Abstract
Inferior alveolar nerve block (IANB) is considered the most widely used anesthetic technique and the gold standard for blocking the hemimandible. This method is used in routine dental and oral surgical practice. The aim of this systematic review was to analyze reports related to the IANB technique combined with different local anesthetics. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were adopted to identify relevant studies, and the PICO (Patient/Population, Intervention, Comparison, and Outcomes) criteria were used to structure the research question. The literature search was conducted using PubMed/MEDLINE, Cochrane Library and Embase databases. The search was undertaken without temporal constraints. Prospective randomized clinical trials and randomized controlled trials were used as filters. Inclusion and exclusion criteria were chosen to initially select the appropriate articles from the published titles, followed by abstract reading. After evaluating the selected articles, the results of the research indicated that no relevant side effects were noted in any of the groups, irrespective of the anesthetic solution utilized. However, it is important to acknowledge that a follow-up period of 1 day may be too short to observe subsequent complications, evolution, or spontaneous remission of its eventual sequelae. Therefore, future randomized controlled clinical trials with large samples and longer follow-up periods are required to confirm these findings.
Keywords: complications, inferior alveolar nerve block, articaine 4%, lidocaine 2%, mepivacaine 3%
Introduction
Traditionally, inferior alveolar nerve block (IANB) is considered the most widely used anesthetic technique and the gold standard for blocking the hemimandible. This method is employed in routine dental and oral surgical practice. When combined with lingual nerve and long buccal nerve block, it provides adequate anesthesia of a wide anatomical area. This includes one side of the mandibular teeth and gingivae, the body and inferior ramus of the mandible, the anterior two-thirds of the tongue and the floor of the mouth.1, 2, 3
Many surgical procedures on the mandible can benefit from IANB, such as tooth extraction, surgical reconstruction, root canal treatment, periodontal treatment, and stabilization in cases of traumatic injury and fracture.4, 5, 6
The identification of anatomical landmarks is of the utmost importance.7 To improve upon the conventional IANB technique, microprocessor-aided electronic devices with digital controls can be used to facilitate aspiration and continuous delivery of local anesthetic solution. This approach is assumed to be less threatening and less painful.8, 9
However, the use of this technique has been previously associated with risks and complications, and the precise mechanism of nerve injury is still discussed. The potential consequences of this procedure may manifest as direct trauma or be caused by the neurotoxicity of the local anesthetic solution chosen.10, 11
As a result of direct trauma, the potential sources of injury include the injection needle, which can cause neural or vascular injury (with the facial nerve being the most frequently affected when the anesthetic solution is applied inside the parotid gland). Other possible causes of injury include hematoma and associated trismus, intravascular injection, mucosal and muscular injury, needle fracture, and post-injection infection related to its contamination.3, 12, 13
The occurrence of adverse effects has been associated with the neurotoxicity of the local anesthetic solution. Allergic reactions have been observed in association with amide local anesthetics. Furthermore, the presence of high concentrations of any local anesthetic in the bloodstream has been documented in cases of multiples injections, excessive doses of the anesthetic solution, or intravascular injection. Also, methemoglobinemia is a reported side effect resulting from an accumulation of metabolites from the anesthetic solution.3, 14, 15
Local anesthetics are differentiated based on their chemical structure, specifically the linkage (the amide linkage vs. the ester linkage) between the elements of the compound. Articaine, lidocaine and mepivacaine are the most commonly used local anesthetic agents in clinical dentistry. Lidocaine and mepivacaine are classified as amide-type local anesthetics. However, articaine, another amide-type anesthetic agent, contains an additional ester linkage. While both types of local anesthetics share the same mechanism of action, they differ slightly in their metabolic processes, binding to cellular sodium channels, and inhibiting the influx of sodium into the cell. This inhibition prevents cell depolarization and subsequent transmission of the previously propagating action potential.8, 16
The selection of an appropriate local anesthetic for a patient necessitates the consideration of several factors, such as surgical time extension, the possibility of self-mutilation in the postoperative period, the necessity for hemostasis, the potential need for post-treatment pain control, and the presence of any relative or absolute contraindications to the local anesthetic solution selected for administration.15
As adverse events can occur due to trauma or the anesthetic solution, it is important to carefully select the injection method and solution. These factors are essential for a successful and secure procedure.
Thus, the objective of the present study was to extract and analyze available data on the IANB technique combined with different local anesthetics (2% lidocaine with 1:80,000 epinephrine, 2% lidocaine with 1:100,000 epinephrine, 3% plain mepivacaine, 4% plain articaine, 4% articaine with 1:100,000 epinephrine, 4% articaine with 1:200,000 epinephrine, and 2% articaine with 1:200,000 epinephrine) in pediatric and adult patients. The study aimed to provide valid evidence for comparing results concerning possible complications.
Material and methods
Methodology
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were adopted for the current review.17, 18
Formulation of research question and keyword selection
The PICO (Patient/Population, Intervention, Comparison, and Outcomes) approach was used to structure and respond to the research question. It was found that higher precision and improved relevance of search results can be achieved through the use of PICO templates.19
The research question was formulated using the PICO criteria, as follows: “Are there different complications (O) reported by patients (P) who underwent IANB (I) with different anesthetics (C)?”. The following keywords and Medical Subject Headings (MeSH) were used for the search according to the research question: (“complications” OR “side-effects” OR “adverse reaction”) AND (“IANB” OR “inferior alveolar nerve block”) AND (“anesthetics” OR “articaine 4%” OR “mepivacaine 3%” OR “lidocaine 2%”). The applied filters included clinical trials and randomized controlled trials.
Search strategy
A literature search was performed using the PubMed/MEDLINE, Cochrane Library and Embase databases. Keywords and MeSH terms were searched individually and combined with Boolean operators (AND and OR). No systematic review was found that specifically addressed our research question under the defined criteria, which further justifies our decision to conduct this review. The literature search was carried out from January 24 to February 8, 2022.
Eligibility criteria
The following inclusion criteria were used in the study: articles with patients who underwent IANB; articles published worldwide and written in English with full access; no timeline restrictions; prospective, randomized clinical trials, or randomized controlled trials; articles reporting complications associated with IANB.
Animal studies, books, case–control studies, case reports and case series, cross-sectional studies, cohort studies, commentaries and conference papers, gray literature, meta-analyses, policies and guidelines, unpublished data, and review articles were excluded from the study.
Study selection process
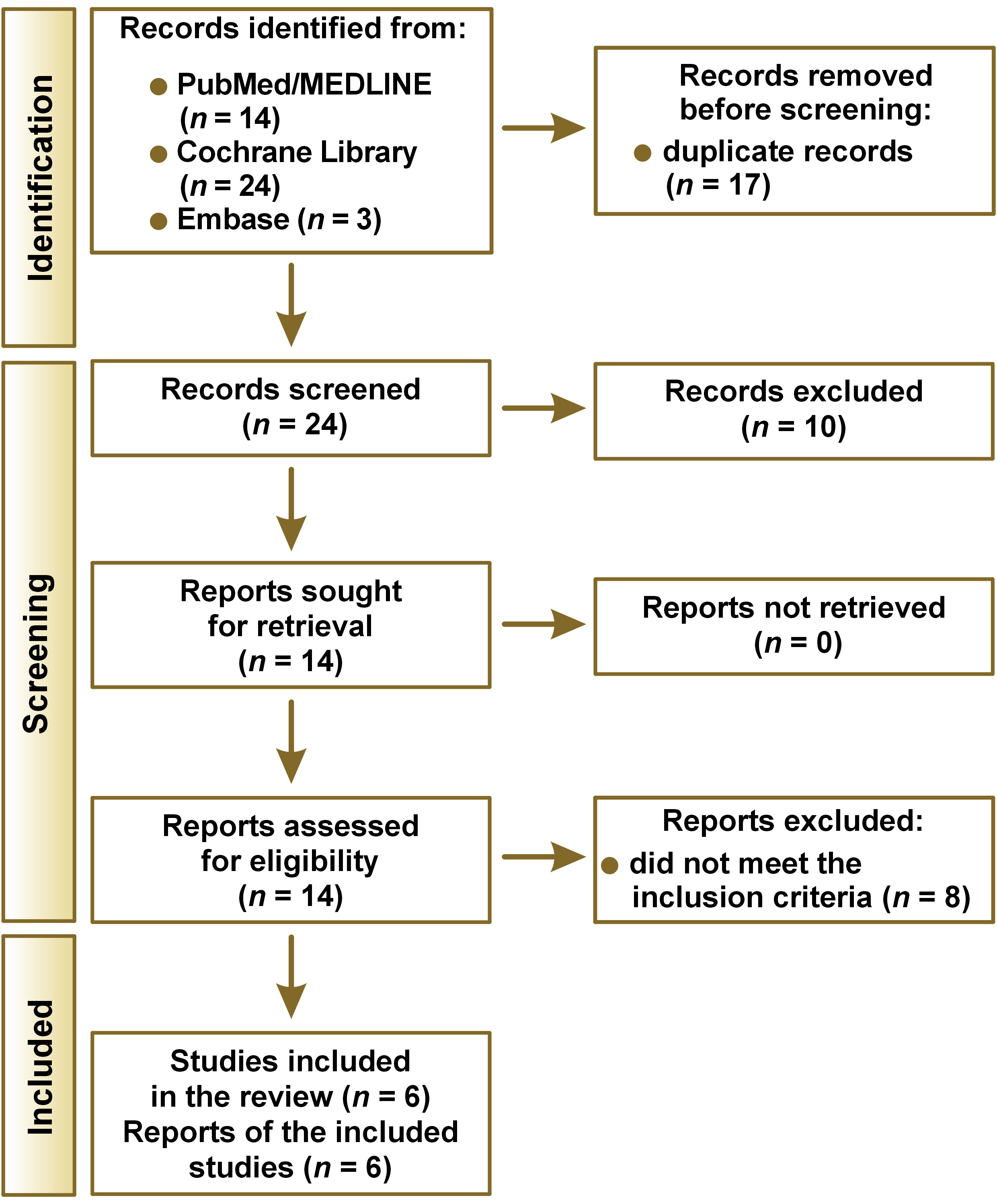
As a result of the systematic literature review, 41 articles were identified: 14 from PubMed/MEDLINE; 24 from Cochrane Library; and 3 from Embase. After removing the duplicates (n = 17), a preliminary screening of titles and abstracts was performed. Ten articles were excluded because they did not meet the eligibility criteria. After revising the full texts of the remaining 14 articles, 8 studies were excluded because they did not meet the inclusion criteria for this systematic review. A total of 6 studies met the inclusion criteria and were selected for analysis and data extraction in accordance with the PRISMA recommendations. A flowchart of the study is presented in Figure 1.
Quality assessment tool
The Cochrane risk-of-bias (RoB) tool for randomized controlled trials was used to assess the quality of the included studies. If all criteria were met (low risk for every domain), the study was labeled “good”. If 1 criterion was not met (high risk in any domain), then the study was considered “fair”, and if 2 or more criteria were not met (high risk or unclear risk in more than 2 domains), the study was labeled “poor”.20
Results
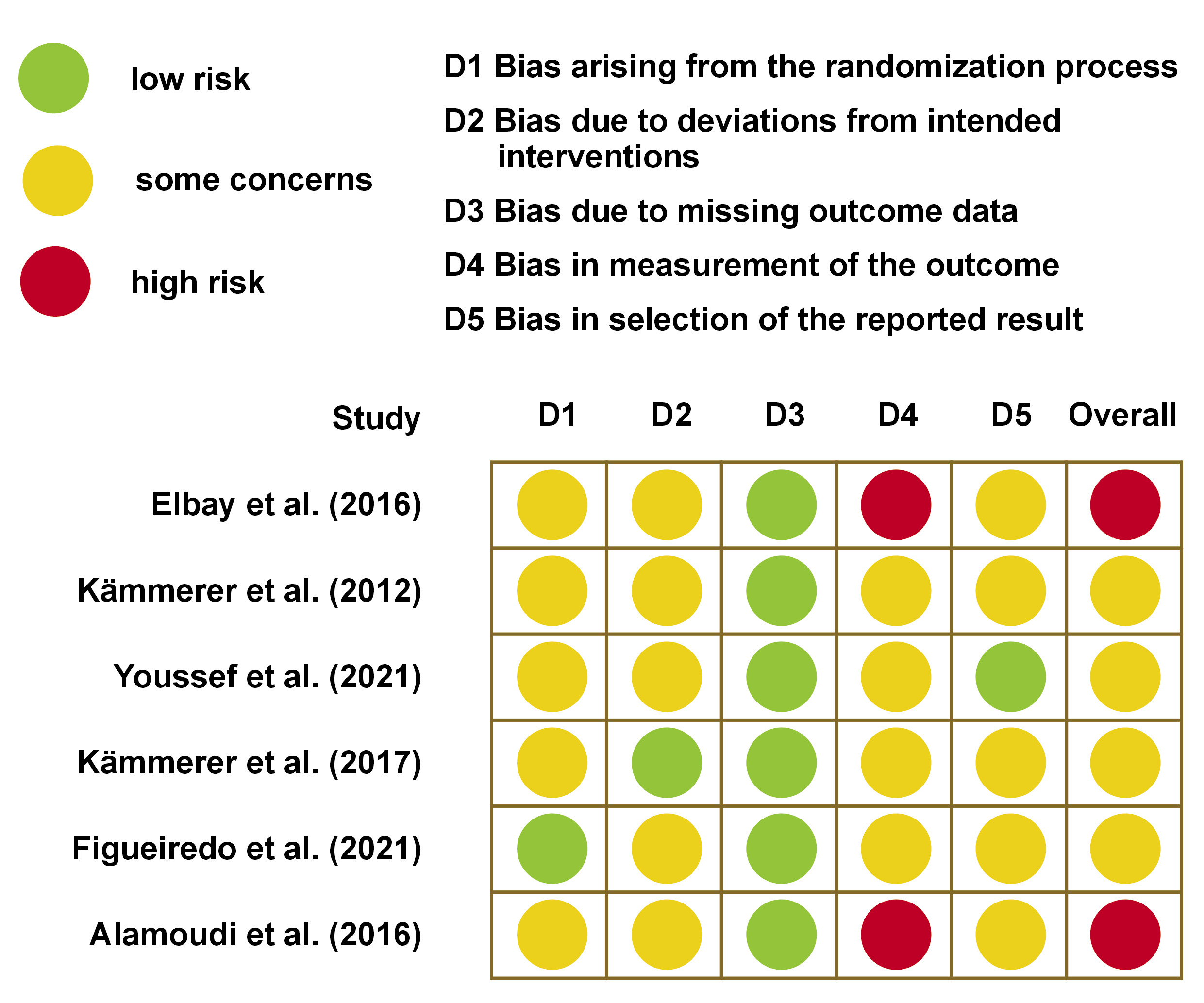
Following a thorough examination of titles, abstracts and full texts of the articles, 6 following studies were identified and included in the systematic review: Elbay et al.21 (study 1); Kämmerer et al.22 (study 2); Youssef et al.23 (study 3); Kämmerer et al.24 (study 4); Figueiredo et al.25 (study 5); and Alamoudi et al.2 (study 6). These studies were categorized as randomized clinical trials. A comprehensive overview of the 6 studies, accompanied by a quality analysis, is presented in Table 1 and Figure 2, Figure 3, Figure 4, Figure 5.
Characteristics of the included studies
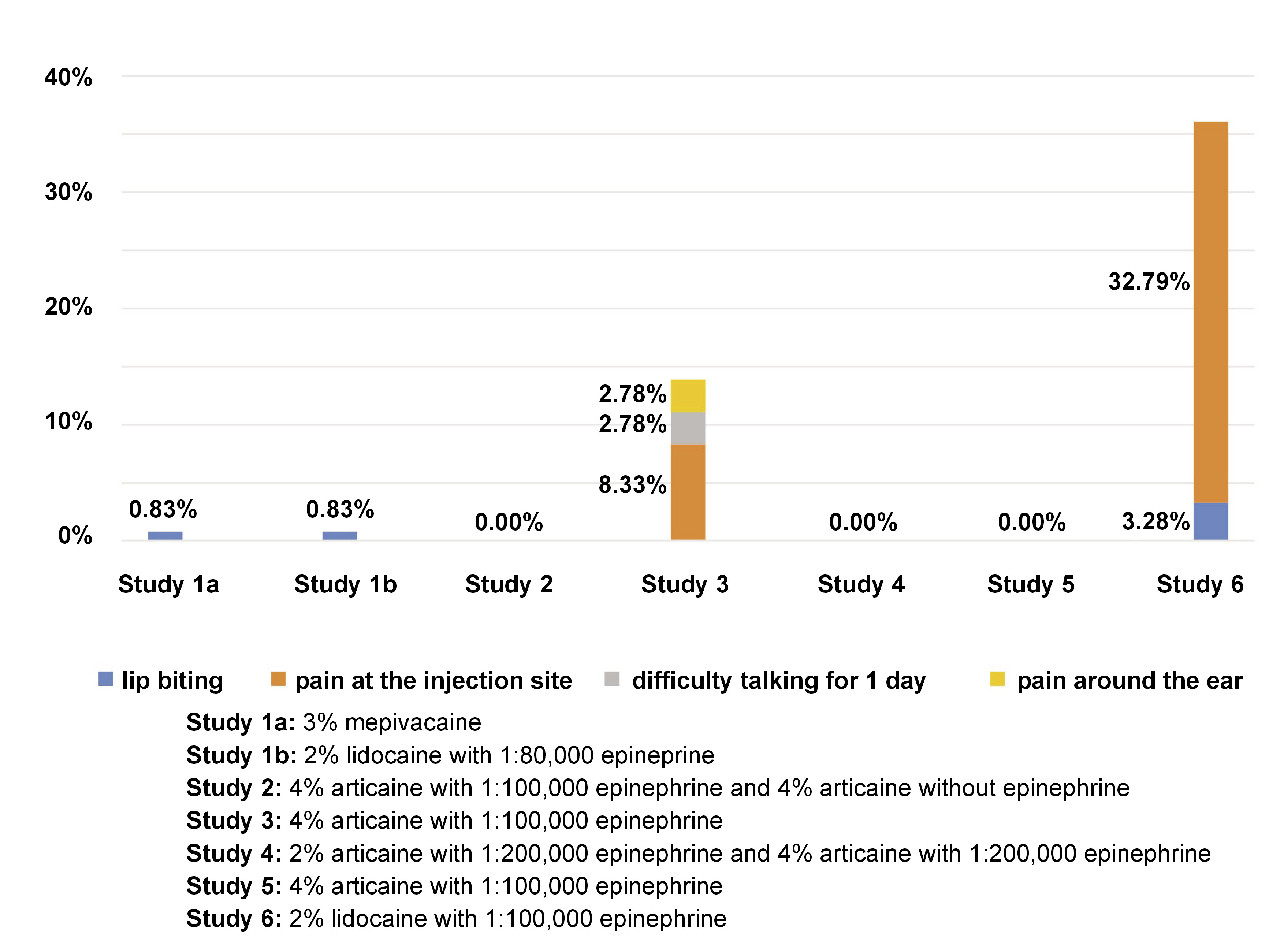
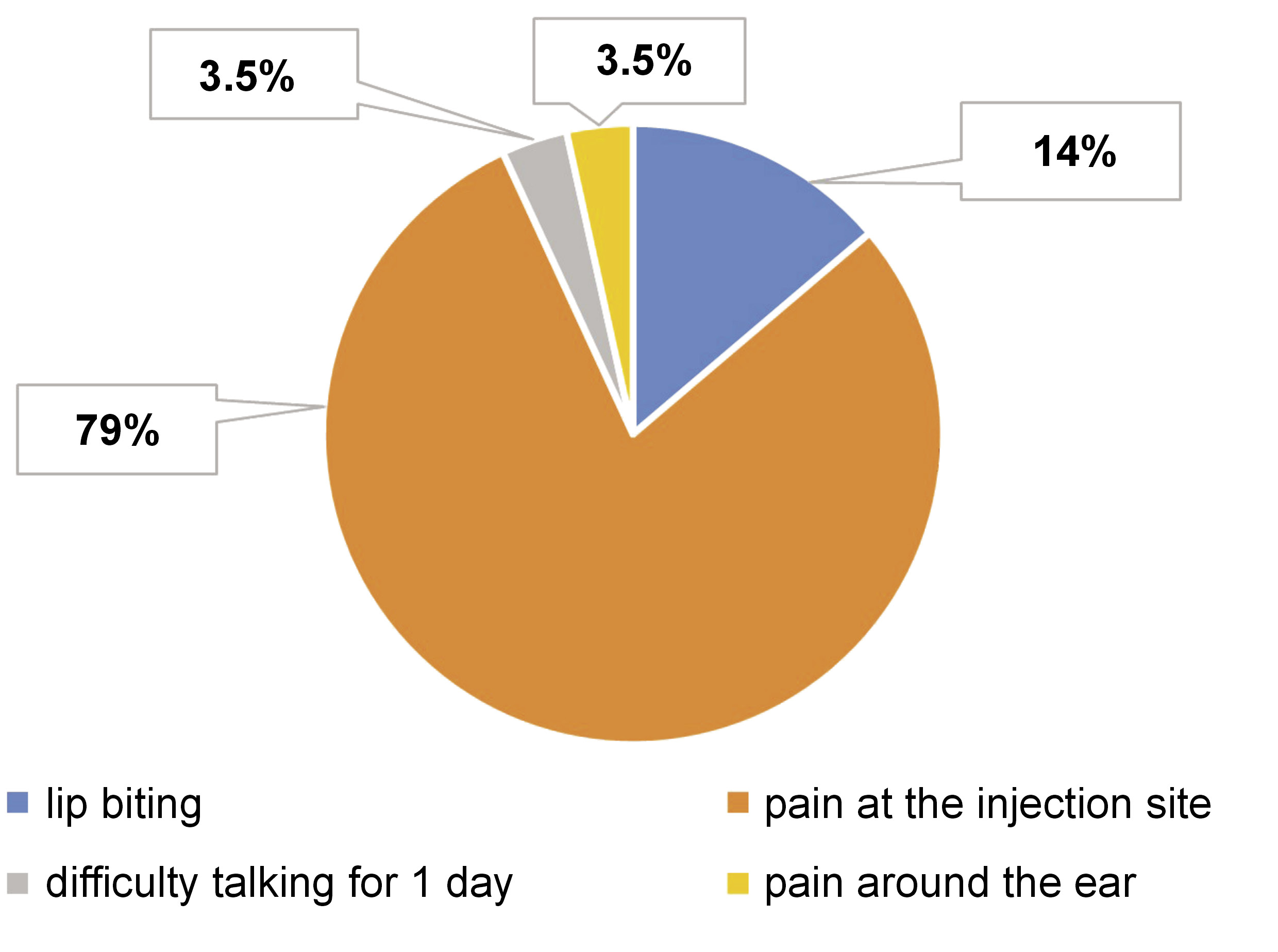
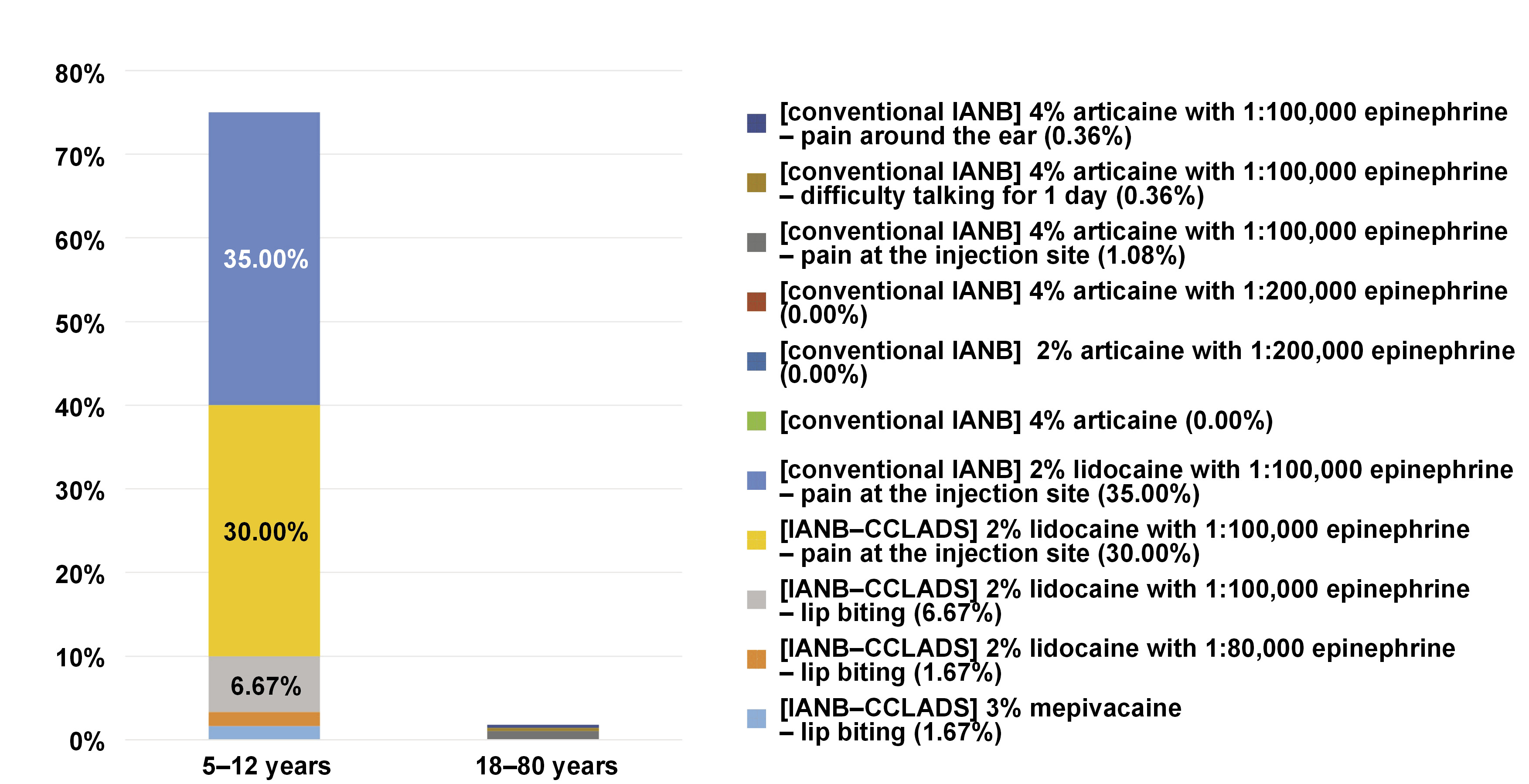
The characteristics of the studies included in this review are summarized in Table 1. Study 1, by Elbay et al., compared the behavior of 3% mepivacaine vs. 2% lidocaine with 1:80,000 epinephrine in computer-assisted IANB.21 The study by Kämmerer et al. (study 2) compared 4% articaine/1:100,000 epinephrine vs. 4% articaine without epinephrine in IANB.22 Study 3, by Youssef et al., compared the use of 4% articaine/1:100,000 epinephrine in intraligamentary anesthesia vs. IANB.23 The study by Kämmerer et al. (study 4) compared 2% vs. 4% articaine/1:200,000 epinephrine in IANB.24 Figueiredo et al. (study 5) compared infiltrative anesthesia vs. 4% articaine/1:100,000 epinephrine in IANB.25 The study by Alamoudi et al. (study 6) compared 2% lidocaine/1:100,000 epinephrine in traditional IANB with computer-assisted IANB and computer-assisted intraligamentary anesthesia.2 Of the 524 randomized subjects, 459 underwent IANB and reported 29 complications (studies 1, 3 and 6), corresponding to 6.32% of all IANB procedures. Telephone calls were made in studies 2, 3, 4, and 6 to assess participants within the first 24 h. Study 1 reported 2 cases of side effects, 1 using 3% mepivacaine and 1 using 2% lidocaine with 1:80,000 epinephrine. Study 3 reported 5 cases using 4% articaine with 1:100,000 epinephrine, while study 6 reported 22 cases using 2% lidocaine with 1:100,000 epinephrine (Figure 3). The most common side effect was pain at the injection site, corresponding to 5.01% (n = 23) of all cases, followed by lip biting with 0.87% (n = 4), difficulty talking with 0.22% (n = 1), and pain around the ear with 0.22% (n = 1) (Figure 4). The age range of participants in the 6 included studies was 5–80 years. All of the studies included in this review were published between 2012 and 2021.
Quality assessment of the included studies
The quality assessment of the included studies, along with the most relevant elements of the systematization process, are presented in Figure 2. The RoB 2 tool was used to evaluate the risk of bias of each study across 5 domains, and to provide an overall evaluation for each trial.26
In study 1, details regarding randomization of allocation are given explicitly, which makes this study free from allocation bias. However, the methodology does not include any information regarding the concealment of allocation. The sample size of the study, the age and sex of the participants, as well as the study source are given. The anesthetic solution was composed of 3% mepivacaine and 2% lidocaine. The double-blindness of the study is mentioned. The study revealed a lack of information concerning patient/caregiver awareness regarding the nature of the intervention being conducted. Additionally, practitioners were blinded to this information. Thus, there is an indication of concerns regarding bias arising from deviations from intended interventions. The outcomes of the randomized participants were documented, and the bias resulting from missing outcome data was low. Parents were informed and advised to call if they observed any postoperative complications. They were also instructed to document the levels (none, mild, moderate) of complications. Parents who are emotionally attached to their child often report complications in excess, resulting in a potential for bias in the measurement of the outcome. Bias in the selection of the reported result shows some concerns. Thus, the overall risk of bias in the study is considered high.
In study 2, the randomization of allocation is described in detail, thereby ensuring that the study is free from allocation bias. The allocation process was performed through the utilization of an online randomization generator, and no information was disclosed regarding the concealment of allocation. The sample size of the study, the age and sex of the participants, and the source of the study are given. The anesthetic solution was composed of 4% articaine and 1:100,000 epinephrine or 4% articaine without epinephrine. The information regarding the blinding of participants and caregivers was not provided. However, the study was double-blind, and the individuals who delivered the intervention were blinded in the study. The absence of information regarding the deviations has given rise to some concerns regarding the possiblity of bias due to deviations from intended interventions. The outcomes of the randomized participants were documented, and the bias resulting from missing outcome data was low. The study did not provide any information regarding outcome measurement. Furthermore, the outcome assessor was blinded. This study did not address the methodology employed to assess complications, which suggests potential concerns regarding the bias in measurement of the outcome. Similarly, concerns have been raised regarding the selection of the reported results. Thus, the overall risk of bias in the study is indicative of some concerns.
In study 3, the randomization of allocation is explicitly delineated, thereby ensuring the study is free from allocation bias. The sample size of the study, as well as the age and sex of the participants, are given. The anesthetic solution was composed of 4% articaine and 1:100,000 epinephrine. The blinding of the participants and caregivers was not mentioned in the study. However, the study was double-blind, and the individuals who delivered the intervention were blinded in the study. The absence of information regarding deviations gives rise to some concerns regarding bias resulting from deviations from intended interventions. The outcomes of the participants who were randomized were disclosed, and the bias resulting from missing outcome data was low. The study does not provide any information regarding the measurement of the outcomes. Furthermore, the individual responsible for assessing the outcomes was blinded. The study did not address the methodology employed to assess complications, which suggests some concerns regarding the bias in measurement of the outcome. The selection of reported results exhibited a low risk of bias. Thus, the overall risk of bias in the study is indicative of some concerns.
In study 4, the details regarding randomization of allocation are provided, which makes the study free from allocation bias. The sample size of the study, as well as the age and sex of the participants, are given. The anesthetic solution was composed of 2% articaine or 4% articaine. The study in question was a double-blind clinical trial. Informed consent was obtained from all eligible participants. However, the study’s methodology did not address whether the participants and caregivers were blinded. The individuals responsible for delivering the intervention were blinded, leading to a low bias owing to deviations from intended interventions. The outcomes of the randomized participants were disclosed, and the bias resulting from missing outcome data was low. No information regarding the measurement of outcomes was given. Furthermore, the outcome assessor was blinded. The methodology regarding the assessment of complications was not addressed in the study. Consequently, some concerns regarding bias in the measurement of the outcome were identified. Similarly, the selection of the reported results shows some concerns. Thus, the overall risk of bias in the study is indicative of some concerns.
In study 5, the randomization of allocation is described in detail, thereby ensuring that the study is free from allocation bias. The sample size of the study, as well as the age and sex of the participants are given. The anesthetic solution was composed of 4% articaine and 1:100,000 epinephrine. The study in question was a double-blind clinical trial. Informed consent was obtained from all eligible participants; however, it was not mentioned whether participants and caregivers were blinded or not. The individuals who delivered the intervention were blinded. Therefore, the bias caused by deviations from intended interventions has given rise to some concerns. The outcomes of all randomized participants were documented, and the bias resulting from missing outcome data was low. The study does not provide any information regarding the measurement of outcomes. The individual responsible for assessing the outcomes was blinded. The study did not address the methodology employed to assess complications, which suggests potential concerns regarding the bias in the measurement of the outcome. Similarly, concerns have been raised regarding the selection of the reported results. Thus, the overall risk of bias in the study is indicative of some concerns.
In study 6, the randomization of allocation was transparent, employing a block randomization technique to assign participants to one of the study groups. However, no information was given regarding the concealment of the allocation sequence until the participants had been enrolled and assigned to interventions. The sample size of the study, as well as the age and sex of the participants, are given. The anesthetic solution was composed of 2% lidocaine and 1:100,000 epinephrine. The study in question was a double-blind clinical trial. Informed consent was obtained from all eligible participants; yet, the study did not mention whether or not the participants and the caregivers were blinded. Nonetheless, the individuals responsible for delivering the intervention were blinded, and no information was disclosed regarding any deviations from intended interventions, resulting in some concerns in this aspect. The outcomes for all randomized participants were documented, and the bias resulting from missing outcome data was low. Potential complications were assessed after 24 h on phone call. The presence of a potential risk of information bias was observed, and a high degree of bias was identified in the measurement of the outcome. Similarly, concerns have been raised regarding the selection of the reported results. Therefore, the overall risk of bias in the study is high.
Overall, 2 trials were identified as being at high risk of bias (33.3%), while 4 trials were evaluated as having some concerns (66.7%). All studies demonstrated a low risk of bias for missing outcome data (100.0%). Five studies indicated some concerns regarding the randomization process and deviations from intended interventions (83.3%), while only 1 study exhibited a low risk of bias for the randomization process (16.7%), and 1 study demonstrated a low risk of bias for deviations from intended interventions (16.7%). Of the 6 trials, 2 showed a high risk of bias for measurement of the outcome (33.3%), and 4 studies demonstrated some concerns (66.7%). One study (16.7%) indicated a low risk of bias due to the selection of the reported result, and 5 studies exhibited some concerns (83.3%) (Figure 2).
Discussion
Inferior alveolar nerve block is the most frequently used technique for achieving local anesthesia for restorative and surgical procedures, especially in mandibular molars.1 The main goal of this technique is to effectively anesthetize all teeth in the same mandibular quadrant, as well as the gingival mucosa, the body and inferior ramus of the mandible, the anterior two-thirds of the tongue, and the floor of the mouth. Despite its reputation as a safe technique, there is a degree of risk involved.27, 28
A total of 524 patients (151 children and 373 adults) were evaluated and 7 different anesthetic solutions were included in this systematic review (2% lidocaine with 1:80,000 epinephrine, 2% lidocaine with 1:100,000 epinephrine, 3% mepivacaine, 4% plain articaine, 4% articaine with 1:100,000 epinephrine, 4% articaine with 1:200,000 epinephrine, and 2% articaine with 1:200,000 epinephrine).
With regard to studies conducted on children’s groups, all of them employed the following exclusion criteria: children who were medically compromised (i.e., those with allergies to local anesthetics or sulfites, or a history of significant medical conditions); and children who demonstrated uncooperative behavior.
Elbay et al. conducted a study with 60 children ranging in age from 6 to 12 years.21 The study compared IANB using 2% lidocaine with 1:80,000 epinephrine and 3% plain mepivacaine. The results indicated that none of the patients reported postoperative complications severe enough to require clinical treatment.21
In regard to the experience of pain, the postoperative pain exhibited no significant variation between the 2 anesthetics or the 2 groups observed by Elbay et al., the first group undergoing pulpotomy and the second one under extraction.21 In contrast, Alamoudi et al. associated 35.5% of postoperative pain after IANB procedure with 2% lidocaine and 1:100,000 epinephrine.2
In the study conducted by Alamoudi et al., no complications or side effects were observed immediately after the procedure.2 After 24 h, all legal guardians of the children were contacted to ascertain whether any postoperative complications had been observed. Two patients (6.66%) in the IANB group reported lip biting.2
Elbay et al. found no significant differences between the 2 groups in terms of postoperative complications such as lip or tongue biting, bleeding or hematoma.21 The occurrence of lip biting was documented in only 1 patient treated with 2% lidocaine and 1:80,000 epinephrine, and 1 patient treated with 3% mepivacaine. With regard to the occurrence of bleeding, no significant difference was observed between the 2 solutions. None of the patients required surgical procedures for hemostasis; however, 5 patients treated with 2% lidocaine and 1:80,000 epinephrine, and 8 patients treated with mepivacaine alone required a change in sponge to achieve hemostasis. All studies conducted on children have shown that patients in all groups have not reported cases of hematoma, swelling or infection.21
The 2% lidocaine with 1:80,000 epinephrine was hypothesized to demonstrate reduced bleeding compared to 3% mepivacaine, given that epinephrine is a vasoconstrictor used to minimize blood loss during surgical procedures. However, no differences were observed related to hemostasis with either anesthetic solution.21
Additionally, Elbay et al. found that 2% lidocaine with 1:80,000 epinephrine and 3% mepivacaine administered alone performed similarly when delivered as IANB anesthesia for primary mandibular molars requiring extraction or pulpotomy in children. These results are consistent with the findings of several other studies conducted on adults.21
Regarding studies conducted on adult groups, a study by Kämmerer et al. was an exception in that it included patients with healthcare conditions (e.g., hypertension, carcinoma in remission, hepatitis, epilepsy, hypothyroidism, and migraine) in the study group.22 All other studies included patients with any systemic disease as an exclusion criterion. The studies selected the patients based on diseases requiring special considerations during their dental treatment or patients with contraindications for any of the anesthetic solution, besides patients requiring open surgical extractions or teeth with signs of severe acute infection.
A study by Kämmerer et al. revealed that patients in the higher risk group did not exhibit any adverse reactions during or after the procedure.22 The article concluded that this finding is in accordance with the established low allergenic and toxic potential of articaine.22 However, it is important to note that a follow-up period of 1 day may not be sufficient to detect potential complications.
According to the study by Kämmerer et al., none of the groups (2% articaine with 1:200,000 epinephrine or 4% articaine with 1:200,000 epinephrine) exhibited any significant superior side effects.24 Other clinical trials comparing 2% and 4% articaine solutions yielded similar results.24 However, this study detected a slightly better anesthetic effect of the solution with the higher concentration, though it was not significant. Additionally, the duration of soft tissue anesthesia was significantly shorter with 2% articaine, consistent with the results of other researchers. Furthermore, the authors reported that no significant neurotoxicity was observed in either articaine group, in accordance with several other studies.24
As stated by Youssef et al., 5.4% of patients reported high scores of pain during IANB injection. However, no evidence of detrimental nerve contact or other complications was observed in any patient.23 Temporary irritations were reported by 5 patients in the IANB group 24 h after the procedure. A single case documented difficulty talking for 1 day after the anesthesia, 3 cases reported pain at the site of injection, and 1 case reported pain around the ear after the injection. With regard to the anesthetic solution, no significant transoperative or postoperative complications were observed in any of the patients who received 4% articaine with 1:100,000 epinephrine.23
A comparative analysis of the IANB technique and local infiltration revealed that both methods were found to be safe, with no significant difference in the perception of pain during the injection. Furthermore, no adverse effects were observed, including local complications (local irritation or discomfort) or systemic side effects (palpitations, nausea, vomiting, or dizziness). However, a greater volume of anesthetic solution was used in the local infiltration group, which has been demonstrated to increase the risk of complications.25
The current review revealed that a substantial percentage of studies exhibited some concerns regarding the risk of bias. Two studies were identified as being at high risk of bias. The primary cause of high risk in trials is the measurement of the outcome, that is, the method by which complications are assessed. However, a majority of the trials exhibited some concerns regarding the randomization process and deviations from intended interventions. All trials have adequately reported the results, and there was no missing data. Therefore, the risk of bias for missing outcome data was rated as low in all trials.
Conclusions
The reports related to the IANB technique combined with different local anesthetics in pediatric and adult patients have demonstrated that no relevant side effects were observed in any group, irrespective of the anesthetic solution employed. Nonetheless, it is important to acknowledge that the prevalence of temporary or even permanent injury due to IANB is considered to be very low, though not non-existent. Furthermore, it is also relevant to note that a follow-up period of 1 day may not be sufficient to observe the development of subsequent complications. Additionally, the fact that most of the studies excluded patients with systemic diseases could be a limitation of this study, both for adults and children.
The recommendation of local anesthetics is contingent upon the existence of high-quality trials with a low risk of bias. Future randomized controlled clinical trials with large samples are necessary to confirm these findings. Additionally, further studies are needed to enhance our comprehension of the distribution of complications associated with IANB using different local anesthetics at varying concentrations, with or without vasoconstrictor association, depending on patient age, the duration of action, the chronology of their onset and remission, and extended monitoring periods.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.