Abstract
Background. In the field of pediatric dentistry, preventing microleakage of glass ionomer cement (GIC) is important for clinical success. The abrasion and roughness of the surface of the restorative material that results from brushing can cause microleakage. The application of surface protection is intended to prevent this situation.
Objectives. The aim of the study was to evaluate the levels of microleakage following toothbrushing after the application of GICs with or without surface protection.
Material and methods. Cavities formed on the buccal surfaces of 180 extracted primary teeth were restored with resin-modified glass ionomer cement (RMGIC), and the teeth were divided into 3 groups according to the surface protection application, with an equal number of samples in each group (n = 60). The thermal cycle was applied to all samples. Subsequently, the groups were divided into 5 subgroups (n = 12/group) according to the brushing simulation (no brushing, and 1, 3, 6, and 12 months of brushing). The samples were stored in 2% methylene blue for 24 h and sectioned in the buccolingual direction. The presence of microleakage was determined with the use of a stereomicroscope. The data was statistically analyzed.
Results. No statistically significant differences were observed between the main groups at all brushing times (p > 0.05). However, higher microleakage results were obtained in the group without surface protection. When the groups were evaluated according to the duration of brushing, no statistically significant differences were identified (p > 0.05), but higher microleakage results were obtained in the samples that underwent brushing for 12 months.
Conclusions. Although statistically significant results were not obtained in terms of microleakage regarding surface protection application and brushing, it should be noted that coating restorations with surface protectants may contribute to a smoother surface and marginal integrity, and may be beneficial in reducing microleakage.
Keywords: dental leakage, toothbrushing, dental cements, deciduous tooth
Introduction
Dental caries is a multifactorial, preventable and common childhood disease.1, 2 The condition can cause pain, difficulty in eating, malnutrition, aesthetic problems, decreased self-confidence, and, therefore, a decrease in quality of life.2, 3, 4 In order to prevent the occurrence or progression of dental caries, the treatment of decayed teeth should be performed promptly.5 Amalgams, glass ionomer cements (GICs), compomers, and composite resins are used as restorative materials in the treatment of primary teeth.6 Glass ionomer cements are used in the treatment of primary teeth and are considered an alternative restorative material that is frequently used in pediatric dentistry.7 Glass ionomer cements were first introduced by Wilson and Kent in 1972.6 These materials are formed by the curing reaction between powdered aluminosilicate glasses and an aqueous solution of polyacrylic acid.8 Glass ionomer cements allow for conservative preparation. They can chemically bind to dental tissues, release fluoride, and be placed in a single step.7 Conversely, studies have highlighted several drawbacks, such as low wear resistance, short working and long curing time, high initial moisture sensitivity, and the occurrence of microleakage.8 To address the limitations of conventional glass ionomer cements (CGICs), resin-modified glass ionomer cements (RMGICs) have been developed. It has been documented that RMGICs have better adaptation, adhesion and aesthetic properties than CGICs.9 Although RMGICs demonstrate resistance to early contact with water, it is not clear how sensitive these materials are to hydration or dehydration immediately after light activation.10 Upon exposure to moisture, the mechanical resistance of GICs decreases, and the surface experiences accelerated wear.11 The use of Vaseline®, cocoa butter, varnishes, and various surface-covering agents is recommended to prevent early contact of GICs with water. Among these, light-curing resin-containing sealants are particularly noteworthy.11
One of the most important factors affecting the success of restorative materials is microleakage. Microleakage is defined as the passage of bacteria, molecules, liquids, or ions between the cavity wall of the tooth and the filling material applied to it.12 Microleakage negatively affects the success of the restorative material by causing problems such as secondary caries, sensitivity, diseases affecting the pulp, and marginal discoloration in the restoration.13 Microleakage may occur due to thermal changes, loss of contour as a result of wear in the restorative material, mechanical stress, or a lack of adaptation of the restorative material, which can result in a gap at the tooth–material junction.14 Restorative materials are exposed to chewing forces, dietary habits and brushing forces in the oral cavity. These factors can lead to wear of restorative materials over time and loss of anatomical form.15 Toothbrushing has been shown to cause adverse conditions, including wear that leads to roughness and microleakage on the surface. This is due to the abrasive content of toothpastes and the mechanical effect of the brush.16, 17 It has been reported that the application of surface protection is effective in preventing microleakage by improving the mechanical and physical properties of materials.11, 18 During maturation, surface protectants isolate the GIC from saliva contamination, increase the durability of the restoration, occlude the surface cracks, and protect the restoration against abrasion.19 However, the effect of applying surface protection to prevent microleakage as a result of the abrasive effect of toothbrushing needs to be investigated. Therefore, the aim of the study was to examine the levels of microleakage following toothbrushing after the application of GICs with or without surface protection, which are frequently used in the restorative treatment of primary teeth.
Material and methods
This study was conducted at the Department of Pedodontics of Zonguldak Bülent Ecevit University, Turkey. It was approved by the Clinical Research Ethics Committee of Zonguldak Bülent Ecevit University (protocol No. 2021-09; May 5, 2021).
A total of 180 lower and upper primary second molars, which were indicated for extraction due to infection, periodontal tissue loss or orthodontic purposes were included in the study. Teeth that were damaged during extraction, had caries on their crowns, or fractures/cracks in the dental crown before extraction were excluded from the study.
The number of samples to be used in our research was determined to have 95% test power (1−β), 95% confidence (1−α), an effect size (f) of 0.677, and at least 10 samples in each of the subgroups. The statistical power was calculated using the G*Power software (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower). The study was performed using a total of 180 samples, with 60 samples allocated to each of the main groups, and 12 samples included in each of the subgroups.
Soft tissue remnants and debris were removed from all studied teeth with a scaler. The extracted teeth were stored in distilled water until the beginning of the experimental phase. The roots of the teeth were cut out with a diamond separator (Komet USA, Rock Hill, USA), under water cooling, and apical to the cementoenamel junction. The crowns of the teeth were cut into two on the mesiodistal axis, parallel to their long axis. In all groups, only the buccal surfaces of the teeth were examined. Class V cavities were created on the buccal surfaces of the deciduous teeth, with the dimensions of 3 mm in mesiodistal width, 2 mm in occlusogingival height, and 1.5 mm in depth. All cavosurface angles were precisely measured to be 90 degrees under water cooling. The prepared cavities were restored with RMGIC (Fuji II LC; GC Corp., Tokyo, Japan).
Glass ionomer cement in capsule form, which does not require an adhesive, was prepared by mixing for 10 s in a mixer, and then applied to the prepared cavities using a capsule applier. After shaping the initial contour, the glass ionomer was polymerized by applying 470-nm wavelength light for 20 s (ELIPAR S10; 3M, Maplewood, USA). The teeth were not polished after glass ionomer polymerization. A total of 180 teeth were randomly divided into 3 equal groups.
The surfaces of the teeth in 2 of the 3 groups were treated with 2 different protective agents, while 1 group was left untreated. The present study employed 2 agents, namely a nanofilled light-curing surface protectant (Equia Forte Coat; GC Corp.) and a light-cured adhesive material (Heliobond; Ivoclar Vivadent, Schaan, Liechtenstein) for the primary teeth.
Following the formation of the groups, all teeth were stored in distilled water at 37°C for 24 h. Then, the teeth were subjected to 500 thermal cycles between 5°C and 55°C, with a 10-s transfer and 30-s holding period. After thermal cycling, each main group was randomly divided into 5 equal subgroups (n = 12/group) based on the time spent in the brushing simulator. It was determined that 1 year of toothbrushing was equivalent to 10,000 cycles, 6 months equaled 5,000 cycles, 3 months equaled 2,500 cycles, and 1 month equaled 840 cycles. The brushing simulator was applied to both groups treated with protective agents, with each subgroup undergoing equivalent brushing cycles for 1 month, 3 months, 6 months, and 1 year. The control group was not subjected to brushing. For the brushing simulation, each tooth sample was placed in the center of an acrylic block, which was prepared to fit the sample cups in the brushing simulator (DentArGe TB-6.1 Brushing Simulator; Analitik Medikal, Gaziantep, Turkey). A single tooth was embedded within each block, with the buccal surface of the teeth exposed and fixed horizontally. The brushing simulation was conducted using a children’s toothpaste (Colgate-Palmolive, New York, USA) mixed with distilled water (1:1) and a children’s toothbrush with medium hard bristles (Denta, Istanbul, Turkey). The brushes were replaced after 2,500 cycles. Brushing was performed for each sample under the following conditions: a vertical force of 200 g (2 N); a cycle speed of 60 mm/s; a stroke length of 20 mm; and standardized back-and-forth movement. Following the brushing simulation, the samples were removed from the sample cups, and each specimen was washed with running tap water for 20 s before being preserved in distilled water.
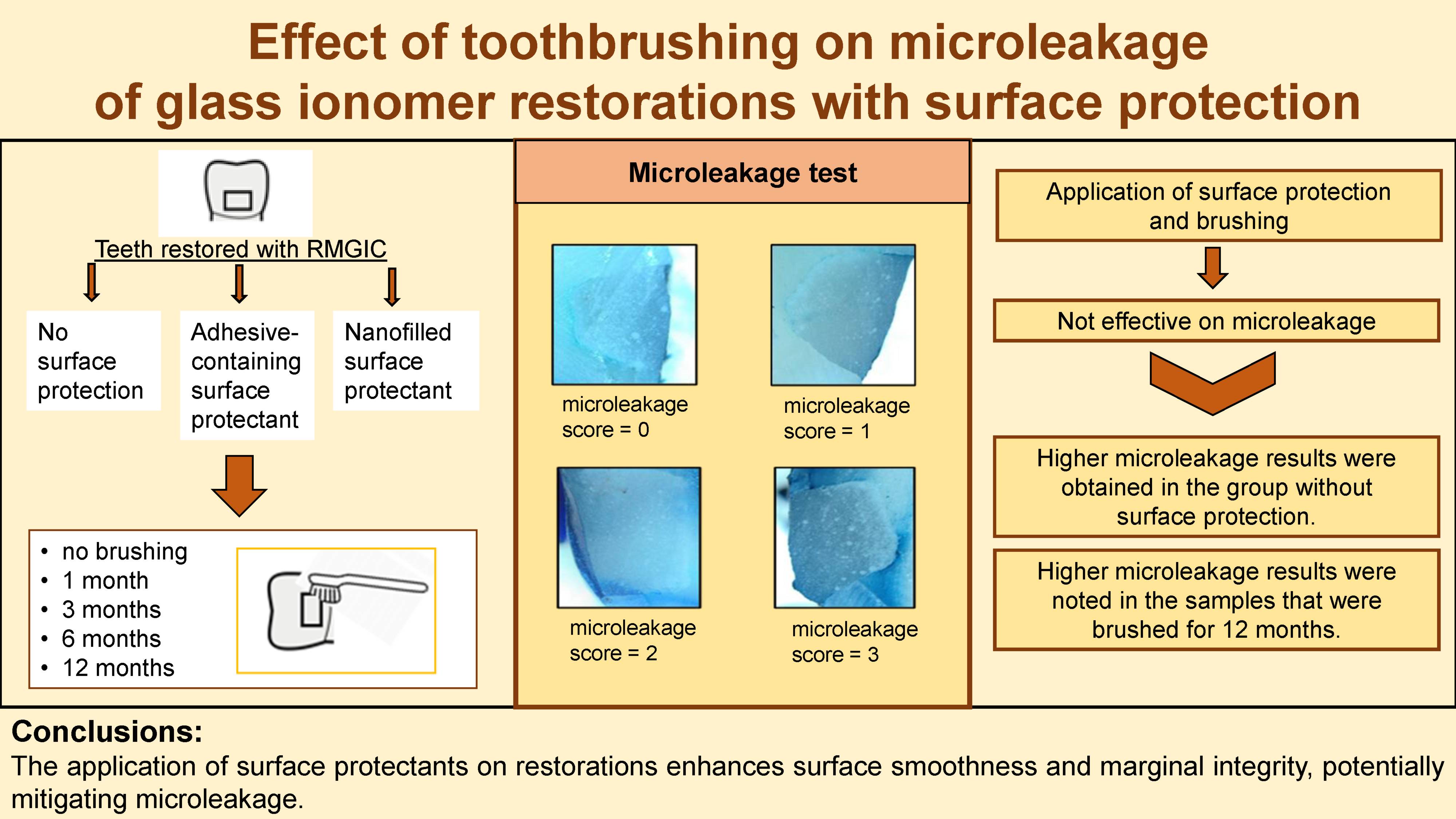
All teeth were then tested for microleakage. Two coats of nail polish were applied to all teeth surfaces, with the enamel surface of the restorations exposed by up to 1 mm. The teeth were stored in containers with 2% methylene blue solution at 37°C for 24 h. Following this, the teeth were removed from the solution, washed under running tap water for 5 min, and dried. Then, the samples were bisected in a buccolingual direction under water cooling. A 0.2-mm thickness diamond separator was used to examine potential microleakage. The occurrence of dye leakage in the obtained sections was examined with a stereomicroscope (Olympus SZ61; Olympus Corporation, Tokyo, Japan) at ×20 magnification. The assessment of dye leaks in the cavities was conducted using a qualitative scoring method, as outlined by Sidhu20:
– 0: no dye penetration;
– 1: dye penetration in less than ½ of the cavity wall;
– 2: dye penetration in more than ½ of the cavity wall;
– 3: dye penetration seen throughout the cavity wall.
The collected data was recorded digitally, and the highest score noted for each sample was evaluated.
Statistical analysis
The data analysis was performed using the IBM SPSS Statistics for Windows software, v. 23.0 (IBM Corp., Armonk, USA). The evaluation of conformity to the normal distribution was performed using the Shapiro–Wilk test. The Kruskal–Wallis test was employed to compare the microleakage values that were not suitable for normal distribution according to the groups differing by surface protection application and brushing times. The results of the quantitative data analysis were expressed as median (Me) (minimum–maximum) and mean ±standard deviation (M ±SD). The significance level was set at p < 0.05.
Results
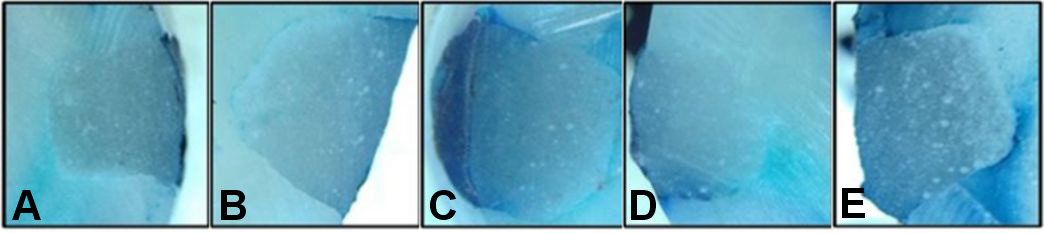
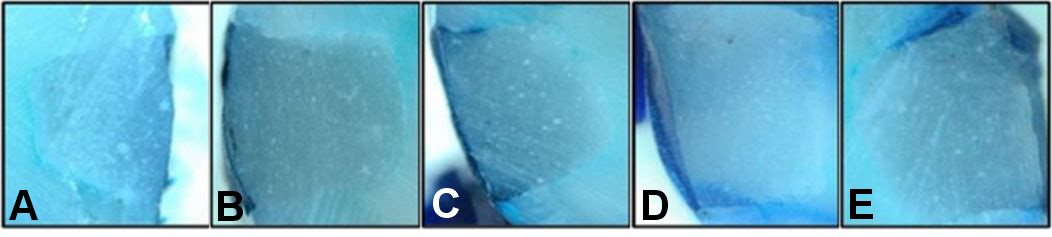
The microleakage scores of the samples with and without surface protectants according to the brushing time are shown in Table 1. The median microleakage score was 0 in unbrushed samples for all 3 main groups. Higher microleakage scores were observed in teeth without surface protectants, although the difference between the groups was not significant (p > 0.05). Furthermore, higher microleakage levels were observed after 1, 3, 6, and 12 months of brushing in the control group. However, the difference between the time points was not statistically significant (p > 0.05).
The microleakage scores of the samples at varying brushing times in relation to the application of surface protectants are shown in Table 2. Higher microleakage scores were observed after 12 months of brushing for all 3 groups. However, the difference between the median values of microleakage scores according to the brushing time was not significant (p > 0.05) (Figure 1, Figure 2, Figure 3).
Discussion
In pediatric dentistry, composite resins, compomers, amalgams, CGICs, high-viscosity GICs (HVGICs), and RMGICs are the preferred materials in the treatment of dental caries.21 Currently, due to the spread of minimally invasive dentistry, the preference for tooth-colored restorative materials and their ability to bond with dental tissues, GICs have become prominent in the field of pediatric dentistry.22, 23 In order to improve the properties of CGICs, various modifications have been made and RMGICs have been developed.24 Resin-modified glass ionomer cements contain the same main components as CGICs (basic glass powder, water and polyacid), 2-hydroxyethyl methacrylate (HEMA) as a monomer component, and camphorquinone as an initiator.25
Glass ionomer cements are sensitive to the presence of early moisture, which can lead to water absorption and hygroscopic expansion. To prevent this, it is important to protect the cement by covering it with a suitable varnish or petroleum jelly (Vaseline®). Recently, nanofilled, self-adhesive and light-cured surface protecting agents have been developed for the use with CGICs, RMGICs, composite resins, and compomer restorations. These agents have been designed to enhance the mechanical properties of the restorations, increase their wear resistance and improve their appearance. Surface protectants contribute to the clinical success of restorations by filling small surface voids and cracks and reducing discoloration.26, 27, 28
The tooth surface and dental materials encounter the abrasive effects of brushing with paste.29 The increase in surface roughness is important for the clinical life, microhardness and abrasion resistance of restorative materials, secondary caries risk, coloration, and aesthetics.30, 31 Studies have revealed that wear, roughness, color change, and microleakage occur after the application of surface protectants to GICs or other restorative materials. Additionally, studies have examined the effect of brushing on the roughness and wear resistance of restorative materials. However, there is a paucity of research examining microleakage in GICs with surface protectants after brushing. Therefore, our study evaluated the effect of toothbrushing on microleakage of RMGICs with adhesive surface protectants and nanofilleld surface protectants.
Microleakage can be observed in RMGICs. Therefore, it is recommended that a surface protectant be applied to teeth after polymerization to prevent this situation.32 Surface protective agents, which contribute to ensuring marginal sealing and improving surface properties in restorations, are fluid materials that can penetrate gaps and restore resin-containing materials or GICs.33, 34 Oba and Aras compared polyacid-modified composite resins (PMCRs) applied in the restoration of class V cavities in extracted primary teeth and RMGICs that were covered with nanofilled surface protectants, similar to our study. The study demonstrated a reduced incidence of microleakage in RMGIC samples, suggesting that the restorations are protected with a surface-protective agent during the cement curing process, thus preventing moisture contamination and microcracking. Similarly, Agnihotri et al. reported that the application of surface protection was effective in reducing microleakage in the RMGIC group.36 The present study found no statistically significant differences in terms of microleakage in the samples that were not brushed, between the surface-protected and unprotected groups. However, higher microleakage was noted in teeth without surface protection compared with surface-protected teeth. In agreement with the findings of previously published studies, our results indicate that the coating of the material surfaces with protective agents has a positive, even if not statistically significant, effect on microleakage. This finding could be related to the prevention of early moisture contamination and the filling of microvoids in RMGICs when surface protection was applied.
In RMGICs, it is preferred to coat the surfaces with filler-containing agents, varnishes or adhesive-containing surface protectants in order to prevent water absorption of HEMA, improve the quality of the material, and reduce dimensional changes.37 Chuang et al. examined the microleakage of RMGIC and reported that the adhesive-containing surface protectant is the most effective in preventing microleakage.38 A study by Ribeiro et al. using different RMGIC materials found no statistical differences in dye uptake between RMGICs.39 However, surface protectant application was required in all samples, and the best results were obtained with the adhesive-containing protectant.39 Erhardt et al. reported that adhesive protectants were not effective in reducing microleakage and had a high probability of abrasion from heat exposure or intraoral abrasive forces.40 Urquía-Morales et al. tested the effect of different surface protectants on the efficacy of composite resins in mitigating microleakage.41 The study found that the utilization of surface protectants significantly reduced microleakage in all experimental groups compared to the control group.41 In contrast to the aforementioned studies, Pacifici et al. evaluated HVGIC and RMGIC with a nanofilled surface protectant, an adhesive-containing surface protectant, and an unapplied surface protectant by scanning electron microscopy.42 The authors found that regardless of the type of surface protection, it was successful for marginal sealing due to its high hydrophilicity and low viscosity.42 In the present study, it was observed that the microleakage scores of samples that were not brushed and specimens to which surface protectants were applied yielded similar results. Furthermore, no statistical difference was detected between the groups. However, there was a discrepancy between the adhesive content and the nanofilled surface protectant with respect to the microleakage score, despite the fact that both materials yielded successful results. The present study revealed no significant difference between 2 surface protectants. The lower microleakage values of both materials were compared to the group that did not receive a surface protectant. However, the lower microleakage scores are likely attributable to the effective coverage of the surface protectants, which exhibited good fluidity and penetration on the surface of the restored teeth.
Abrasion has been reported as a undesirable condition that increases surface roughness and causes the restorative material to separate from the surface.43 The separation of material from the surface may lead to the formation of new undesirable margins that can cause bacterial retention and subsequent microleakage.44 Momoi et al. demonstrated that the wear rate increased significantly after brushing in CGIC, amalgam and composite resin materials.43 When evaluating various effects of toothbrushing on microleakage, Goldstein et al. reported no statistically significant difference between the brushing group and the control group of class V composite resin restorations after using a sonic toothbrush.45 Similar to our results, this study has shown that brushing does not have a significant effect on microleakage.45 The prevention of early moisture contamination of materials allows for better abrasion resistance and marginal integrity, which, in turn, leads to improved sealing restorations. The application of surface protection is recommended to prevent microleakage.46, 47 Kanık compared a nanofilled surface protectant and varnish application on 2 different HVGICs with non-preserved composite resin for abrasion resistance as a result of brushing.48 It was reported that with increasing brushing cycles, the teeth applied with varnish showed significantly more wear than the teeth applied with the nanofilled surface protectant. In the context of our study, which examined brushing simulations at different time points, no statistically significant difference in terms of microleakage was observed between the non-preserved group and the protected groups with respect to brushing times. Our observations revealed that neither brushing nor the duration of brushing exerted any influence on microleakage in all samples. A comparison of our results with other studies was precluded by the absence of research evaluating the effect of brushing on microleakage in RMGICs treated with surface protection. Although our study did not identify statistically significant differences, higher microleakage levels were observed in the group that did not utilize surface protection. Consequently, the utilization of surface protectants may enhance the wear resistance of RMGICs.
Toothbrush wear and the resulting surface roughness cause changes to the surface properties of different materials. Studies have reported that surface protectants undergo a gradual deterioration due to the effects of abrasive factors over time.48, 49 Kanık and Türkün examined the surface protective activity after brushing simulation and observed that the protective agents exhibited signs of wear.50 In their evaluation of the effectiveness of surface protectants, Lohbauer et al. reported that nanofilled surface protectants underwent partial or complete erosion from the restoration surface at 6 months due to brush abrasion and occlusal contact.49 While our study did not yield significant results, higher microleakage results were observed in samples that underwent brushing for 12 months when compared to the 1-, 3- and 6-month brushing periods. The observed increase in microleakage results at 12 months of brushing is likely due to the roughness and abrasion caused by the abrasive forces of toothbrushing over time, the effect on the resin matrix, and deterioration of the surface of the restorative material.
The study was conducted in vitro, under the influence of brushing only, while other conditions in the oral environment were ignored. Therefore, further clinical studies should be conducted on the topic.
Conclusions
After analyzing the collected data, it is predicted that the application of surface protectants on RMGIC restorations will reduce microleakage through the filling of microvoids and the enhancement of the wear resistance of the restorative material. The investigation revealed no statistically significant differences in microleakage outcomes between nanofilled and adhesive surface protectants, indicating that both materials are suitable for clinical use. While the impact of brushing on microleakage is not significant, it is crucial to note that the extent of wear and leakage in RMGIC materials can increase with the increased duration of brushing time.
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Zonguldak Bülent Ecevit University (protocol No. 2021-09; May 5, 2021).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.