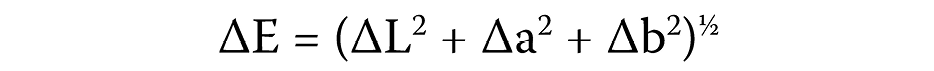Abstract
Background. Comparing the new and existing products is essential to identify the one that minimizes risks to the dental structures while effectively fulfilling its intended purpose.
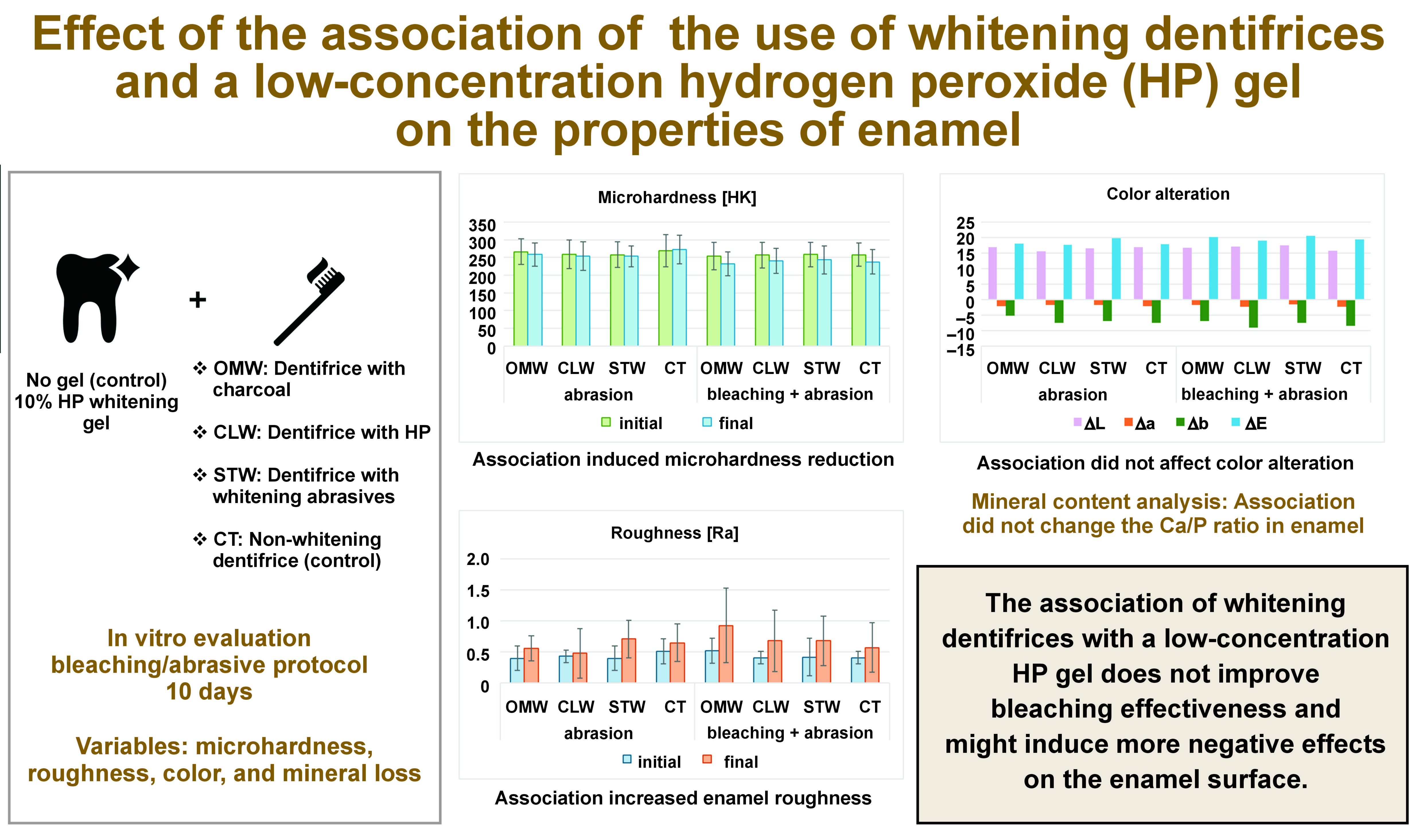
Objectives. The aim of the present study was to evaluate possible changes in the surface properties, mineral loss and color of bovine enamel subjected to bleaching dentifrices used in combination with a low-concentration hydrogen peroxide (HP) bleaching gel.
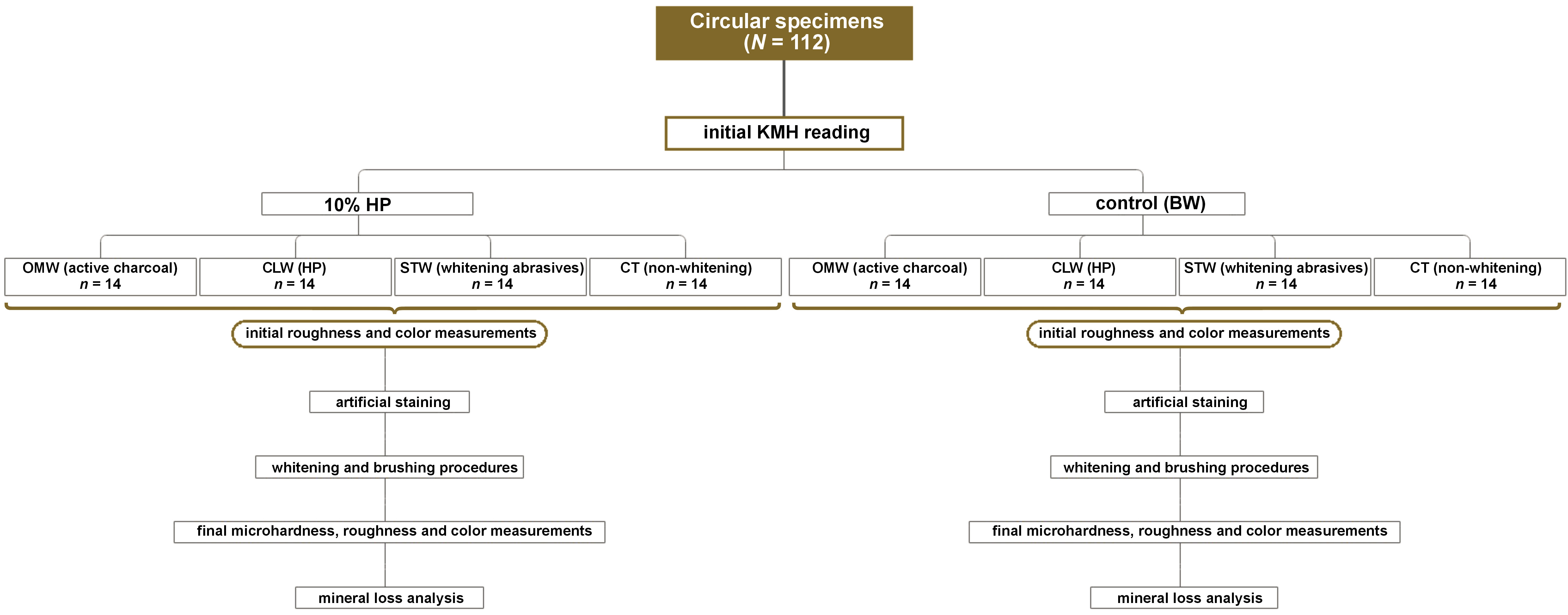
Material and methods. Bovine tooth substrates disinfected with thymol were used to make 112 circular samples with a diameter of 4 mm. After the samples were embedded in transparent acrylic resin, they were polished with grit of decreasing granulation and divided into 8 groups (n = 14 per group), according to the bleaching treatment (Opalescence Go (OpGo) – 10% HP or immersion in buffered water (BW) – control) and the toothpastes used (OMW – Oral-B 3D Mineral White Clean; CLW – Colgate Luminous White Advanced; STW – Sensodyne True White; or CT – Colgate Total 12). The bleaching gel was used for 30 min daily for 10 days. The samples were brushed using an electric brush and a slurry (3:1 ratio) for 120 s twice a day, with an interval of 12 h, with the first brushing immediately after the bleaching treatment. Prior to the commencement of the treatment, the initial microhardness, surface roughness and color data was evaluated.
Results. For microhardness, a reduction in values was observed for all groups, except for the control (CT + salt), whereas for roughness, there was an increase in the final values for all groups. A significant difference in the post-treatment values was observed only for the lightening treatment factor (p = 0.0079).
Conclusions. There was a reduction in enamel microhardness for all groups, except for the group that used a non-bleaching dentifrice and was treated with BW.
Keywords: microhardness, roughness, over-the-counter products
Introduction
Tooth whitening is based on the oxidation reaction of hydrogen peroxide (HP). This reaction releases free radicals of low molecular weight, capable of penetrating the dental structures and causing the chemical degradation of the chromogens. The action of HP promotes the reduction of chromophore molecules, modifying the refractive index of light and causing the lightening effect.1, 2 Bleaching can be done in-office, using high-concentration HP gels or at home with lower-concentration gels. However, even though whitening is considered the most cost-effective procedure for treating chromatic changes, access to is restricted to part of the population, as it requires professional consultation and supervision.3 In this context, alternative methods for tooth whitening are frequently desired by patients. Over-the-counter (OTC) products comprise toothpastes, whitening tapes, whitening pens, and mouthwashes, and are sold without professional prescription.4, 5
Dentifrices with whitening properties are in high demand among patients.3 They contain chemical and optical agents, usually with a high amount of abrasives and detergents as compared to non-whitening toothpastes.6 The combination of different abrasives is usually responsible for removing stains,3 but their continuous use may be related to the abrasive wear of the tooth surface,7 which can cause a progressive dental structure loss. Consequently, dentin hypersensitivity may develop in teeth with non-carious cervical lesion or gingival ressession.8, 9 Bleaching procedures can also increase the surface roughness of enamel, dentin and composite restorations,10, 11, 12 as well as enhance the cytotoxicity of certain restorative materials when they come into contact with saliva.13, 14, 15, 16, 17
According to the literature, the effectiveness of OTC whitening products is controversial. Karadas and Duymus reported a positive effect of a toothpaste with a whitening effect on removing superficial enamel stains.18 Rached Dantas et al. showed opposite results, concluding that whitening dentifrices were not effective in terms of staining removal.19 A recent systematic review showed that whitening dentifrices could produce some level of whitening effect on enamel, yet with considerable adverse effects, with low to moderate quality of evidence.20 Most of the patients do not have knowledge about the deleterious effects the prolonged use of these products can generate.
Beside dentifrices, strips with low-concentration HP gels are often available as an OTC option for tooth whitening. Recent evidence suggests that their use presents lower whitening efficacy than dentist-supervised at-home bleaching, although with a lower incidence of tooth sensitivity and gingival irritation.21 The associated use of whitening dentifrices and HP strips might induce further adverse effects on enamel, which are yet to be defined. The industry innovations regarding OTC products always demand updated research to evaluate their adverse effects on the dental structures. In addition to abrasives, some whitening dentifrices have active ingredients in their composition, such as HP or activated carbon, and the side effects on enamel related to their constant use have not been established yet. Comparing the new and existing products is essential to identify the one that minimizes risks to the dental structures while effectively fulfilling its intended purpose.
The aim of the present study was to evaluate the effects of the concomitant use of different whitening dentifrices and a low-concentration HP gel on the surface properties, mineral loss and color change (ΔE) of bovine enamel.
Material and methods
Sample size calculation
The sample size was calculated based on the study by Borges et al., considering the microhardness values for calculating f (effect size).22 The G*Power software, v. 3.1 (Heinrich-Heine-Universität, Düsseldorf, Germany), was used with a 95% significance level and 80% test power. The calculation obtained was 14 samples per group.
Sample preparation
Sound bovine incisor teeth, freshly extracted and acquired, were cleaned with periodontal curettes to remove any gum tissue residues adhered to the surface, and polished with a rubber cup and pumice paste and water, using Robinson brushes (Microdont, São Paulo, Brazil) in low rotation. Subsequently, 0.9% saline solution was used to store the teeth under refrigeration until used.
The crown was separated from the root at the cementoenamel junction (CEJ) with a diamond disk (KG Sorensen, Cotia, Brazil), and then, a circular enamel/dentin sample with a diameter of 4 mm was obtained using a diamond trephine mill. Subsequently, the enamel and dentin thickness were standardized at 2 mm (1 mm of enamel and 1 mm of dentin). The sample surface was polished in a circular polishing machine (Aropol; Arotec, Cotia, Brazil) with a speed of 600 rpm and constant irrigation, with 600-, 800- and 1,200-grit silicon carbide sandpaper (Extec Corp., Enfield, USA) for 60 s, 90 s and 120 s, respectively, resulting in parallel surfaces.
Study groups
The samples were stratified and divided into 8 groups (n = 14), considering their initial Knoop microhardness (KMH) values for enamel. The initial KMH of all specimens was measured using a microdurometer with a Knoop indenter (HMV-2T; Shimadzu, Kyoto, Japan), with a load of 50 g and a residence time of 15 s, following ISO 28399 (2011). Three indentations were made for each sample, and the average with regard to them was considered. The samples showing outliers in 20% were excluded.
The group division followed the whitening treatment and the toothpaste products. For the whitening treatment variable, the use of a low-concentration HP gel (Opalescence Go (OpGo), 10% HP; Ultradent Products, Inc., Indaiatuba, Brazil) was considered, with the negative control (buffered water (BW), pH 7.0). For the dentifrice factor, the dentifrices used were as follows: OMW – Oral-B 3D Mineral White Clean (Procter & Gamble Company, Cincinnati, USA); CLW – Colgate Luminous White Advanced (Colgate–Palmolive Company, New York, USA); STW – Sensodyne True White (GSK, Philadelphia, USA); and CT – Colgate Total 12 (non-whitening; Colgate–Palmolive Company). The composition of all dentifrices is presented in Table 1. Figure 1 shows the group division and the study flowchart.
Initial roughness and color measurements
The initial roughness values were obtained with a roughness tester (Surftest SJ-301; Mitutoyo, Tokyo, Japan) and measured by the parameter Ra. Three readings were made per sample and their average value was considered according to the provisions of ISO 28399 (2011).
The initial color data was collected using a colorimetric reflectance spectrophotometer (CM-2600d, Konica Minolta, Osaka, Japan), according to the CIE L* a* b* system (Commission internacionale de l’eclairage – CIE, International Commission on Illumination). In this, the L* axis represents the degree of luminosity and varies from 0 (black) to 100 (white), the a * axis represents the degree of the green/red color and the b * axis – the degree of the blue/yellow color. The initial color coordinates were measured with the equipment adjusted to the use of the D65 light source, with 100% ultraviolet, and the specular component included (SCI) mode. The observer’s angle was adjusted to 2° and the reading area was 12.56 mm2 (considering π = 3.14 and the radius of 2 mm), since the reading was made considering the internal diameter of 4 mm of the sample. To standardize the position of the samples in the spectrophotometer, each sample was marked with a diamond tip #1012 (KG Sorensen) on one of its sides, and a mark was made on the equipment.
Artificial sample pigmentation
The samples were immersed in a black tea coloring solution for extrinsic staining. The solution was prepared with 1.6 g of black tea (Leão Junior S.A., Curitiba, Brazil) in 100 ml of boiling distilled water (100°C), brewed for 5 min. The solution was renewed every 24 h and the samples were immersed for 6 days.23 After the pigmentation process, the samples were submerged for 7 days in artificial saliva, which was daily changed.
Whitening and brushing procedures
In the bleached groups, the whitening gel was used according to the manufacturer’s instructions in terms of time and number of applications. A 1-millimeter-thick layer of gel was applied over the samples for 30 min, totaling 9.4 mm3, once a day for 10 days. In the control groups, the samples were immersed in BW, with pH 7.0, for 30 min per day for 10 days, instead of the bleaching treatment. Afterward, the samples were washed with mineral water and submerged in artificial saliva (Byofórmula, São José dos Campos, Brazil) for 30 min.
Regarding the abrasion protocol with the tested dentifrices, they were applied as a slurry (3:1 with artificial saliva). The samples were brushed with the toothpaste corresponding to each group twice a day, with the first brushing immediately after the bleaching procedure, and the other one 12 h later. The sample was in contact with the slurry for 120 s, comprising 15 s of abrasion with an electric brush (Procter & Gamble) and 105 s of immersion in the slurry.
After the first brushing, the samples were washed with mineral water and submerged in artificial saliva for 12 h, when they were again brushed with the toothpaste corresponding to each group. In the intervals, the samples were kept immersed in artificial saliva. The artificial saliva used was composed of sodium carboxymethylcellulose (CMC, 10 g/L), sorbitol (30 g/L), potassium chloride (1.2 g/L), monobasic potassium phosphate (3.42 g/L), calcium chloride dihydrate (1.46 g/L), magnesium chloride (52 g/L), sodium chloride (84 g/L), sodium benzoate (1 g/L), sodium fluoride (1.25 g/L), methylparaben (1.5 g/L), and distilled water.
Final microhardness, roughness and color measurements
The final KMH and Ra measurement was made 7 days after the bleaching/abrasive procedures to enable the rehydration and stabilization of the samples. The samples were submerged in artificial saliva, which was changed daily.
The final color measurement was also made 7 days after the bleaching/abrasive procedures were completed, for rehydration and color stabilization. The same parameters were used as in the initial readings, and the final color was defined by calculating the variation of L* (∆L), a* (∆a) and b* (∆b). The total color change was calculated by parameter ∆E, using the following formula (Equation 1):
where:
∆E – color change;
∆L* – difference in lightness;
∆a* – difference in the green–red axis; and
∆b* – difference in the blue–yellow axis.
Mineral loss analysis
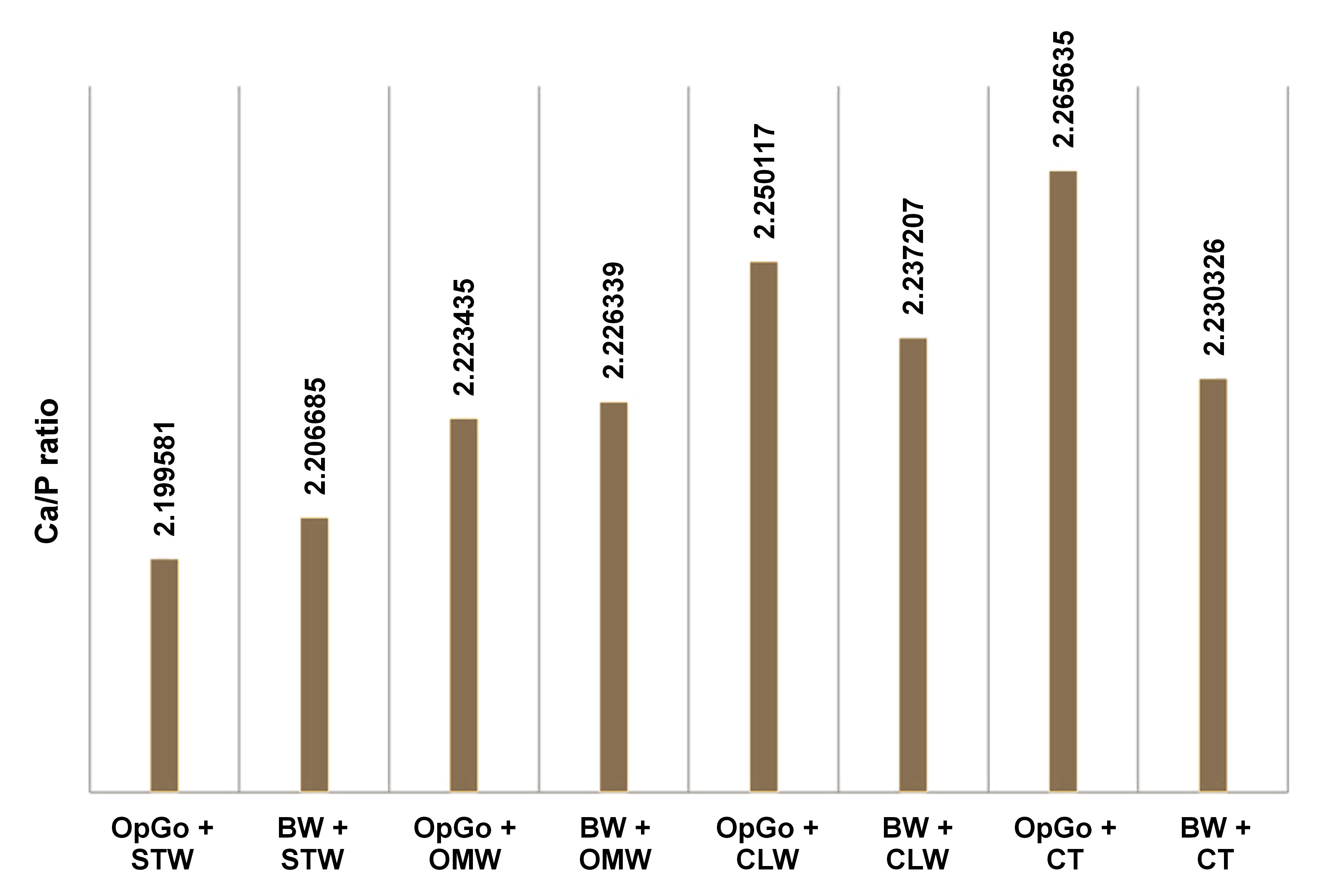
Five samples per group were randomly selected and were subjected to the micro-energy dispersive X-ray (µEDX) analysis, evaluating the mineral loss as the ratio between calcium (Ca) and phosphorus (P). The readings were performed using an energy dispersive X-ray fluorescence spectrometer (µEDX-1300; Shimadzu). The device is equipped with a rhodium (Rh) X-ray tube and a silicon (Si) detector, cooled by liquid nitrogen (N), and is associated with a computer and specific software for processing the collected data. The samples were placed on a glass plate sequentially in the order of each group. In each specimen, 3 readings were performed on the enamel surface with a voltage of 15kV and current of 100 µA, for 100 s with dead time 25%. The equipment was calibrated with stoichiometric hydroxyapatite, a certified commercial reagent (Ca10(PO4)6(OH)2 – synthetic hydroxyapatite, 99.999% purity grade, lot 10818HA; Sigma-Aldrich, St. Louis, USA), as a reference. The measurements were made using the fundamental emission parameters characteristic of the elements Ca and P. The element oxygen (O) was used as a chemical balance.
Statistical analysis
After analyzing data normality, the KMH values were subjected to the two-way repeated measures analysis of variance (ANOVA) with the Bonferroni correction. For the Ra values, the Friedman and Kruskal–Wallis tests were used, and the color data was analyzed with the one-way ANOVA and Tukey’s test (∆L and ∆E), or the Kruskal–Wallis test and Dunn’s test (∆a and ∆b). For the EDX values, the data underwent a descriptive analysis. The confidence level was set at α = 0.05.
Results
The results of the analysis of surface microhardness are available in Table 2. After bleaching, the samples showed a significant difference in microhardness after the abrasive protocol, regardless of the dentifrice tested (p < 0.05). When the samples were not bleached, there was a significant difference only in the case of the abrasive protocol carried out with the toothpaste containing charcoal (p < 0.05). A statistically significant difference was found comparing the bleached and non-bleached groups, regardless of the toothpaste used. The dentifrices showed no statistically significant difference between each other in the final values. When whitening was used concomitantly with the toothpaste with charcoal, a significant difference was found as compared to the use of charcoal alone, without bleaching taking place.
As the roughness data did not meet the normality criteria, non-parametric tests were applied; the Friedman test was used to compare between the time points, and for groups, the Kruskal–Wallis test was employed. There were statistically significant differences between the baseline and final values in the bleached groups using the OMW and CLW toothpastes (p < 0.05) (Table 3).
Regarding ∆E, there were no statistically significant differences between the tested groups (p > 0.05) (Table 4).
The Ca/P data revealed no differences between the dentifrices studied (p > 0.05). Figure 2 shows the absolute mean values of the 5 samples tested in each group, assessing the mineral loss as the ratio between CA and P for the treatment performed.
Discussion
This in vitro study evaluated the effects of whitening strips with 10% HP associated or not with brushing with toothpastes containing different active ingredients on enamel. Regarding toothpastes, apart from active ingredients, they also presented differences in their formulation; however, the results were interpreted considering that differences in performance between the formulations were due exclusively to their operating principles (charcoal, low-concentration HP and whitening abrasives).
Regarding microhardness, the differences found for all the samples bleached with 10% HP were significant in relation to the dentifrices tested; for roughness, there were significant differences for the groups of samples bleached with 10% HP, and brushed with the toothpastes containing activated charcoal (OMW) and a low concentration of HP (CLW); the color analysis being the exception, since between the baseline and final values, there were no differences, regardless of the dentifrice used. As mentioned above, for the samples brushed with the toothpaste containing HP, the difference was statistically significant. There is evidence that HP can increase enamel roughness due its ability to demineralize hydroxyapatite crystals,22 and the association of the gel and the toothpaste containing this ingredient induced a more pronounced adverse effect. There is no specific clinical protocol described to overcome this, and patients should be aware that increased roughness can enhance biofilm formation and staining.
When the toothpastes were used immediately after whitening with 10% HP, a decrease in the surface microhardness values of tooth enamel was observed when compared to the groups where there was no whitening, with the exception of the dentifrice containing activated charcoal, which showed a reduction in microhardness (p < 0.05).
The methodology in the present study was designed to simulate the performance of supervised home whitening treatment, in which the patient uses a tray pre-loaded with the gel, and, in an attempt to enhance the effect of this whitening product, without the guidance and permission of their dentist, makes use of toothpastes freely available in the market that are advertised as making the teeth whiter.
In this study, the reduction in enamel microhardness promoted by the whitening gel was less than 10%, which is below the safety limit allowed by the American Dental Association (ADA). In fact, previous data showed that the use of HP does not promote significant changes in the histomorphology and microhardness of enamel,24, 25 and even if any changes occur with regard to the initial microhardness and roughness, they are reversed by the action of saliva within a certain time.26 As the toothpaste was used immediately after removing the gel, there may not have been enough time for saliva to produce its remineralizing effect.
It is argued that the viscosity of the whitening gel may be directly related to the deleterious effects.27 The pH of Opalescence Go used in this study, according to the manufacturer, is neutral. Therefore, any expected deleterious effect on enamel was minimal, which corroborates previous studies.27, 28, 29 Bistey et al. reported that, in addition to these factors, structural changes to the enamel surface also depend on the contact time, with considerable alterations occurring when the time of exposure to HP exceeds 60 min.30 The exposure time used in this study was 30 min, which may also have been the reason for not promoting changes in the studied surface properties.
However, there was a statistically significant difference in microhardness in the group with no bleaching, using the toothpaste containing activated charcoal. Charcoal is an abrasive that can be manufactured from a variety of carbon-rich materials, including walnut shells, coconut shells, bamboo, peat, and wood.31 When it is used for toothbrushing, it is manufactured as a fine powder of varying abrasiveness, depending on the source and the methods used to prepare and grind it.32 The charcoal-based toothpaste used in this study has also other abrasives in its formulation, such as silica, titanium dioxide and mica, enhancing the abrasive potential of this toothpaste, thus making it more harmful to enamel. It is known that the abrasiveness offered by toothpastes is normally the determining factor for the removal of extrinsic stains and, consequently, the whitening sensation promoted.33, 34 However, the findings of this study indicate that the combined effects of toothpaste abrasiveness and pH can lead to greater tooth tissue loss when applied to enamel with a softened surface layer.
Although the toothpaste used in this study contains fluoride, its high adsorption capacity raises concerns about the actual availability and benefits of fluoride and other active ions in the dentifrice, as these may have been absorbed by charcoal.32 Furthermore, despite fluoride sodium present in the composition of the toothpaste used in this study, it is reported in the literature that only 8% of charcoal toothpastes contain fluoride.31, 32 Fluorides that are present in conventional toothpastes have an acknowledged anti-cariogenic potential, and offer protection, even if limited, against erosion and cariogenic microorganisms, forming fluoride precipitates of calcium (CaF2).35 These benefits are not available if fluoride inactivation by charcoal occurs, leaving the tooth more susceptible to acid attacks.
Brushing with the toothpaste containing a low concentration of the active ingredient HP immediately after using the whitening gel resulted in significant changes in the surface roughness of enamel. This tested toothpaste, in addition to having 3% HP, contains a combination of abrasives (sodium and tetrasodium pyrophosphate and silica), which may have been responsible for the significant increase in roughness.
The μ-EDX analysis indicates the Ca/P ratio in the dental structures. The literature shows that a sound enamel has similar Ca and P concentrations.26 In this study, the analysis was conducted after the procedures, and the results indicated no significant changes to enamel in all groups after the bleaching treatment and the abrasion with the tested dentifrices.
Finally, regarding color alteration, there were no significant differences between the groups, indicating that the toothpastes tested did not enhance color alteration when used with the bleaching tray tested. All kinds of treatment produced high ∆E values, indicating effective whitening; however, these results should be interpreted with caution, as the staining protocol used may have overestimated the extent of extrinsic staining. Future studies should include polishing of the enamel surface previous to the initial color measurement to remove loosely bounded staining, and be closer to clinical conditions.
The aim of this study was to evaluate the concomitant effect of the whitening treatment and the abrasive action of toothpaste; therefore, the brushing period was limited to the recommended period of the whitening treatment used, i.e., 10 days. Hence, the results obtained after longer periods of use could demonstrate greater deleterious effects on the surface of tooth enamel. Even though it is an in vitro study, the findings show that the use of toothpastes without guidance from a dental surgeon, especially when it is carried out concomitantly with supervised whitening treatment, can be harmful to enamel. As a limitation, this study was conducted in vitro, using artificial saliva in the bleaching and abrasion protocol; therefore, the presence of the acquired pellicle was not considered. Additionally, although the staining protocol employed is commonly cited in the literature, it may overestimate the physiological staining that occurs in the oral cavity.
Conclusions
Within the limitations of this study, it might be concluded that the association of whitening dentifrices with a low-concentration HP gel does not improve bleaching effectiveness, and might induce more negative effects on the enamel surface. Thus, this combination might not be clinically viable, and patients shall be advised of possible side effects when using OTC whitening products without a proper supervision of a dentist.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.