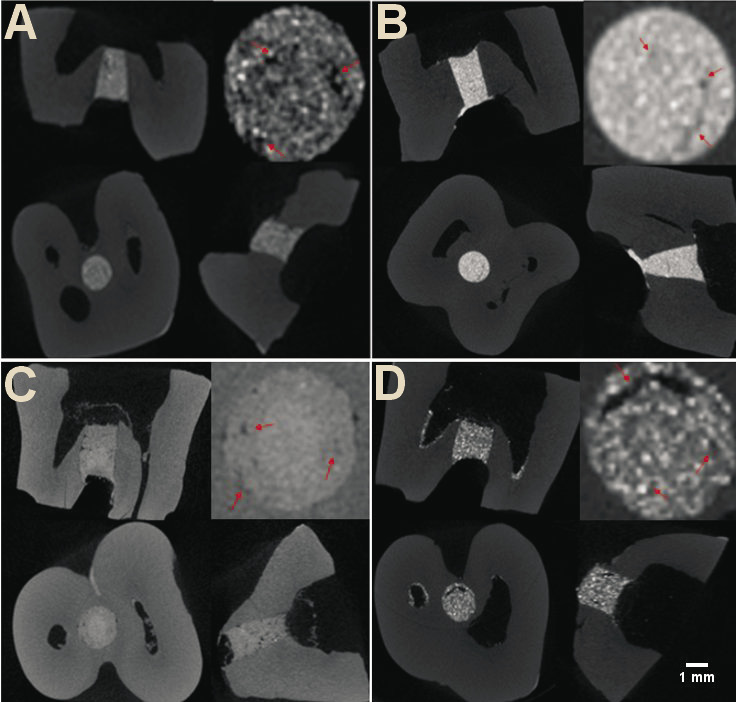
These fractions were then imported into the CTAn software, v. 1.16.4.1 (Bruker), which is used to create quantitative parameters and visual models, and enables densitometric and morphometric measurements. The images were monitored in the X, Y and Z planes using the DataViewer software (Bruker) (Figure 2,Figure 3).
Statistical analysis
The non-parametric Kruskal–Wallis test was performed due to the non-normal data distribution, which was confirmed by the Shapiro–Wilk test. Intergroup correlations were evaluated using the Mann–Whitney U test, while the Kruskal–Wallis H test with Bonferroni correction assessed intergroup differences. All analyses were conducted using the IBM SPSS Statistics for Windows software, v. 20.0 (IBM Corp., Armonk, USA), and a p-value <0.05 was considered statistically significant.
Results
Significant differences in the closed pore volume were found between group II and other experimental groups (p < 0.05), while no significant differences were observed between groups I, II and III (p > 0.05). Group II showed the lowest values for the closed pore percentage, and the differences was significant, while the highest closed pore percentage values were found in group IV, with the differences being significant (p < 0.05) (Table 1).
A statistically significant difference was found between group IV and other experimental groups regarding the open pore volume and percentage, with the BioAggregate group having the lowest values (p < 0.05) (Table 1).
Group II had the lowest values for the total pore volume, and the differences with regard to other groups were significant (p < 0.05). No statistically significant differences in the total pore volume were observed between groups I, III and IV (p > 0.05) (Table 1).
While no statistically significant differences were found between groups I, III and IV for the total pore percentage (p > 0.05), group II had the lowest total pore percentage as compared to other groups, and the differences were significant (p < 0.05) (Table 1).
Discussion
Due to the complex anatomy of the root canal, perforations may occur at the base of the pulp chamber, or on the coronal, medial or apical parts of the roots during preparation. Various materials, such as GIC, zinc oxide–eugenol cement, calcium hydroxide, and composite resins, have been used for the repair of root perforations. However, none of them have ideal features to meet the characteristics and conditions of root repair. Mercury oscillation and poor esthetics have limited the use of amalgam. Composite resins release toxic monomers and exhibit constriction during polymerization. Other materials are inadequate in terms of impermeability and coating, while having osteogenic, cementogenic and antibacterial effects.16
Impermeable materials with high mechanical endurance should be used to repair iatrogenic furcation perforations. The use of bioceramics is considered appropriate in areas not directly related to resistance, particularly for perforation repair and pulp treatment, and in retrograde cavities to ensure hermetic obturation.17
Porosity depends on the gaps between the anhydrite cement granules, and is a natural characteristic of bioceramic-based cements. As the hydration reaction progresses, the hydration products fill the gaps, reducing porosity. However, if the water–cement ratio is too high during the mixing process, porosity increases. High liquid–powder ratios may increase porosity and solubility, and the use of a greater amount of liquid increases calcium (Ca) release from MTA.18 Moreover, the amount of air in the mixture during the mixing process and the pH of the environment also affect porosity.18, 19
Three different types of pores are observed: closed; through; and blind.20, 21 Closed pores begin and end inside the material, and are therefore not related to the surface or leakage. However, they decrease the mechanical strength of the material.22 In cements, 10% porosity may reduce mechanical endurance by 50%.23 Through pores look like tunnels that pass straight from one outer surface of the material to the other. Blind pores start at the outer surface of the material and end inside the material. Through and blind pores are related to the surface, and are called open pores. The presence of open pores negatively affects the adaptation and impermeability of the material.24 Although porosity has a negative effect on the physical characteristics of the material, it has a positive effect on reactivity and biological characteristics by increasing the release of bioactive ions.21 The presence of porosity can cause microcracks in the material, and the expansion of these microcracks leads to the deterioration of the structure of the material. Such microcracks cause microleakage. Thus, the functionality of materials changes in the long run.25
Gandolfi et al.10 mixed calcium silicate- and calcium hydroxide-based materials, and found that Biodentine had the highest mechanical endurance due to its low liquid–powder ratio.26 Camilleri et al. reported that Biodentine was denser and less porous than MTA, but more susceptible to microorganism colonization, which negatively affected its hermetic impermeability.27 Gandolfi et al. found that the porosity of MTA Angelus decreased over time due to hydraulic expansion and the formation of calcium phosphate, which closes open pores.28
Porosity is directly affected by the content of the material, the liquid–powder ratio, and the problems that occur during mixing and handling.29 In this study, we tested several different materials and analyzed them using micro-CT to obtain 3D, detailed and reliable data on their internal structures.30 No specific data on the percentage ratios or stable levels of porosity of dental cements, obtained by micro-CT scanning, have been reported in the literature.31 For this reason, no control group was used in this study, and the porosity measurements of the tested materials were compared with each other.
Two studies evaluated the porosity of bioceramic-based cements. Gonçalves De Souza et al. used micro-CT to evaluate the porosity of iRoot® BP Plus, Biodentine, Ceramicrete, and ProRoot MTA.29 However, they did not find any significant differences between these materials. Biodentine tended to have the lowest porosity, which was related to the trace amount of water that it contained during the mixing process.29 Guerrero and Berástegui evaluated only the total porosity of the materials, and found that it was lower in Biodentine as compared to MTA.32 Silicone blocks were used in both studies. In our study, open, closed and total porosity was calculated separately to provide individual views of the leakage and mechanical endurance of the materials placed inside lower molars.
Our study revealed that ERRM demonstrated significantly lower closed pore volume and percentage as compared to MTA Angelus, Biodentine and BioAggregate. This may be attributed to the fact that ERRM consists of nanoparticles, is prepared as a paste and is easier to handle than other powder–liquid systems. BioAggregate exhibited the poorest results in terms of closed pore volume and percentage. This could be due to the larger particle size as compared to MTA Angelus and Biodentine. As suggested in previous studies, cements with larger particles tend to exhibit more internal porosity when mixed.27, 33 Our results are in line with these findings.
On the other hand, BioAggregate showed significantly better results than MTA Angelus, ERRM and Biodentine for the open pore volume and percentage. The reason why a bioceramic-based cement would exhibit undesirable results for the closed pore volume, but desirable results for the open pore volume can be explained by the fact that materials with higher water absorption tend to expand more. Water absorption may have occurred due to the water present in the dentin tubules and the dentin barrier, rather than the water added during the mixing process. Likewise, BioAggregate shows strong adhesion to the dentin barrier, which is facilitated by humidity absorption in the dentin tubules.34, 35
The total porosity of ERRM was significantly lower as compared to other materials. When powder and liquid are added separately, and mixed afterward, air bubbles may form during the mixing process, making it harder to obtain a homogeneous mixture and leading to operator errors. EndoSequence Root Repair Material maintains its crystallized structure unless exposed to an acidic environment, absorbing water owing to its crystallized structure and forming a residue similar to hydroxyapatite. The release of calcium ions (Ca+2) reduces porosity.36, 37, 38
Conclusions
For the treatment to be successful, perforation repair materials need to be durable and leak-proof. Porosity is an important physical feature of bioceramic materials. Our findings indicate that ERRM had significantly lower total porosity than the other materials tested. Within the limitations of this in vitro study, our results can promote further in vivo studies.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.