Abstract
Background. Secondary caries is one of the main reasons for the clinical failure in dental restorations. Therefore, it is preferable for restorative materials to possess antibacterial properties, which support a long-lasting restoration.
Objectives. The present in vitro study aimed to evaluate the effect of both the polyamidoamine (PAMAM) liquid and bioactive glass (BAG) powder added to glass ionomer cements (GICs) on their antibacterial properties.
Material and methods. Polyamidoamine was prepared and characterized. Four groups were distinguished, as follows: GI – samples of commercially available GIC (control); GII – samples of GIC mixed with PAMAM; GIII – samples of GIC mixed with BAG; and GIV – samples of GIC mixed with PAMAM and BAG.The biofilm assessment test was conducted using a colony forming unit (CFU) count, and the ion release test was used to quantify the amount of released silica (Si), calcium (Ca), phosphorus (Ph), and sodium (Na) ions in mg/L. Thirty-six samples were prepared for each test. Furthermore, the pH of the soaking solution was measured for each sample in the ion release test. The parametric data was examined using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test.
Results. The biofilm assessment test revealed a significant reduction in the recovered Streptococcus mutans counts in all modified groups compared to the control group (p < 0.05). Also, the ion release test demonstrated a significant increase in the release of Si and Na ions for all modified groups compared to the control group (p < 0.05).
Conclusions. The modification of GICs with PAMAM and BAG enhances their antibacterial properties.
Keywords: bioactive glass, glass ionomer cement, ion release, antibacterial, polyamidoamine
Introduction
Glass ionomer cements (GICs) have a wide range of clinical applications due to the possible modification of their physical properties or chemical formulations. Furthermore, the chemical adhesion of GICs to enamel and dentin, the material’s good marginal integrity, its dimensional stability at high humidity, as well as its fluoride (F) release and recharge are positive characteristics of the material.1 Moreover, GICs are considered the best materials of choice for tooth repair by the atraumatic restorative treatment (ART) and the restoration of tooth surfaces with minimal preparations.2
Dental caries is one of the most prevalent and significant dental problems. According to the World Health Organization (WHO), approx. 60–90% of children worldwide, and nearly 100% of adults, suffer from dental caries, with secondary caries being one of the main reasons behind the clinical restorative failure of GICs.3 As Streptococcus mutans is among the main microorganisms considered the etiological factors for dental caries,4 the prevention of bacterial invasion, in the case of microleakage, becomes the key to long-lasting dental restorations.5 Glass ionomer cements have been reported to be the most cariostatic and antibacterial due to their F release,6 exhibiting no or low cytotoxicity.7 However, annual clinical surveys have declared that secondary caries remains the main reason for GIC failure, indicating that the F release from GICs is insufficient to inhibit bacterial growth or prevent the bacterial destruction of the tooth structure.8
Glass ionomer restorations with antibacterial properties are highly desirable. Consequently, researchers have investigated various modifications to increase the antibacterial and physical properties of GICs, including the addition of zirconium,9 glass fiber,10 hydroxyapatite,11 bioactive glass particles,12 casein phosphopeptide-amorphous calcium phosphate,13 cetrimide, cetylpyridinium chloride, chlorhexidine (CHX),14 and chitosan (CH).15
Bijle et al. investigated the influence of incorporating L-arginine (Arg), a semi-essential amino acid, into GIC.16 They found that adding 4% Arg to GIC increased its antibacterial activity via a biofilm modulatory effect for microbial homeostasis.16 Additionally, Neelima et al. investigated the antimicrobial efficacy of conventional GIC with the addition of propolis, CH and CHX against S. mutans and Lactobacillus acidophilus.17 It was concluded that GIC with CHX revealed higher antimicrobial activity against both strains. Streptococcus mutans and L. acidophilus were effectively inhibited by GIC with propolis and GIC with CH.
In several studies, bioactive glass (BAG) has been added to GICs to improve their bioactivity and capacity for tooth regeneration. Additionally, it has demonstrated antimicrobial properties within the oral cavity and low cytotoxicity to dental pulp cells.18 Polyamidoamine (PAMAM) dendrimer, on the other hand, is a highly developed polymer with reactive endings. Among the PAMAM dendrimers that harbor a variety of functional groups, the amino-terminated PAMAM dendrimers (PAMAM-NH2) revealed the strongest antibacterial activity. Functional amine groups get adsorbed to the bacterial cell surface, diffusing through the cell wall, with subsequent bacterial death.19 The antibacterial mechanism of PAMAM dendrimers reveals an apparent lack of inducing bacterial resistance to antibiotics,20 a phenomenon that may have negative consequences for society.21 With an increase in the generation number (G) of PAMAM, the number of functional groups doubles.22 It has been stated that G2 or higher generation of PAMAM-NH2 can significantly damage bacteria, while G3 or higher generation of PAMAM-NH2 has the potential to damage mammalian cells.23 Therefore, it has been proposed that a range of generations of PAMAM-NH2 exists between both thresholds, possibly having significant antibacterial activity while maintaining tolerable toxicities. According to previous studies, G3 or higher generation of PAMAM-NH2 dendrimers exhibited excellent antibacterial activities, though they also demonstrated high toxicity.23
Gou et al. examined the long-term antimicrobial effect and antiproteolytic potential of PAMAM-NH2 cavity cleanser, evaluating its binding capacity to dentin surfaces to fulfill these effects.24 For antibacterial testing, colony forming unit (CFU) counts and live/dead bacterial staining were performed. Antibacterial testing indicated that PAMAM-NH2 significantly inhibited bacterial growth on the dentin disks compared with the control group, which exhibited a similar level of antibacterial activity to that of 2% CHX (p > 0.05). Also, evidence has demonstrated the low cytotoxicity level of PAMAM-NH2 toward human dental pulp cells.24
To the best of our knowledge, no published study has evaluated the effect of adding both PAMAM and BAG on the antibacterial properties of GICs. Therefore, given the importance of antibacterial properties in determining the success and longevity of dental restorations, this study was conducted to evaluate the effect of the addition of both G2 PAMAM-NH2 liquid and BAG powder to GICs on their antibacterial properties. The effect of pH and ion release ability of the modified GICs on their antibacterial properties was also determined.
Material and methods
According to the calculated sample size, a total of 72 samples were prepared. The materials used in the study are listed in Table 1. First, a pilot study was conducted through a biofilm assessment test (CFU counts) to select the optimum concentration of PAMAM (4, 8, or 16 multiple its minimum bactericidal concentration, which was equal to 6.2%, 12.4% and 24.9% by volume (v), respectively) that would be added to the cement liquid, and the optimum concentration of BAG (5% or 10% by weight (w)) that would be mixed with the cement powder.
Based on the results of the pilot study, the addition of 6.2% PAMAM and 5% BAG was discarded due to their ineffective antibacterial effect, while 12.4% and 24.9% concentrations of PAMAM were selected for incorporation into the cement liquid. Additionally, 10% BAG was added to the cement powder.
Synthesis of polyamidoamine
To obtain the desired second-generation (G2) PAMAM-NH2 dendrimer, the hyperbranched PAMAM polymer was synthesized by 4 steps of successive Michael addition and amidation reactions. The starting reactants were ethylenediamine as the core and methyl acrylate. Firstly, the Michael addition reaction was executed in a 500-mL round flask, in accordance with the following protocol: 7.5 mL (0.12 mol) of ethylene diamine in 100 mL of methanol was added dropwise to a solution of 42 mL (1.25 eq, 0.46 mol) of methyl acrylate in 25 mL of methanol over 1 h at 0°C. The mixture was stirred for 48 h at room temperature. The reaction mixture was then subjected to a process of evaporation using a rotary evaporator (R-210 Buchi; BÜCHI Labortechnik GmbH, Essen, Germany) to achieve a state of complete dryness. The addition step was followed by the amidation step, which entailed adding a solution of ethylene diamine in 50 mL of methanol at 0°C to 12.5 g (0.03 mol) of the methyl-terminated core in 50 mL of methanol. The reaction mixture was heated to room temperature and stirred for 12 h. The unreacted amine and methanol were removed under a vacuum. Two consequent steps of the Michael addition and amidation reactions were carried out as previously described.25 The G2 PAMAM-NH2 was prepared and characterized by Fourier transform infrared analysis (FTIR) (wavelength range: 400–4,000 cm−1, spectrum resolution: 4 cm−1) (VERTEX 80 V; Bruker, Bremen, Germany) and proton nuclear magnetic resonance (1H NMR) (Jeol 500 MHz; Jeol, Freising, Germany).
Grouping of the study samples
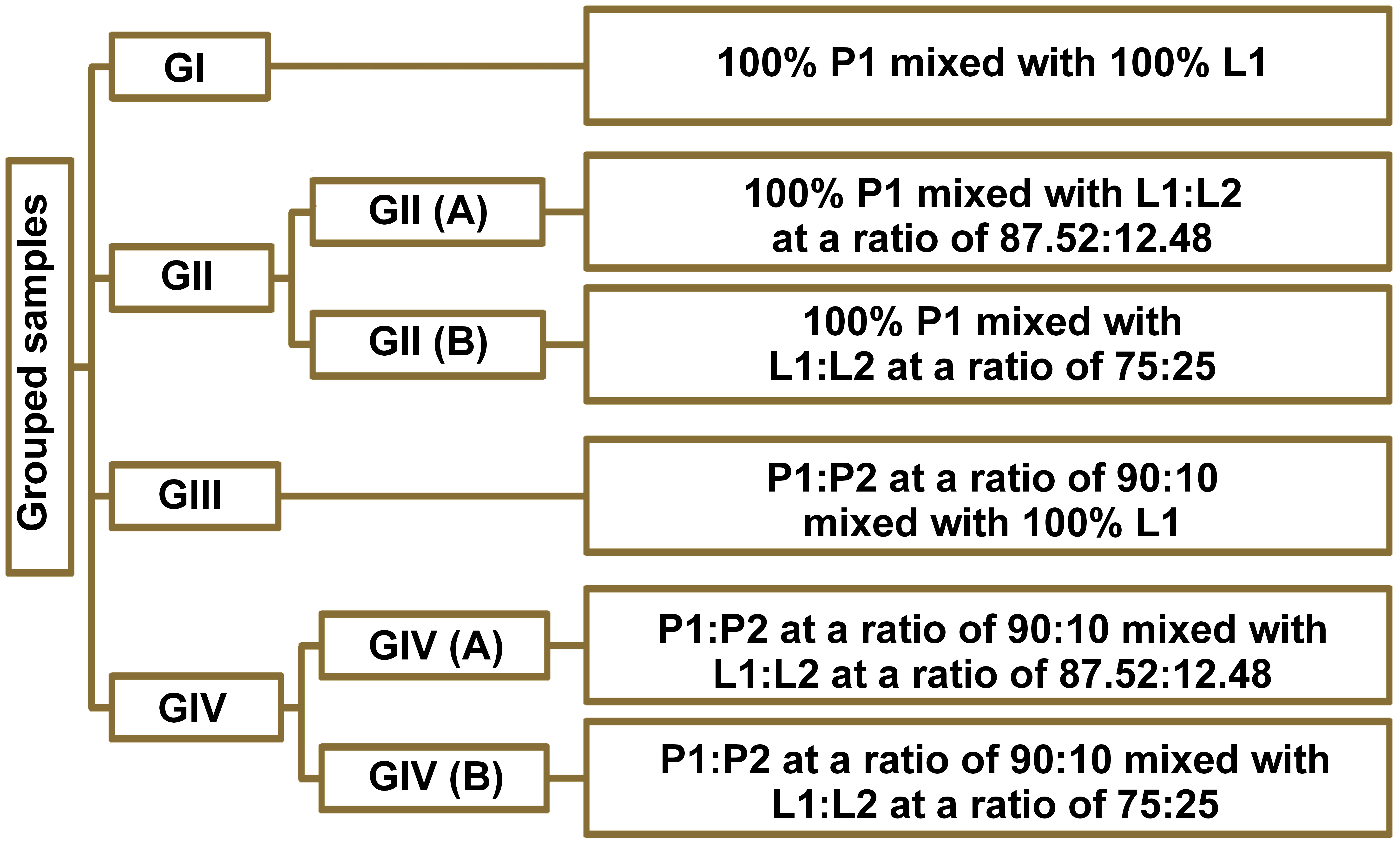
A total of 72 samples were divided into groups and subgroups, based on the volume percentage of PAMAM liquid (L2) added to the GIC liquid (L1), and the BAG powder (P2) added to the GIC powder (P1). The groups were prepared as follows (Figure 1):
– GI: 100% P1 mixed with with 100% L1;
– GII: • GII (A): 100% P1 mixed with L1:L2 at a ratio of 87.52:12.48;
• GII (B): 100% P1 mixed with L1:L2 at a ratio of 75:25;
– GIII: P1:P2 at a ratio of 90:10 mixed with 100% L1;
– GIV: • GIV (A): P1:P2 at a ratio of 90:10 mixed with L1:L2 at a ratio of 87.52:12.48;
• GIV (B): P1:P2 at a ratio of 90:10 mixed with L1:L2 at a ratio of 75:25.
Preparation of samples
According to the manufacturer’s instructions, the GIC samples were prepared by mixing at a powder–liquid (P/L) ratio of 3.6 to 1.0 (g/g). A digital balance (A200S; Sartorius, Göttingen, Germany) was used to weigh each sample individually. The P/L ratio was consistent across samples in each group. For the samples modified with PAMAM liquid and BAG powder, the GIC liquid was manually mixed with PAMAM, and the GIC powder was manually mixed with the BAG particles. Afterward, the cement powder was mixed with the cement liquid to form a paste, which was placed in a Teflon mold with specific dimensions, according to each test.
Bacterial strain and growth conditions
Streptococcus mutans ATCC 25175 was used as the test organism in the study, and tryptic soy broth (TSB) supplemented with 0.3% w/v yeast extract was employed as its culture medium. Whenever necessary, TSB was supplemented with 2% w/v agar. Streptococcus mutans was cultured in a 5% carbon dioxide incubator (Binder GmbH, Ulm, Germany) at 37°C under microaerophilic conditions.
Antibacterial tests
Determination of the minimum inhibitory concentration and minimum bactericidal concentration
The antibacterial activity of PAMAM against S. mutans was assessed. According to the Clinical and Laboratory Standards Institute (CLSI) guidelines, the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of PAMAM were determined by the broth microdilution method.26, 27
GIC biofilm assessment through CFU count
A total of 36 samples (6 disks/group) were prepared according to the manufacturer’s instructions in a custom Teflon mold with a diameter of 7.5 mm and a thickness of 1.5 mm. The materials were mixed by a single operator, packed into the mold, covered, and pressed flat with a glass slide.
The test was conducted within the first 24 h after sample preparation, with slight modifications. Briefly, the disks from each group were placed in a 2-mL Eppendorf tube containing 500 μL of TSB supplemented with 0.3% w/v yeast extract and inoculated with a 10% v/v S. mutans suspension (107 CFU/mL). In each trial, 2 disks of each group were tested. Afterward, the Eppendorf tubes were incubated for 24 h at 37°C in a 5% carbon dioxide incubator (Binder GmbH). Growth control was performed by incubating 2 Eppendorf tubes without adding disks to the bacterial suspension.28
After incubation, each disk was rinsed with 1 mL of sterile phosphate-buffered saline (PBS) and vortexed for 10 min at maximum speed in a new 2-mL Eppendorf tube containing 500 μL of PBS. The resulting bacterial suspensions were subjected to a 10-fold dilution with sterile PBS. Ten microliters from each dilution were spotted on tryptic soy agar plates supplemented with 0.3% w/v yeast extract, for the purpose of the viable count. The CFU of S. mutans/disk were counted, and the results were tabulated and statistically analyzed.
Growth control tubes were used to measure the bacterial count and to verify whether the experimental conditions would affect the growth of S. mutans.
Ion release test
A total of 36 samples (6 disks/group) were prepared using a split Teflon mold with a diameter of 7.5 mm and a thickness of 1.5 mm. During the preparation of the samples, a convenient length of stainless steel orthodontic ligature wire was inserted into the sample before its final setting. A 10-mm piece of stainless steel wire was projected from the ring to ensure proper handling of the sample and its complete immersion in the deionized water (Figure 2).
The soaking water was collected after 7 days. The analyzed solution was used to quantify the released silica (Si), calcium (Ca), phosphorus (Ph), and sodium (Na) ions in mg/L using inductively coupled plasma optical emission spectrometry (ICP-OES) (Agilent 5100; Agilent Technologies Inc., Petaling Jaya, Malaysia).29 The results were tabulated and statistically analyzed.
Measurement of the pH
The soaking solution obtained from each sample of the ion release test was further used to measure its pH with an Adwa AD8000 electrode (Adwa Instruments, Nușfalău, Romania) by the immersion in the central part of the solution. Assessments of pH were performed 24 h after specimen preparation.30 The electrode was cleaned and recalibrated, and the results were tabulated and statistically analyzed.
Statistical analysis
The numerical data was assessed for normality by checking its distribution and using Kolmogorov–Smirnov and Shapiro–Wilk tests. All values were expressed as mean ±standard deviation (M ±SD). The SPSS Statistics for Windows software, v. 17.0 (SPSS Inc., Chicago, USA) was used for the analysis. The parametric data was examined with one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test for multiple comparisons. A value of p < 0.05 was considered statistically significant. Pearson’s correlation and linear regression analysis were used to test the relation between parameters.
Results
Characterization of the prepared PAMAM dendrimers
Fourier transform infrared analysis
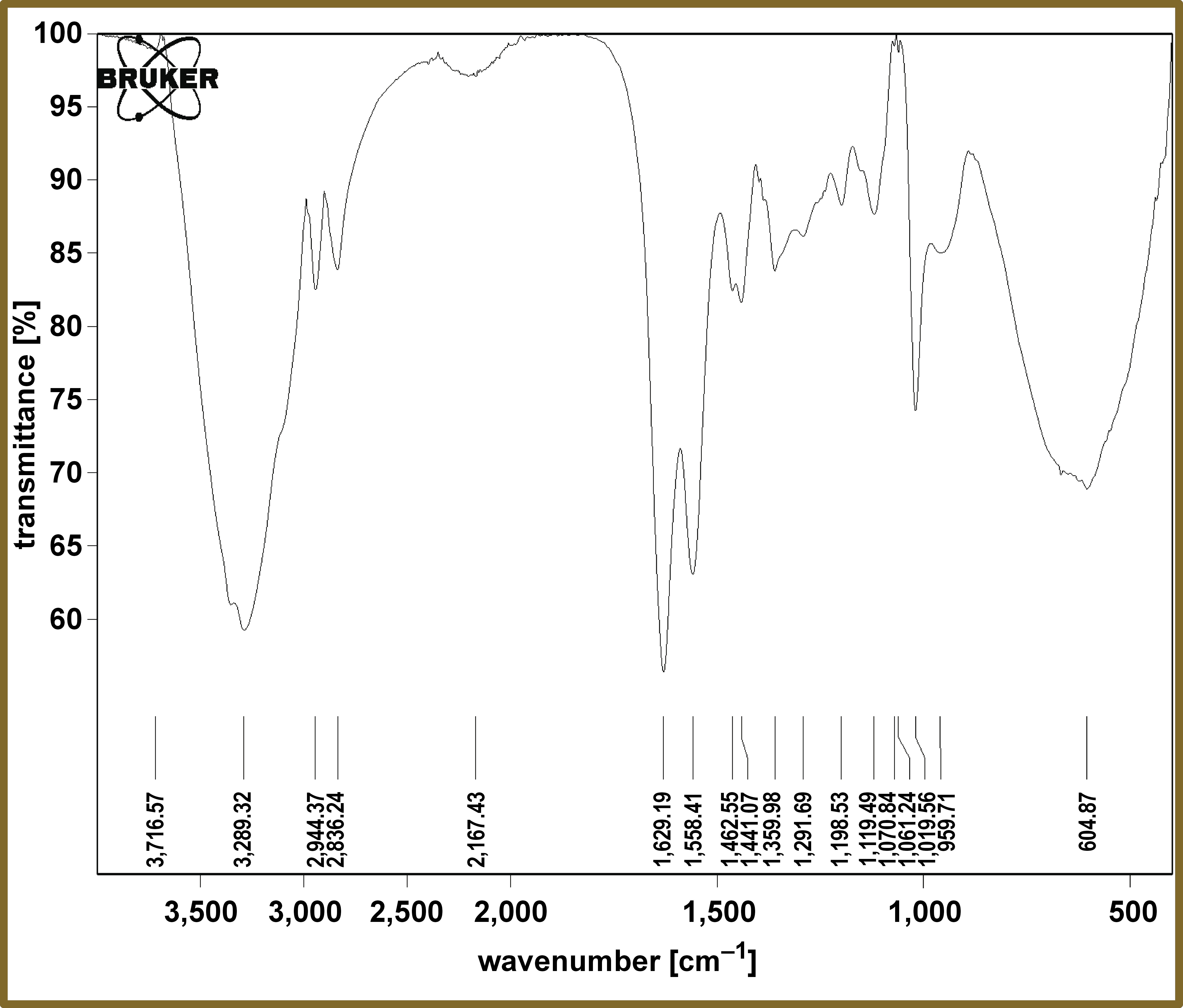
The FTIR spectrum for the prepared PAMAM is shown in Figure 3. The peaks at 3,289 cm−1, 2,944–2,836 cm−1 and 1,629 cm−1, were assigned to N–H, C–H Aliphatic and C=O, respectively, indicating the main constituent chemical functional groups of the prepared PAMAM.
Proton nuclear magnetic resonance
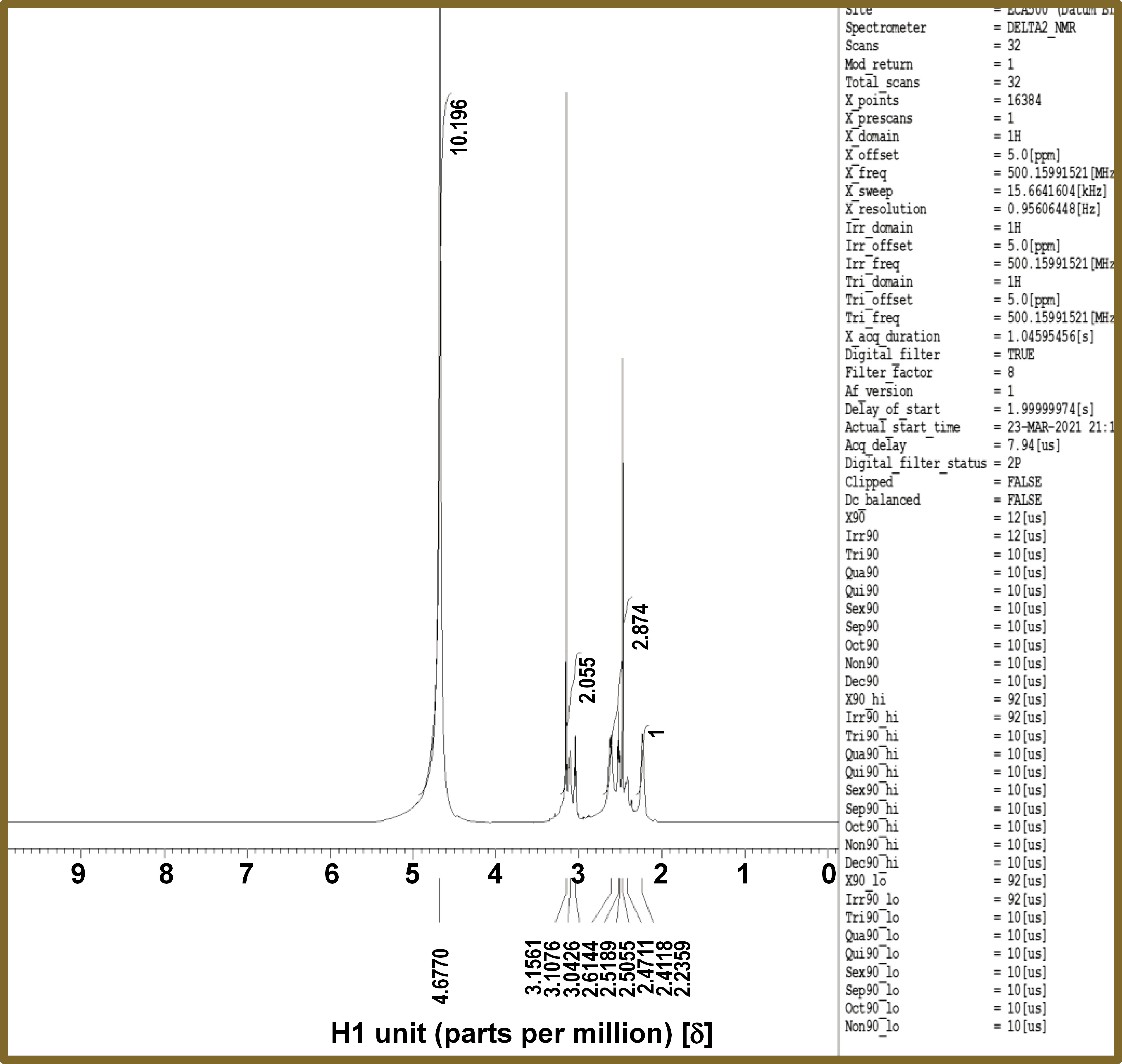
The 1H NMR spectrum for the prepared PAMAM liquid is shown in Figure 4. The peaks at 4.67 δ, 3.15 δ and 2.61 δ, were assigned to NH, CH2–N and CH2–CO, respectively, ensuring thorough characterization of the composition of the prepared PAMAM.
Antibacterial tests
Minimum inhibitory concentration and minimum bactericidal concentration
The recorded MIC and MBC for PAMAM against S. mutans were 0.78125 ±0% v/v and 1.5625 ±0% v/v, respectively. The MBC was used to determine the optimal concentration of PAMAM that would be added to GICs.
Biofilm assessment assay
The results of the biofilm assessment test are listed in Table 2. A significant decrease in the number of recovered bacteria was detected in all modified groups compared to the control group (GI) (p < 0.05). In addition, there was a significant reduction in bacterial counts in GIV (B) in comparison to GII (A) (p < 0.05).
The experimental conditions did not affect the growth of S. mutans, as indicated by the bacterial count recovered from the growth control tubes (log10 8.33 ±0.124 CFU).
Ion release test
The results of the ion release test are listed in Table 2.
Silica and sodium ion release
There was a significant increase in the release of Si and Na ions in the solution for all modified groups compared to the control group (GI) (p < 0.05). A significant increase in the amount of Si ions released in GIV (A) was observed in comparison to GI, GII (A and B) and GIII (p < 0.05). At the same time, GII (B) showed a significant increase in the amount of released Na ions compared to the other groups (p < 0.05).
Calcium ion release
There was a significant increase in the amount of the Ca ions released in GIII and GIV (A and B) compared to the control group (GI) (p < 0.05). An insignificant difference in the amount of Ca ions was observed between GI and GII (A and B) (p > 0.05).
Phosphorus ion release
There was a significant increase in the amount of the released Ph ions in GII (B), GIII and GIV (A and B), compared to the control group (GI) (p < 0.05). An insignificant difference in the amount of released Ph ions was noted between GI and GII (A) (p > 0.05).
pH test of the soaking solution after 24 h
The pH test results for the soaking solution after 24 h in the various investigated groups are listed in Table 3. There was an insignificant difference in the pH between the control group and all modified groups (p > 0.05). A significant decrease in the pH of the solution of GIV (A and B) was observed when compared to the GII (A) and GIII solutions (p < 0.05).
Discussion
The most common reason for restorative failure is secondary caries, which is mainly caused by oral bacteria.31 In recent years, a significant body of research has been conducted to develop antibacterial dental restorative materials that could enable the eradication of the cause of dental caries.32 An effective antibacterial lining material may address potential issues such as the remnant cariogenic microorganisms after partial caries removal.33 Therefore, this study aimed to investigate the interaction of S. mutans with GICs modified with BAG and PAMAM in terms of bacterial growth.
The conventional glass ionomer (GC Gold Label 9, high-strength posterior restorative) was selected due to its high P/L ratio, which offers significant advantages over other commonly used restorative dental materials. It has become the material of choice in the ART technique.34, 35 Furthermore, it is a resin-free material presented in powder and liquid formats for manual mixing, and it sets chemically, without the need for light curing. This characteristic facilitates the ART procedures for young and old individuals, as well as in low-income countries.36 The material was preferred over encapsulated materials because it more readily permitted modification of its constituents by the inclusion of PAMAM and BAG additives.
In this study, G2 PAMAM-NH2 was selected as an antibacterial agent due to its 16 primary amine surface functional groups, which result in lower toxicity,23 and its relatively simple and cheap synthesis when compared to higher generations.
In the present study, finishing and polishing procedures for the samples were not performed to avoid surface contamination before interaction with the S. mutans biofilm, which should require sterilization of the samples.37 Sterilization methods were not preferred as they might have an impact on the properties of the studied restorative materials, degradation, crack formation, as well as other surface modifications of the GIC.38, 39
In the pilot study, MBC was selected as the starting concentration of PAMAM on 4 occasions due to its established efficacy in in vivo settings.40 As no effective difference in the recovered bacterial count was achieved between the control group and the modified groups, the added concentrations of PAMAM and BAG were doubled.
In the biofilm assessment assay, the control group exhibited antibacterial properties, likely attributable to the release of F in the vicinity of the cement, and the low pH of GICs during the setting process. This result is consistent with the findings reported by Shashibhushan et al.41 and Krämer et al.42
Also, the antibacterial properties of GII might be due to the antibacterial properties of PAMAM-NH2 and its ability to disrupt the bacterial cell,20 an outcome that aligns with the observations reported by Maleki et al.43 and Matboo et al.44 The results of the ion release test indicated a greater release of Si, Na, Ca, and Ph ions from GII compared to the control group, a phenomenon that could potentially explain their enhanced antibacterial properties.
Additionally, the antibacterial properties of GII may be due to the antibacterial effects of BAG particles on S. mutans.45 The ion release test results indicate a higher Si, Na, Ca, and Ph ion release, which may account for its enhanced antibacterial effect compared to the control group. Furthermore, it was revealed that needle-like BAG debris on the bacterial surfaces disrupted the bacterial structure, indicating that debris from BAG-modified GIC was among the causes of bacterial death.46
Moreover, the elevated antibacterial properties of GIV, which surpass those of all other groups, might result from the increased release of Si, Ca and Ph ions, as well as the potential antibacterial effect of the added BAG and PAMAM.
In the ion release test, the modified groups showed higher Si, Na, Ca, and Ph ion release in comparison to the control group. This may be due to the addition of PAMAM aid in the initial attack of the glass particles by both polyacrylic acid and PAMAM, causing the chelation of more ions that ionize during the test. It has been stated that PAMAM with different terminal groups possesses calcium coordination sites that attract free calcium and phosphate from the saliva.47 Furthermore, the bioactive nature of BAG is related to its ion release ability.48 The modification of GIC by adding BAG particles composed of Si, Na, Ca, and Ph,36 which dissolve in the solution, may lead to an enhanced amount of ion release compared to GIC alone. The results on Si ion release were consistent with those reported by Yli-Urpo et al.,29 though they did not align with their observations on Ca and Ph ion release.
The pH of the soaking solution was measured to investigate its effect on the antibacterial activity of the modified GIC. The study revealed an insignificant difference in the pH of the soaking solution after 24 h between the control group and all modified groups. This finding suggests that the antibacterial activity of the modified groups may be due to their chemical composition and ion-releasing ability, rather than the pH.
Limitations
The present research is not without its limitations. First, sterilization of the samples was not performed before conducting the antibacterial test. Also, biocompatibility tests for the PAMAM and BAG-modified GIC samples were not conducted. In addition, the pilot study indicated that the added percentages of PAMAM and BAG had to be selected as the least effective antibacterial concentration to prevent alterations in mechanical properties and setting time.
Conclusions
The modification of GICs with PAMAM and BAG has been demonstrated to enhance their antibacterial properties. Also, it has been shown that the higher the ion release ability of the modified GICs, the more pronounced their antibacterial effect, independent of pH. The hypothesis of the present study was rejected, as the PAMAM and BAG added to GICs enhanced their antibacterial properties.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.