Abstract
Background. Denture adhesives promote greater stability and retention of dentures. However, they can also facilitate biofilm formation related to oral diseases.
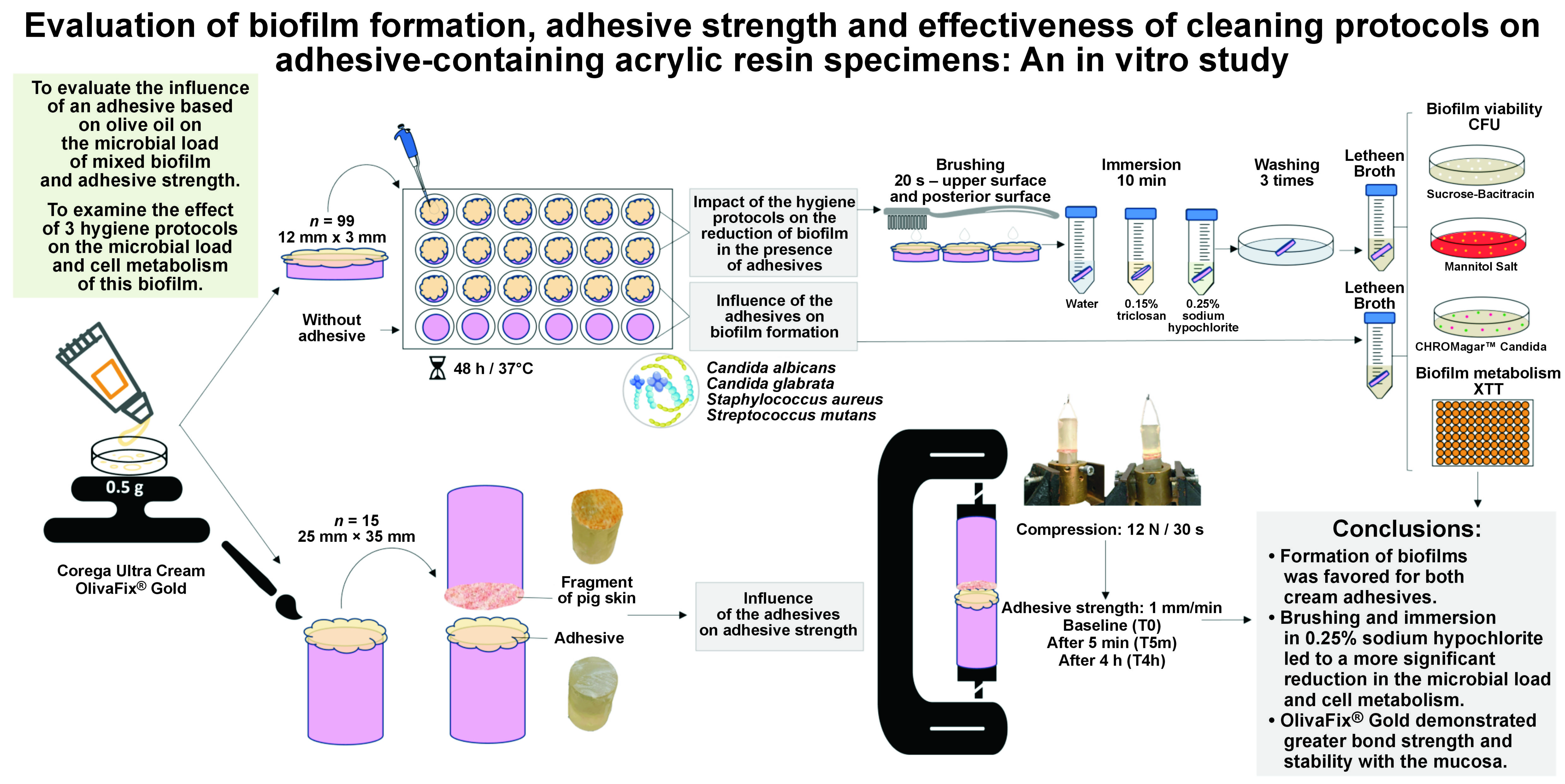
Objectives. The study aimed to evaluate the influence of 2 adhesives on the microbial load of mixed biofilm and adhesive strength. Additionally, the objective was to assess the effect of 3 hygiene protocols on the microbial load and cell metabolism of this biofilm.
Material and methods. The study compared Corega Ultra Cream (CCA) and OlivaFix® Gold (OFA) adhesives by evaluating the biofilm formation of Candida albicans, Candida glabrata, Staphylococcus aureus, and Streptococcus mutans by colony-forming unit (CFU), as well as adhesive strength. The implemented hygiene protocols included brushing and immersion in water (BW), 0.15% triclosan (BT0.15%), or 0.25% sodium hypochlorite (BSH0.25%). The control groups were either without adhesive (CG) or without any hygiene protocols (CGwH). The one-way and two-way analyses of variance (ANOVAs) with Tukey’s post hoc test and a generalized linear model with Bonferroni adjustment were used for statistical analysis (α = 0.05).
Results. The microbial load of C. albicans was higher when OFA was used (p < 0.001). The microbial loads of C. glabrata and S. mutans were similar between adhesives and higher in the CG (p < 0.001). The influence of the adhesives on the microbial load of S. aureus was not statistically significant (p = 0.287). The adhesive strength promoted by OFA was greater and more stable than when CCA was used (p = 0.007). The immersion in sodium hypochlorite led to a reduction in the microbial load of C. albicans (p < 0.001), C. glabrata (p = 0.002) and S. mutans (p = 0.012), independent of the adhesive. For S. aureus, the microbial load was lower with OFA/BSH0.25% (p = 0.022). All hygiene protocols resulted in a decreased cell metabolism when compared to the CGwH (p < 0.001).
Conclusions. Brushing with BSH0.25% solution was the most effective hygiene protocol, resulting in a reduction in the microbial load and metabolism. This protocol may be recommended as a first-line option for the disinfection of dentures.
Keywords: adhesives, denture, hygiene, biofilm
Introduction
The rehabilitation of edentulous individuals can be achieved through the use of complete dentures.1, 2 However, the support tissues undergo continual remodeling after tooth loss, compromising retention and support for dentures, as well as affecting quality of life.1 This problem can be addressed by using dental implants in conjunction with complete dentures.2, 3, 4, 5 Nonetheless, this treatment is not universally applicable due to various psychological, anatomical, systemic, and social factors.2, 3
An alternative approach involves the use of denture adhesives,6 which enhance retention and stability, increasing comfort, confidence, satisfaction, and, consequently, the quality of life related to oral health.6 However, a disadvantage of this method is the difficult removal of the adhesive from the denture surface. Moreover, the repeated use of adhesives can lead to the growth of microorganisms, such as Candida albicans, Candida glabrata, Staphylococcus aureus, and Streptococcus mutans, which have been associated with the development of denture stomatitis.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22
An effective hygiene method for removing adhesive residues and biofilm is essential to maintain health of the oral mucosa. However, few studies have evaluated adhesive removal methods.23, 24, 25, 26 The literature suggests that brushing the denture along with its immersion in 0.25% sodium hypochlorite or 0.15% triclosan provides effective anti-biofilm action.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 In addition to effective hygiene methods, the incorporation of antimicrobial agents into the adhesive has also been proposed.38 OlivaFix® Gold (bonyf AG, Vaduz, Liechtenstein) is an adhesive with 30% organic extra virgin olive oil and an absence of petroleum derivatives and zinc, making it a natural alternative on the market. A number of studies have been conducted to assess the anti-biofilm properties of OlivaFix® Gold11, 38, 39, 40, 41, 42, 43, 44, 45, 46 in comparison to other well-established adhesives.6, 10, 12
Further research employing a standardized methodology and utilizing the most prevalent microorganisms10, 11, 14, 15 in complete denture biofilm, as well as adhering to hygiene protocols accessible to patients is necessary to confirm the safe use of cream adhesives. Thus, the present study evaluated the influence of an adhesive based on olive oil on the microbial load of a mixed biofilm and adhesive strength. In addition, the study examined the effect of 3 hygiene protocols on the microbial load and cell metabolism of this biofilm. The evaluated adhesive was then compared with an adhesive that is commonly recommended in the literature. The null hypothesis posits that microbial load and adhesive strength are similar between the adhesives, as well as that the hygiene protocols have comparable effects on biofilm control.
Material and methods
Study design and setting
The materials used in the study are presented in Table 1. The quantitative response variables and variation factors were: (1) microbial load of a mixed biofilm evaluated by colony-forming units (CFUs) formed on the surface of acrylic resin specimens without (control group (CG)) or with adhesive – Corega Ultra Cream (CCA) (GlaxoSmithKline, Buenos Aires, Argentina) or OlivaFix® Gold (OFA); (2) bond strength of CCA and OFA adhesives; (3) microbial load (CFU) of the biofilm after the application of the hygiene protocols; and (4) cell metabolism (XTT assay) of the mixed biofilm formed on acrylic resin specimens with CCA and OFA adhesives, before and after the use of hygiene protocols. A group that did not undergo the brushing procedure (CGwH) was incorporated into the analysis. The results of the XTT assay were interpreted as the percentage of metabolic reduction found in the groups, considering the cell metabolism in the CG as 100%. The analyses were performed in triplicate on 3 separate occasions, and 1 specimen was used as a sterilized control (free of contamination) on each occasion.
Preparation of specimens
Circular, heat-polymerized acrylic resin specimens (12 mm × 3 mm) were obtained by the conventional technique of using metallic matrices in plaster, pressing, polymerization, and finishing.32 The roughness (Ra) of the surface of the specimens was standardized between 2.7 µm and 3.7 µm with a profilometer (Surftest SJ-201P; Mitutoyo Corporation, Kawasaki, Japan).33 Subsequently, 27 specimens were randomly distributed into 3 groups (n = 9 per group): non-adhesive group (CG); CCA group; and OFA group. The specimens were placed in a beaker with 200 mL of distilled water for sterilization in a microwave oven set at 650 W for 6 min (model Perfect; Panasonic, Tokyo, Japan).33 After cooling, the specimens were distributed into 24-well plates (Techno Plastic Products AG, Trasadingen, Switzerland). A quantity of 0.080 g of the adhesive was applied homogeneously to the surface of the acrylic resin samples to form a thin layer, which was then disinfected using ultraviolet-C (UV-C) light with a power of 60 W for 20 min in a laminar flow chamber (Pa 400-ECO; Pachane, São Paulo, Brazil). Following the disinfection process, the adhesive was distributed into 24-well tissue culture plates.
Analysis of biofilm formation on acrylic resin surfaces
Exponential growth phase cultures of C. albicans (ATCC 10231), C. glabrata (ATCC 2001), S. aureus (ATCC 25923), and S. mutans (ATCC 25175) were obtained. Subsequently, 1.5 mL of brain heart infusion (BHI Broth; HiMedia Laboratories Pvt. Ltd., Thane, India) inoculated with yeast and bacteria in the concentrations of 1×105 cells/mL and 1×106 cells/mL, respectively, was added to the specimens, which were then incubated as previously described.33 After biofilm maturation, the specimens were rinsed 3 times in phosphate-buffered saline (PBS) and inserted into polypropylene test tubes (Techno Plastic Products AG) with 10 mL of Letheen Broth (HiMedia Laboratories Pvt. Ltda.). Then, the specimens were sonicated at 40 kHz and 200 W (Clean 9CA; Altsonic, Ribeirão Preto, Brazil) and vortexed (Phoenix™ AP 56; Phoenix Industria e Comercio de Equipamentos Cientificos, Ltda, Araraquara, Brazil). The suspension was seeded in the specific culture media for the growth of the microorganisms. The biofilm formation was quantified as CFU/mL and presented as log10.33
Evaluation of the adhesive strength of cream adhesives
To assess the adhesive strength, cylindrical specimens (n = 15, 25 mm × 35 mm) were made using a previously described conventional technique with minor modifications.10 A handle was attached to the upper part of the specimens and connected to the tow bar of the mechanical testing machine (EMIC DL 2000; Instron Brasil Equipamentos Científicos Ltda, São José dos Pinhais, Brazil). The specimens were then measured according to the ISO 10873 recommendations.47 To simulate the presence of mucosa, a piece of pig skin with the same diameter as the surface was fixed with cyanoacrylate-based instant adhesive (Loctite® Super Bonder®; Henkel Ltda., São Paulo, Brazil).48 Subsequently, the pig skin-covered surface was moistened with 5 mL of artificial saliva for 1 min, and 0.5 g of cream adhesive was evenly applied. Another acrylic resin specimen was then positioned in contact with this surface, according to the manufacturer’s instructions. The adhesives were compressed with a force of 12 N (1.2 kg of weight) for 30 s.48 The adhesive strength was measured immediately (T0), after 5 min (T5m) and after 4 h (T4h) of application. The assembly was moved in a tensile mode at 1 mm/min, and the maximum force was calculated in Newtons (N).
Evaluation of the effect of hygiene protocols on mixed biofilms
The antibiofilm efficacy of the hygiene protocols on the microorganisms in biofilms formed on the surfaces of acrylic resin specimens with CCA or OFA was determined by means of microbial load (CFU/mL) and metabolic activity (XTT assay) evaluation. Three replicate inter-assays were performed at 3 independent times. Seventy-two specimens (12 mm × 3 mm) were randomly distributed among the following regimens: no hygiene protocol (CGwH); brushing and immersion in water (BW); brushing and immersion in 0.15% triclosan (BT0.15%); and brushing and immersion in 0.25% sodium hypochlorite (BSH0.25%).
In order to implement the hygiene protocols, 2 specimens were removed from the culture plate and placed within orifices, prepared in plexiglass plates (Policarbonato; Day Brasil, Barueri, Brazil), with the dimensions corresponding to those of the specimens. The specimens were manually brushed by the same operator using a dental brush49 (TEK® soft; Johnson & Johnson, São José dos Campos, Brazil) and 1 drop of neutral soap, with standardized movements and pressure. The brushing movement was executed in the same direction for 20 s on both the upper and posterior surfaces of the specimen. Afterward, the specimens were washed 3 times with PBS and immersed in 10 mL of the hygiene solutions for 10 min. Then, the samples were rinsed thrice in PBS and transferred to tubes containing 10 mL of Letheen Broth.33 To analyze the residual microbial load, the procedures for seeding in agar medium and CFU counting were performed as previously described.
Analysis of cell metabolism
The XTT colorimetric assay was used for the analysis of cell metabolism.33 Briefly, after the formation of biofilms, 60 specimens were allocated according to hygiene protocols and transferred to sterile 24-well culture plates containing tetrazolium salt. Following a 2-h incubation period at 37°C, the absorbance of the formazan product was measured in triplicate using a microplate reader (Multiskan GO; Thermo Fisher Scientific, Vantaa, Finland) at 492 nm.
Statistical analysis
The data was tested for normality (Shapiro–Wilk test) and heterogeneity (Levene’s test). The effect of the adhesives on biofilm formation and adhesive strength was analyzed using one-way analysis of variance (ANOVA), two-way ANOVA and Tukey’s post hoc test. The generalized linear model with Bonferroni adjustment, two-way ANOVA and Tukey’s post hoc test were used to compare the effects of the antibiofilm action of the hygiene protocols. All statistical tests were performed using the IBM SPSS Statistics for Windows software, v. 25.0 (IBM Corp., Armonk, USA), considering α = 0.05.
Results
Biofilm formation
The biofilm formation of C. albicans was higher in the presence of OFA compared to the CG and CCA, which demonstrated similar results. There were no significant differences between the adhesives for C. glabrata and S. mutans; however, both presented higher values compared to the CG. Staphylococcus aureus was not influenced by the presence or type of the adhesive (Table 2).
Adhesive strength
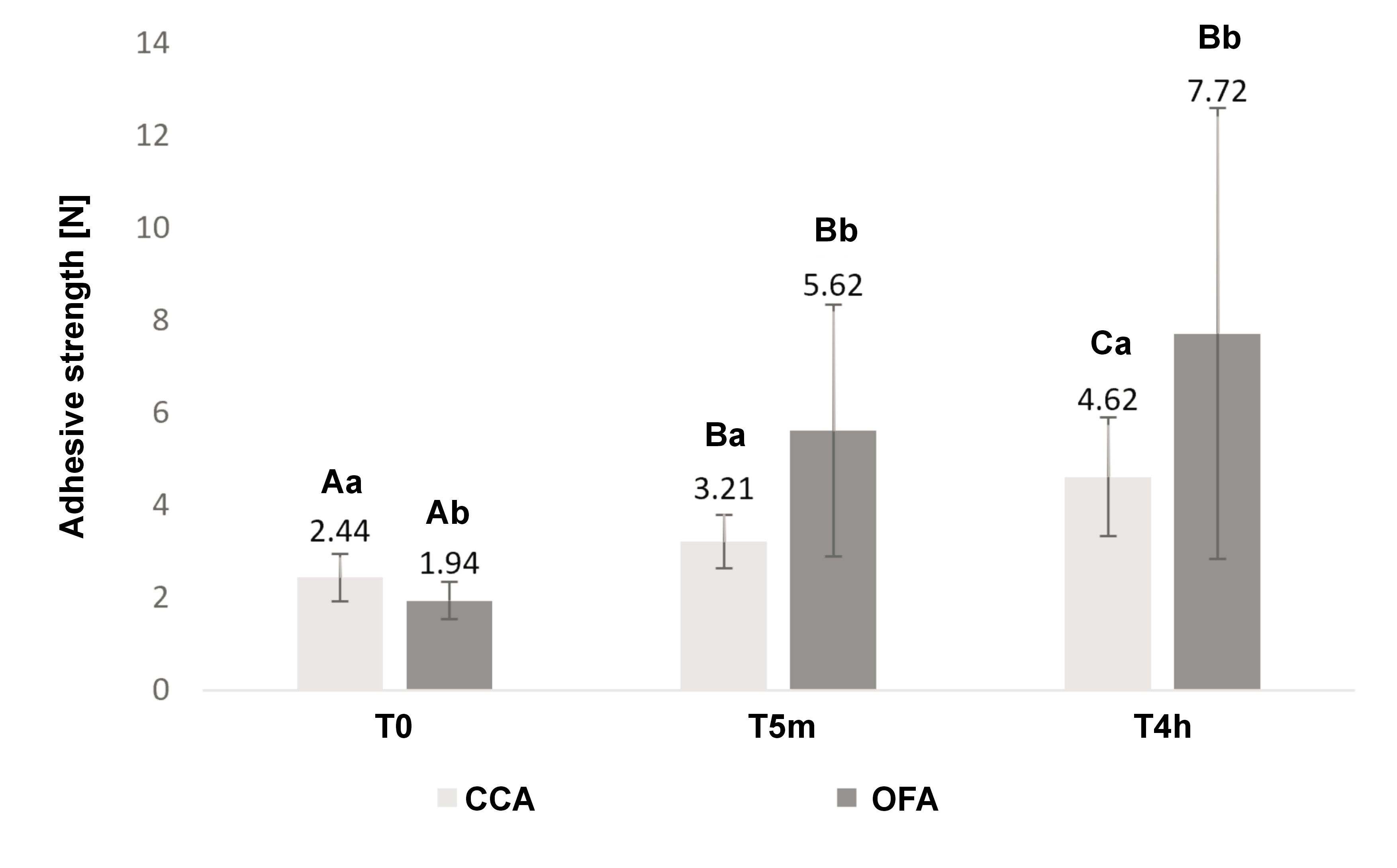
The adhesive strength exhibited a significant interaction with time (p = 0.007). At the initial time point (T0), the bond strength was higher for CCA. However, at T5m and T4h, OFA values were elevated. For CCA, the adhesive strength increased over time. For OFA, the adhesive strength increased between T0 and T5m, and reached comparable levels at T5m and T4h (Figure 1).
Effect of hygiene protocols on mixed biofilms
The implementation of hygiene protocols resulted in a reduction of the microbial load for all microorganisms compared to the CGwH, irrespective of the adhesive used. The BSH0.25% protocol demonstrated the greatest efficacy, causing the inhibition of C. albicans (p < 0.001), C. glabrata (p = 0.002) and S. mutans (p = 0.012), and significantly reducing S. aureus (p = 0.022) when associated with OFA. For C. albicans and C. glabrata, the BT0.15% protocol was more efficient with OFA (Table 3,Table 4). For S. aureus, all protocols were statistically different from each other, and the most significant reduction was promoted by BSH0.25%, followed by BT0.15% and BW. Triclosan caused a decrease in S. aureus CFUs with OFA (Table 5). For S. mutans, BT0.15% was more effective than BW for both cream adhesives and resulted in the inhibition of S. mutans with OFA (p = 0.012) (Table 6).
Cell metabolism
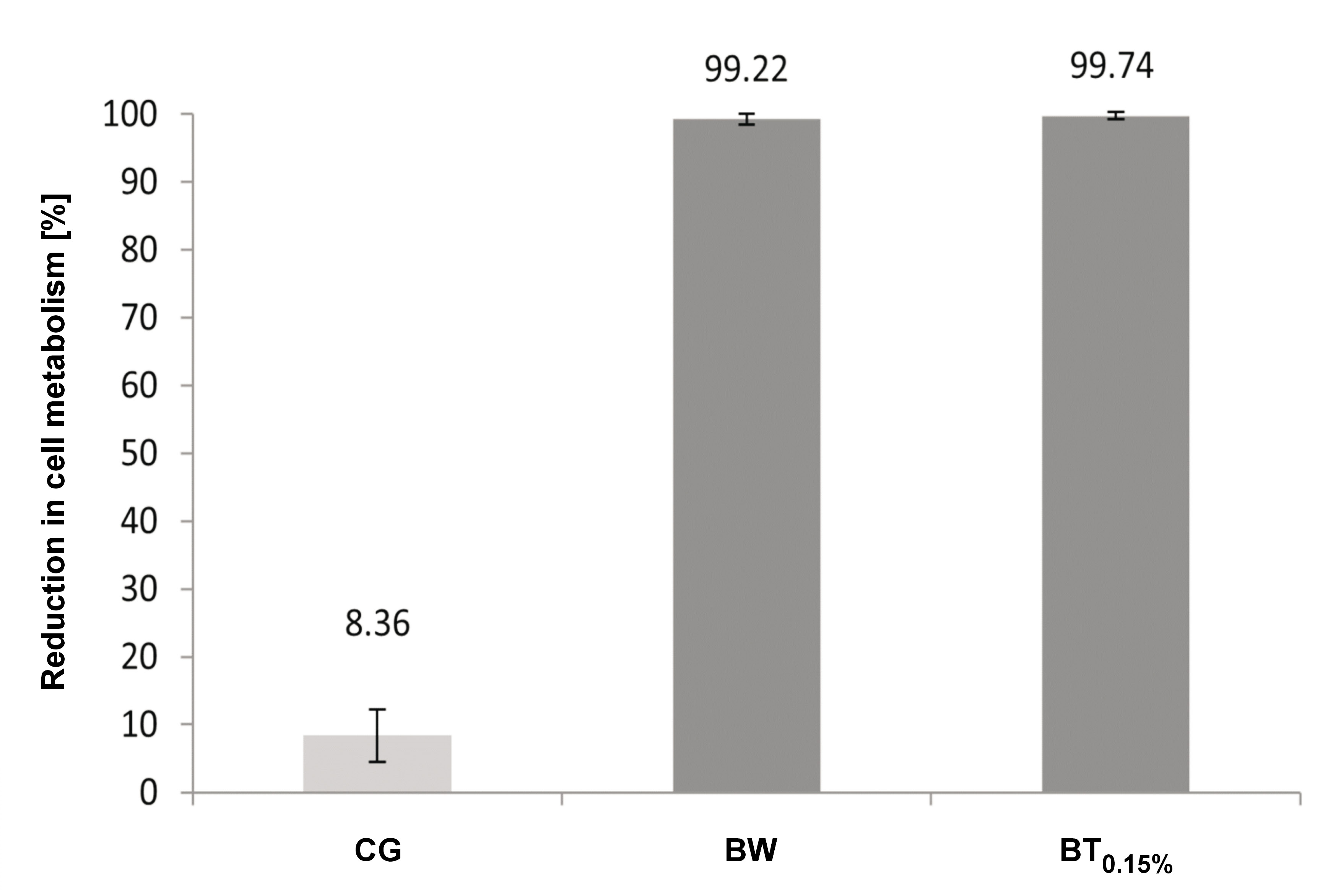
The impact of hygiene protocols on cell metabolism was found to be statistically significant (p = 0.000) (Figure 2). The study revealed no difference between the adhesives (p = 0.124) and no interaction between the hygiene protocols and adhesives (p = 0.260). The microorganisms exhibited no evidence of cell metabolism with BSH0.25%. The use of triclosan and BW yielded analogous outcomes, leading to a more pronounced reduction in metabolism when compared to the CGwH.
Discussion
The null hypothesis was rejected due to the observed difference between the cream adhesives in terms of biofilm formation and adhesive strength, as well as between the hygiene protocols. The results of this study confirm the tendency for greater biofilm accumulation when the adhesive is associated with the prosthesis. However, the findings also reveal that biofilms can be controlled through brushing and the use of sodium hypochlorite. Consequently, the patient’s quality of life can be ensured through the retention facilitated by the adhesive, while concurrently preserving the health of the tissues by the effective regulation of biofilm promoted by hygiene methods.
Candida albicans and C. glabrata are frequently isolated in individuals with denture stomatitis, especially in immunocompromised individuals.14, 15, 22 Furthermore, C. albicans develops a dense, multilayered biofilm with intricate hyphae to support the adhesion of C. glabrata.16 In the present study, the C. albicans count was higher with OFA when compared to the CCA and CG. At the same time, there was no difference in the biofilm formation of C. glabrata between the 2 adhesives. This result may be related to adhesion and cell surface hydrophobicity (CSH), which can suffer environmental variations.13 In a study with a limited number of C. glabrata isolates, the CSH was comparable to that of C. albicans. However, when many C. glabrata isolates were analyzed in comparison to C. albicans, the CSH of C. glabrata exhibited enhanced resistance to the same conditions,14 suggesting that C. glabrata may not be as sensitive or susceptible to environmental factors.
The mean CFU count of C. albicans associated with CCA was analogous to the CG, suggesting that this adhesive did not promote the proliferation of this microorganism. However, it did not hinder its growth, which is consistent with the findings of several studies.8, 9, 10 Another study reported that CCA caused 42% inhibition in the growth of C. albicans using a 1% solution of adhesive in a liquid culture medium, which was more diluted than in our study.7
A higher C. albicans count was observed for the OFA adhesive. This may be related to a highly viscous film formation on the specimens, which possibly affected the adhesion capacity of yeasts. These findings contradict those reported by Azevedo et al.50 The authors conducted a crossover clinical study with 23 patients using 3 groups of cream adhesives: a control (Kukident Pro); an experimental type (OFA); and a placebo (Vaseline). The experimental adhesive demonstrated superior C. albicans growth inhibition and prolonged effectiveness in comparison to the control and placebo groups (p < 0.001).50
Staphylococcus aureus forms a strong biofilm on denture surfaces.17 The effective control measures are highly necessary due to antibiotic resistance.51 The results demonstrated that the microbial load of S. aureus remained consistent across different adhesives, corroborating the observations reported by Costa et al.10 and Ozkan et al.15 The latter study, a clinical investigation, confirmed that there was no difference in the CFU count of S. aureus isolated from biofilms of complete dentures, both with and without adhesive.15
Streptococcus mutans is a precursor of biofilm formation, which can alter the local environment by forming an extracellular polysaccharide matrix-rich and low pH milieu, thereby creating a favorable niche for other acidogenic and aciduric species to colonize hard surfaces, such as dentures.52 This is clinically significant because denture wearers are typically elderly patients who are more likely to develop systemic infections.21 In this study, the CFU count of S. mutans was higher for adhesives than for the CG. These results highlight the need for meticulous removal of adhesives. However, Chen et al. evaluated the growth of S. mutans following the use of 3 denture adhesives (Polident cream, Protefix® cream and Protefix® powder) and did not observe any differences between the adhesives when compared to the control group.19 Additionally, 3 commercial adhesives (CCA, Fixodent Pro Original and Biotene Denture Grip) showed antimicrobial effects against S. mutans.20 The observed discrepancy between the results of the present study and those of other studies may be due to methodological differences.
For CCA, the maximum adhesive strength was reached after 4 h, which is consistent with the findings of the study by Costa et al.10 With regard to OFA, the comparison of results is limited due to the paucity of literature on the subject. However, the manufacturer stipulates an adhesive retention period of up to 24 h. The results of this study could be attributable to variations in composition. Briefly, carboxymethylcellulose (CMC) and poly(methyl vinyl ether-co-maleic acid) (PVM-MA) are classified as short-acting and long-acting salts, respectively.53 The CMC compound exhibits strong initial retention, but due to its high level of solubility, its effectiveness is rapidly diminished.53 The CCA adhesive contains both PVM-MA and CMC, while the OFA adhesive contains PVM-MA.
A number of studies have evaluated different hygiene protocols and found positive results regarding adhesive removal.23, 24, 25 However, these studies did not observe favorable outcomes in terms of the antimicrobial effect.23, 24, 25 Thus, the findings of our study are promising, as the BT0.15% and BSH0.25% protocols promoted a reduction in the microbial load when compared to the CGwH.
Triclosan is a synthetic, lipid-soluble antimicrobial agent of the broad spectrum that has the capacity to inhibit enzymes responsible for fatty acid biosynthesis.34 The agent induces K+ extravasation, leading to cell lysis through its effects on RNA and protein synthesis.36 It can be used as an alternative to hypochlorite for allergic patients and is recommended for wearers of partial dentures.32 In the present study, BT0.15% was more effective when used with OFA. The effect of BW was analogous to that of BT0.15% against C. albicans and C. glabrata when used in conjunction with CCA. This phenomenon may be attributed to the mechanical brushing procedure, which can disorganize the biofilm32, 33 and remove the adhesive component.
Sodium hypochlorite, an oxidizing agent, interferes with the integrity of the cytoplasmic membrane due to its high pH.33 This property renders it effective in sanitizing complete dentures.26, 27, 28, 29, 30, 31, 32, 33, 34 Although one of the disadvantages of sodium hypochlorite is its unpleasant odor, it was well accepted by patients at a concentration of 0.25% and can serve as a positive control in the evaluation of other solutions.29, 30, 31, 32, 33 The results of this study demonstrated a reduction in mitochondrial activity of metabolically active cells, which aligns with the findings on microbial load. Sodium hypochlorite completely inhibited cell metabolism,33 while triclosan or water caused a significant decrease in metabolic activity (99.74% and 99.22%, respectively). However, a direct comparison with the extant literature is precluded by the dearth of studies in the field.38 A notable finding in the CGwH sample is an 8.36% reduction in metabolism, indicating that the adhesives provided a slight imbalance in the metabolic activity of microorganisms without compromising their viability.
Limitations
The present study was subject to certain limitations. First, an adhesive removal test was not conducted, which would have complemented the obtained results. Second, alternative techniques for assessing biofilm quantity, such as fluorescence microscopy, were not employed. This underscores the necessity for further research on the subject. However, the obtained results can inform clinical decision-making regarding the selection of the most suitable adhesive, based on the adhesive strength and hygiene method to be employed with each material.
Conclusions
The formation of biofilms was favored for both cream adhesives; however, the OFA adhesive demonstrated greater bond strength and stability with the mucosa. Brushing and immersion in 0.25% sodium hypochlorite resulted in a more significant reduction in the microbial load and cell metabolism when compared to the use of 0.15% triclosan.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.