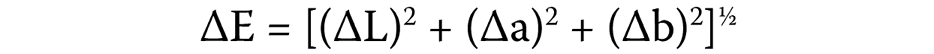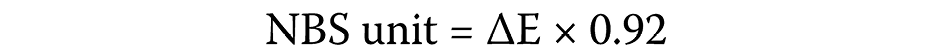Abstract
Background. Denture base materials can be highly sensitive to the effects of daily beverage consumption, manifesting in alterations to their surface texture or color.
Objectives. The study aimed to evaluate the effect of different beverages (Pepsi, coffee and tea) on the surface roughness (Ra) and color stability of 3 types of denture base materials.
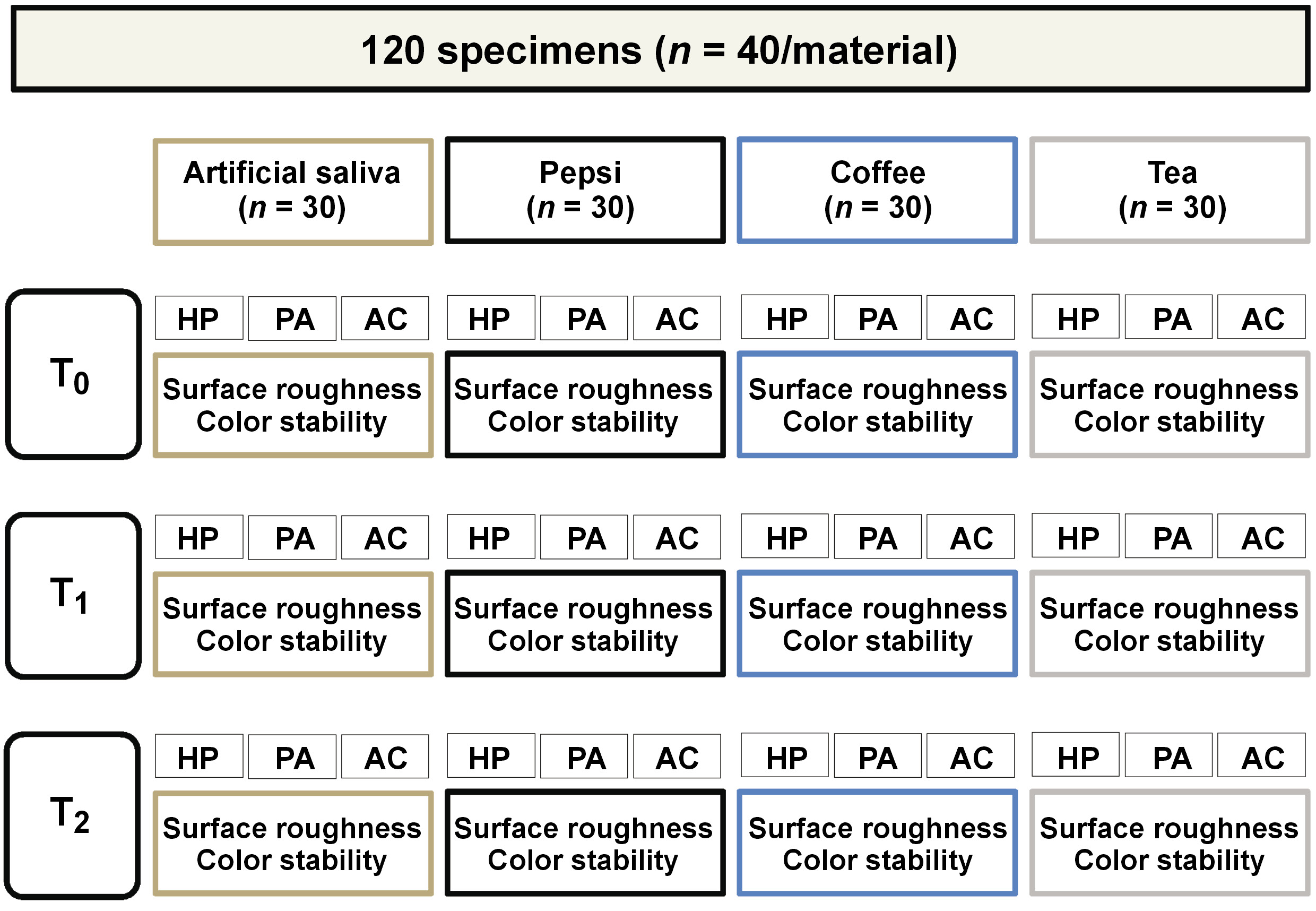
Material and methods. A total of 120 specimens (n = 10/group) were fabricated from 3 different denture base materials, namely heat-polymerized polymethyl methacrylate (HP), thermoformed polyamide (PA) and acetal (AC). The surface roughness and color stability of the specimens were evaluated 3 times: before immersion in beverages; after 30 days of immersion; and after 90 days of immersion in artificial saliva (a control group) and Pepsi, coffee and tea (test groups). The data analysis was performed using two-way analysis of variance (ANOVA) to compare the results of Ra and color change (ΔE) between denture base resins and beverages.
Results. The differences between the materials, beverages and time were significant for Ra values, as well as the interaction between materials and beverages, and between beverages and time. The findings indicated significant differences in ΔE between denture base materials. In comparison to PA and AC, HP exhibited lower ΔE values. A significant change in color was observed over time for all of the tested materials.
Conclusions. The tested beverages increased Ra and caused change in the color stability for all materials. The observed color change was correlated with the duration of the immersion, and was more evident in thermoformed resins.
Keywords: polymethyl methacrylate, denture base, thermoformed resin, surface properties, chromogen solution
Introduction
Removable dental prostheses remain an essential prosthetic treatment in many conditions of oral rehabilitation.1 Although polymethyl methacrylate (PMMA) resin is the material of choice used in the fabrication of denture bases, it possesses certain drawbacks, such as the presence of residual monomer, surface porosity, difficult processing, as well as weak flexural and tensile strength.2, 3, 4
Thermoformed denture base materials are used in the fabrication of dental prostheses, particularly removable partial or complete dentures, preformed clasps, flexible partial denture frameworks, temporary crowns and bridges, orthodontic appliances, antisnoring devices, different types of mouth guards, and splints.4, 5 These materials offer numerous advantages, including light weight, flexibility, good stability, and retention. The most significant advantage of thermoformed resins is the minimal content or absence of free monomers or metal alloys in the material, which reduces the risk of allergic reactions.4, 6 In addition, thermoformed materials possess non-metal clasps and exhibit a natural aesthetic color, which blends with the color of gingival tissues.7 This enables clinicians to use undercut areas for enhanced retention, a property that is unattainable with conventional denture base resins.6
The acetal resin has several advantages, including flexibility and resistance to occlusal wear and fracture. Therefore, it is considered an optimal material for the fabrication of frameworks and clasps for removable partial dentures,8 provisional bridges, artificial teeth for removable dentures, and orthodontic appliances. The main disadvantage of acetal is its lack of translucency, which hinders its ability to match the aesthetic appearance of thermoplastic acrylic and polycarbonate.9
Polyamide (nylon) is a monomer-free substance that is considered suitable for patients allergic to methyl methacrylate. In addition, it is light in weight and impervious to oral fluids.10 However, its stiffness makes it ill-suited for the application in occlusal rests or denture elements that necessitate rigidity.11, 12
Denture discoloration may be attributed to intrinsic and/or extrinsic factors including material composition, wear and exposure to stains.13 Discoloration of denture base resin has been observed following the use of beverages, oral fluids and denture cleansers.14 Accordingly, denture base materials must possess adequate color stability to achieve optimal aesthetics and serviceability.14, 15
Several denture base materials are available on the market, and numerous beverages are consumed by different populations. Therefore, it is important to assess the effect of commonly consumed beverages on the surface roughness (Ra) and color stability of denture base materials.16 Although there have been several studies on Ra and color stability of acrylic resin denture bases, the comparison of these resins with polyamides and acetal after immersion in beverages remains limited.14 Therefore, the current study evaluated the effect of different beverages, specifically Pepsi, coffee and tea, on Ra and color stability of 3 types of denture base materials: heat-polymerized PMMA (HP); thermoformed polyamide (PA); and acetal (AC). The null hypothesis stated that Ra and color stability of the 3 denture base resins would not be affected by immersion in the tested beverages.
Material and methods
A total of 120 specimens made of 3 denture base resins (HP, PA, AC) were used in the study. The specimens were fabricated by pouring melted baseplate wax (Cavex Set Up; Cavex Holland BV, Haarlem, Netherlands) inside silicon molds measuring 20 mm × 20 mm × 3 mm.17 Four wax specimens were invested at a time in dental stone (Denston Turkish Dental Stone Type 3; DentaCarts, Cairo, Egypt) within a metal flask (61B Two Flask Compress; Handler Manufacturing, Westfield, USA) following the conventional method for HP. A special dental flask (SABILEX dental flask; Sabilex, Buenos Aires, Argentina) was designed for the injection molding technique in thermoformed resins. A sprue measuring 4 mm in diameter was attached to each specimen at 1 corner, and the 4 sprues were then integrated into a single sprue, which emerged through the flask orifice at one side. Wax was melted away following the investment in the dental stone. The stone surfaces with mold spaces were coated with a separating medium (Acrostone Separating Medium; Acrostone Dental & Medical Supplies, Cairo, Egypt) and then left to dry. Heat-polymerized PMMA (Acrostone, Cairo, Egypt) powder and liquid were mixed according to the manufacturer’s instructions. Once the dough stage was reached, HP was packed into the mold spaces following the conventional method. Subsequently, it was transferred to a thermostatically controlled water bath, where it underwent a short curing cycle at 70°C for 1.5 h. Thereafter, it was brought to boil at 100°C for 1 h. Thermoformed polyamide (Sabilex FlexiUltra, shade: pink 78; Sabilex) and AC (Bio Dentaplast; bredent GmbH & Co. KG, Senden, Germany) are available in the form of granules, which are contained in cartridges of different sizes. During the injection process, the cartridge was aligned with the opening of the flask within the electric furnace (BIOSTRONG 400; Sabilex). In accordance with the manufacturer’s guidelines, PA and AC were plasticized for 15 min at 280°C under a pressure of 7.5 bars. The specialized dental flask was bench-cooled for 15–20 min before opening.18
The specimens were then subjected to a finishing process that employed the same technique and was carried out by the same operator in order to standardize the pressure exerted. It was achieved by using a tungsten carbide bur (HM79GX-040-HP; Meisinger, Centennial, USA) at a low speed for 2 min, followed by polishing with the use of a new set of a polishing kit for each group material. The polishing procedure was performed using a pre-polishing brown rubber disc at 1,500 rpm for 1 min, followed by a fine pumice for 2 min, and finally using a Tripoli compound for 2 min. The specimens were stored in distilled water at room temperature for 48 h to reduce the residual monomer before testing.
The specimens of each material (n = 40/material) were immersed in artificial saliva (control group), Pepsi, coffee, or tea (test groups) (n = 10/group) (Figure 1). All the steps were accomplished by a single researcher (MMG) to ensure standardization. The composition of the beverage solutions and preparation procedures are delineated in Table 1. The specimens were stored in different containers holding 50 mL of the tested beverages for 15 min (average time for which a beverage is consumed during the day), followed by storage in distilled water until the next day. This procedure was repeated daily for 30 days (T1) and 90 days (T2).19 To prevent fungal growth, the solutions were refreshed and changed daily. Artificial saliva and Pepsi were used at room temperature,20 while coffee and tea were utilized at 50°C21 to mimic the temperature of actual use. The surface roughness values were measured at baseline (T0), T1 and T2 using a non-contact optical interferometric profilometer (Contour GT-K; Bruker Nano GmbH, Berlin, Germany) at a resolution of 0.01 mm. Each specimen was scanned at 5 sites, and the average Ra value of each specimen was calculated.
Color change (ΔE) was evaluated using a spectrophotometer (Color-Eye® 7000A; X-Rite, Grand Rapids, USA). The color variation was calculated according to the International Commission on Illumination (CIE) at T0, T1 and T2. The CIE L*a*b system measures color variation between 2 points, based on the following formula20 (Equation 1):
where:
ΔL – difference in lightness/darkness value;
Δa – difference in the red/green axis;
Δb – difference in the yellow/blue axis.
Before testing, the spectrophotometer was calibrated according to the manufacturer’s instructions. Each specimen was placed against the port, after which the support arm was locked. Four measurements were recorded for each specimen, and the mean ΔL, Δa and Δb values were calculated.20 The color change (ΔE) was quantified between T1 and T0, as well as between T2 and T0.20 Subsequently, the ΔE values were converted to the National Bureau of Standards (NBS) units (trace = 0.0–0.5, slight = 0.5–1.5, noticeable = 1.5–3.0, appreciable = 3.0–6.0, much = 6.0–12, and very much: >12) using the following formula (Equation 2):
Statistical analysis
The IBM SPSS Statistics for Windows software, v. 20.0 (IBM Corp., Armonk, USA), was used for data analysis. The numerical data, which was based on the measurements of Ra and color stability for the 3 denture base materials, was presented as mean (M) and standard deviation (SD). The Kolmogorov–Smirnov test revealed that the tested datasets conformed to the normal distribution. A two-way analysis of variance (ANOVA) was applied to compare the results for Ra and color stability for each material and beverage. Tukey’s post hoc test was applied for pairwise comparisons. The values were considered statistically significant at p ≤ 0.05.
Results
A two-way ANOVA for Ra, followed by Tukey’s post hoc test, revealed significant values for materials, beverages and time (all p < 0.001) (Table 2). The interaction between materials and beverages (p < 0.001) and the interaction between beverages and time were significant for Ra values (p = 0.007). However, the relationship between materials and time was not statistically significant (p = 0.340).
A comparison of Ra of denture base materials subjected to immersion in different beverages is outlined in Table 3. Significant differences were observed for HP between the time points in comparison to baseline (T0) after immersion in different beverages (p < 0.05), with the exception of the difference between T1 and T0 after immersion in artificial saliva (p = 0.573). The time factor exhibited non-significant differences between T1 and T2 for all tested liquids (p > 0.05). The highest Ra value was observed in coffee at T2 and T1, followed by Pepsi at T2 and tea immersions. Significant differences in Ra were noted for PA between the evaluated time points and baseline for all tested solutions (p < 0.05), with the exception of Pepsi at T1 and T2 (p = 0.401 and p = 0.064), and tea at T1 (p = 0.404). The impact of the time interval on Ra of PA did not show significant differences after immersion in the tested solutions. The highest Ra values at T2 were observed for coffee, followed by tea, saliva and Pepsi. Statistically significant differences in Ra were noted for all beverages when AC was examined (p < 0.05). However, between T1 and T2, a significant difference in Ra was noted only after immersion in saliva (p = 0.012). The highest Ra values were reported at T2 with saliva, followed by coffee, tea and Pepsi.
A two-way ANOVA for color stability revealed significant values for materials, beverages and time (all p < 0.001) (Table 4). The interaction between materials and beverages, materials and time, as well as the correlation between beverages and time were significant for ΔE values (p < 0.001). A comparison of the color stability of denture base materials subjected to immersion in different beverages is summarized in Table 5. The results indicated significant differences in the color stability of HP after immersion in the tested liquids between baseline and all time points (p < 0.05). For HP, the highest color difference was recorded at T2 with tea, followed by Pepsi and coffee. For PA and AC, significant differences in color stability were observed with all beverages (p < 0.05) except for T1 in saliva (PA: p = 0.101; AC: p = 0.263). In PA at T2, the highest color difference was documented with Pepsi, followed by the coffee and tea immersions. In AC at T2, the most significant color change was noted with coffee, followed by Pepsi and tea.
The results indicated significant differences in ΔE between denture base materials (p = 0.001). Specifically, HP exhibited lower ΔE values when compared to PA and AC. A variation was observed in the conversion of mean color difference values to the NBS units, contingent upon the type of material. For HP, the change in color was slight with all tested beverages at T1 and T2, with the exception of tea at T2, where a noticeable change was observed. A noticeable change at T1 and appreciable at T2 were evident for PA and AC across all tested beverages, except for tea at T2, which exhibited a noticeable color change for AC. For all materials, color changes were slight for the control group, with a noticeable change observed only for PA at T2.
Discussion
The current study evaluated the effect of Pepsi, coffee and tea on the color stability and Ra of different denture base materials. The null hypothesis of the study was rejected, as Ra and color stability were affected by the process of immersion.
The beverages selected for the present study are the most commonly consumed worldwide. Carbonated drinks, including Pepsi and Coca-Cola, are widely consumed, with more than 1.8 billion servings per day.22 The exposure of acrylic to different beverages for a minimum of 56 days causes a clinically perceptible color change.19 Consequently, in this study, the immersion period was extended to 90 days. The color stability was evaluated using the spectrophotometer due to its accuracy in measuring color coordinates.23 Certain foods and beverages cause changes in the surface properties of denture base resin.24, 25 Studies found that increased staining was correlated with an elevated Ra of dental prostheses.25, 26, 27
In the present study, the immersion of denture base materials in artificial saliva and beverages increased Ra of all materials. Coffee elicited the highest Ra values in all denture base materials, followed by tea for AC and PA, and Pepsi for HP. Among the tested materials, PA and AC exhibited the highest Ra values after being immersed in coffee. This phenomenon may be caused by the adsorption and absorption of coffee, which penetrated deeper than tea.14, 28 Particularly, both coffee and tea were used at high temperature in the present study, a condition that is known to promote water absorption. Moreover, the tannic acid present in tea and coffee has demonstrated to exert harmful effects on polymer surfaces.29 Pepsi demonstrated an increase in Ra of HP and AC compared with the control group. These results are consistent with the findings of Ikram, who reported increased Ra in PMMA after immersion in a carbonated beverage (Coca-Cola).25 The observed changes in denture base resin might have resulted from the acidity of the beverages due to the presence of phosphoric acid, which acts as a plasticizer.30
Intraoral dentures are subject to absorption and adsorption due to their contact with saliva, food, beverages, and denture cleansers.24 Staining occurs due to the physical penetration of pigments between the molecular lattices or the adsorption of pigments on the surface of specimens.14 The clinically acceptable value for color stability is 3.3.20 Similarly to the results of the previous studies,31, 32, 33 the present study demonstrated color changes even in specimens that were immersed in saliva (the control group). This observation may be attributable to the yellow color of mucin, a component of saliva, and the absorption of water molecules due to the polarity of acrylic resin.
In the present study, the tested beverages significantly altered the mean color stability values of HP, PA and AC at T1 and T2 in comparison with the control group. However, the consumption of the tested beverage for 30 days caused only a noticeable change in color for all materials, falling below the clinically acceptable value. However, after the increase in duration of immersion to 90 days, an appreciable change in the ∆E values was observed for PA and AC. The color change for HP was slight with Pepsi and coffee and noticeable with tea. Our results are in line with those of several previous studies, which indicated a correlation between the immersion time and increased discoloration of denture base materials.28, 34, 35 Similarly, studies reported a decreased color stability of polyamides with an extended duration of staining solution,7 and a heightened color stability of acrylic resin.4, 36, 37 The color change of polyamide is 2–4 times more pronounced than that of PMMA.4 The cause of the color changes in polyamide denture base material may be attributed to its hygroscopic nature, as its moisture content varies in response to the surrounding conditions.14 In addition to its hydrophilic properties,36 it has increased water sorption and leached plasticizer, in contrast to acrylic resin, which showed moderate sorption.37 Another reason for the greater discoloration observed in thermoformed resin in the present study could be their higher Ra compared to HP. There is a correlation between staining and the Ra value of the denture base material.38, 39
The highest color change of HP was observed after 90 days of immersion in tea, followed by Pepsi and coffee. This result aligns with the study by Hatim and Al-Tahho, who reported that tea induced the most significant color change among denture base materials compared with coffee and Pepsi.13 This observation is consistent with the results of previous studies.40, 41 Um and Ruyter reported that tea and coffee caused color change after 48 h of immersion.21 It was suggested that tannic acid present in tea and coffee is responsible for the observed denture brown discoloration, as it is water-soluble and known to trigger brown pigmentation.15 On the contrary, some previous studies reported a significant color change with coffee compared to tea28 and Pepsi. It should be noted that some of these studies utilized different materials than those tested in the present study.26, 27
Pepsi and coffee caused appreciable change in the color of PA and AC, and tea caused noticeable change at T2. The observed color change may have resulted from the low pH value of Pepsi, which adversely affected the surface integrity of the material.42 Previous studies suggested that a low pH value of Pepsi may act as a contributing factor in color change.13, 29 Our results are in line with those of a previous study that reported a significant color change of polyamide resin after the usage of Coca-Cola and coffee.14, 43 Coffee causes staining of resin because it includes yellow colorants, in addition to caffeine and caffeic acid.28, 29, 42 In the same context, a previous study has demonstrated that the discoloration caused by coffee was more than that of tea due to adsorption and absorption of colorants by resin materials, but the change in color caused by tea was only due to surface adsorption of the colorants.31 In the present study, coffee caused the highest Ra values, which could be a contributing factor that enhanced discoloration. Another variable that may have influenced the results is the high temperature of the beverage. A previous study revealed that the immersion of HP in hot water resulted in the whitening of HP due to water absorption.44
Limitations
The present study tested 3 different denture base materials. In addition, the immersion protocol closely resembled the actual use of beverages. However, the study’s limitations stem from its in vitro nature, which precludes the simulation of the oral environment, including changes in temperature, pH and the presence of oral flora. Another limitation is the relatively short testing period, which simulates 3 months of actual usage. Further studies are required to test the durability of the denture materials under prolonged and simulated oral conditions. In addition to the aforementioned procedures, it is imperative to investigate the effect of denture cleansers and cleaning methods on the restoration of the original color of the tested denture base materials. Also, further research is required to evaluate the effect of other beverages and denture cleansers on other mechanical and physical properties of denture materials.
Conclusions
The findings of the present study indicate that the use of beverages resulted in a substantial increase in Ra. As time exposure to the tested beverages increased, a significant rise in color change was observed, particularly with PA and AC, while HP demonstrated greater color stability. Dentists should educate their patients about discoloration associated with certain beverages and denture base materials, which can potentially compromise the aesthetics and lead to additional costs for replacement.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.