Abstract
Background. This article presents the global consensus report of the World Federation for Laser Dentistry (WFLD) on laser-assisted caries prevention and management. Laser-assisted caries removal is a minimally invasive approach based on selective ablation, targeting demineralized tissues while preserving the adjacent healthy enamel and dentin. This approach aligns with the principles of modern conservative dentistry. Erbium-doped yttrium aluminum garnet (Er:YAG) and erbium, chromium-doped yttrium scandium gallium garnet (Er,Cr:YSGG) lasers are highly absorbed by water and hydroxyapatite (HAP), enabling precise ablation with minimal thermal diffusion and collateral damage. Laser wavelengths can also exhibit bactericidal effects through thermal and photomechanical mechanisms, reducing the microbial load in carious lesions.
Objectives. The aim was to review, evaluate and consolidate the current evidence on the use of laser technologies in caries prevention and treatment, in light of the emerging scientific knowledge and clinical advancements.
Material and methods. This summary is based on the current evidence from in vitro, ex vivo and clinical studies evaluating the interaction of erbium-based lasers (Er:YAG, Er,Cr:YSGG) with enamel and dentin, their effect on the microbial load, fluoride uptake and resin adhesion, and their use in photobiomodulation (PBM) therapy.
Results. Laser irradiation induces physicochemical changes in enamel, such as superficial melting and recrystallization, reducing porosity and increasing resistance to acid attacks. Fluoride uptake is enhanced through microstructural modifications, especially when combined with topical fluoride. Subablative laser settings synergize with fluoride to enhance retention without damaging enamel. Laser conditioning before fissure sealing improves surface energy and resin adhesion, reducing microleakage. Photobiomodulation promotes remineralization in early lesions via cellular stimulation.
Conclusions. Laser-assisted caries treatment offers precise, minimally invasive management of dental caries with added benefits, such as microbial reduction, structural enhancement and improved adhesion. Careful control of parameters is essential to balance efficacy and safety. Further studies are needed to standardize protocols and confirm long-term outcomes in clinical practice.
Keywords: dental caries, lasers, dental caries prevention, evidence-based dentistry
Introduction
Dental caries remains one of the most widespread and burdensome oral diseases globally, affecting both children and adults, and contributing significantly to impaired oral health and reduced quality of life.1, 2 Despite advances in preventive strategies and restorative techniques, including fluoride use, fissure sealants and minimally invasive dentistry, the prevalence of caries continues to be high.3, 4 Traditional approaches are limited by issues such as patient compliance, variable long-term effectiveness and the recurrent need for retreatment. Consequently, there is growing interest in adjunctive methods that can strengthen hard tissues, reduce microbial challenges and support biological repair while preserving tooth structure.
Over the past few decades, lasers have emerged as a promising technology in dentistry. Continuous improvements in laser devices have led to greater safety, precision and versatility, opening new avenues for their use in both the prevention and treatment of caries. A variety of laser systems, including CO2, neodymium-doped yttrium aluminum garnet (Nd:YAG), erbium-doped yttrium aluminum garnet (Er:YAG), erbium, chromium-doped yttrium scandium gallium garnet (Er,Cr:YSGG), argon, and diode lasers, have been investigated for dental applications.5 Their distinct wavelengths and tissue interactions allow them to induce morphological and chemical changes in enamel and dentin, such as melting, recrystallization and the occlusion of dentinal tubules. These modifications can reduce enamel solubility, increase acid resistance and improve patient comfort by reducing hypersensitivity.4
In preventive dentistry, laser irradiation has demonstrated the ability to enhance enamel resistance to demineralization, particularly when combined with fluoride or other remineralizing agents.4 Subablative protocols can promote fluoride uptake without causing structural damage, offering synergistic protection against acid attacks. Certain wavelengths also possess bactericidal effects, reducing the microbial load on tooth surfaces and within incipient lesions. Collectively, these attributes suggest that lasers can play an important role in reducing the risk of caries initiation and progression.5, 6
From a therapeutic perspective, erbium-based lasers allow the selective ablation of carious dentin, facilitating minimally invasive caries removal while preserving healthy tissue. This aligns with the philosophy of modern conservative dentistry. Additionally, photobiomodulation (PBM) therapy, formerly referred to as low-level laser therapy (LLLT), has been explored as a complementary strategy to stimulate pulp and dentin healing, and promote lesion reversal when combined with agents such as casein phosphopeptide–amorphous calcium phosphate (CPP–ACP).6 Moreover, a microscopic and crystallographic analysis of dental enamel melted using a 445-nm diode laser showed a significant increase in acid resistance.7 These innovative applications expand the potential of lasers beyond traditional cutting instruments, positioning them as multipurpose tools in caries management.
Nevertheless, several challenges remain before widespread clinical adoption can be recommended. Outcomes vary considerably depending on the laser type, wavelength, energy settings, and irradiation protocols used. While in vitro and short-term clinical studies are promising, robust evidence on long-term efficacy, cost-effectiveness and biomechanical safety is still limited. The lack of standardized guidelines further complicates clinical decision making and may hinder optimal integration of lasers into daily practice.
In this context, the World Federation for Laser Dentistry (WFLD) has developed the present consensus report on laser-assisted caries treatment and prevention. Drawing on the expertise of an international panel of specialists, this document aims to critically evaluate the available evidence, provide practical recommendations for preventive and therapeutic use of lasers in caries management, and identify key areas for future research. By establishing a unified framework, the WFLD seeks to guide clinicians toward evidence-based adoption of laser technologies, ultimately contributing to improved patient outcomes and advancing minimally invasive dentistry.
Methods
A modified Delphi approach was used to develop the consensus report. The panel consisted of 11 specialists from 8 countries (Belgium, Brazil, Lebanon, the United Kingdom, Thailand, Romania, Japan, and Poland), all of whom are active members of the WFLD. Eight experts were assigned to prepare a section of the manuscript according to their specific field of expertise. The drafted sections were then compiled and redistributed anonymously to all panel members for critical review and comments. One designated coordinator integrated the suggested corrections and produced a revised draft of the manuscript. This version was subsequently reviewed in detail by 2 independent experts, followed by a group discussion to resolve any remaining disagreement. The final approval of the consensus document was obtained once all experts agreed with the revised version. This structured process ensured transparency, minimized individual bias, and guaranteed that the final consensus reflected the collective expertise and international agreement of the panel.
Background on laser applications in dentistry
The use of lasers in dentistry spans several decades, but recent technological advances have significantly improved their safety, precision and versatility. A variety of lasers, including CO2,8, 9 Nd:YAG,10, 11 Er:YAG,12, 13, 14, 15 Er,Cr:YSGG,16 argon,17 and diode lasers,18 have been utilized for caries-related applications. These lasers differ in terms of wavelength, interaction with the tissue and depth of energy penetration, leading to various biological and chemical effects on enamel and dentin substrates. When applied under appropriate parameters, laser irradiation has been shown to induce desirable morphological and chemical changes, including the surface melting, fusion and recrystallization of dental hard tissues.8 These effects are believed to reduce enamel porosity, decrease acid solubility and increase the acid resistance of hydroxyapatite (HAP) crystallites, considered as key factors in caries prevention.
In the preventive context, laser irradiation has been associated with enhanced enamel resistance to demineralization.19 Studies report that laser treatment can either directly alter the crystalline structure of enamel or synergize with topical agents like fluoride to enhance its uptake and incorporation into HAP.20, 21 This dual action not only provides mechanical resistance to acid attacks, but may also alter diffusion pathways for acid, thereby slowing down the progression of incipient lesions. Additionally, certain laser wavelengths exhibit bactericidal properties, making lasers capable of reducing the microbial load on tooth surfaces and within early carious lesions.22 This antimicrobial effect further contributes to the caries-preventive potential of laser applications.
From a therapeutic perspective, lasers offer the possibility of selective caries removal, targeting demineralized tissues while preserving the adjacent healthy enamel and dentin.23 This minimally invasive approach aligns with the principles of modern conservative dentistry. In cases involving dentin, some laser systems have been reported to occlude dentinal tubules, which can reduce hypersensitivity and improve patient comfort. For example, CO2 laser irradiation has demonstrated the ability to increase dentin resistance to artificial caries-like lesions.24 Another emerging area in the context of caries treatment is the use of PBM therapy, formerly known as LLLT. Photobiomodulation does not aim to alter hard tissues directly, but instead stimulates cellular responses that may aid in remineralization or pulp–dentin complex healing. When combined with remineralizing agents such as CPP–ACP or bioactive glass, PBM may accelerate lesion reversal and promote biological repair mechanisms.25
Despite the promising clinical and in vitro results, the underlying mechanisms governing laser-induced beneficial changes in enamel and dentin are not yet fully elucidated. While some studies describe chemical transformations, such as carbonate loss, changes or modifications in the HAP crystallite lattice, spectroscopic and ultrastructural evidence remains incomplete. Effects vary not only with the laser type, but also with specific parameters, such as pulse duration, energy density, repetition rate, and the presence of adjunctive agents like fluoride.
Moreover, the long-term clinical efficacy and cost-effectiveness of laser-assisted caries management remain topics of ongoing research. Standardization of protocols and deeper understanding of laser–tissue interactions at the molecular level are essential before widespread clinical adoption can be recommended.
Laser-assisted caries prevention
Tooth decay remains one of the most common oral diseases worldwide.26 In response to the limitations of traditional preventive methods, dental research has turned to innovative approaches, including the use of lasers. This technology, originally reserved for restorative treatment, is increasingly being recognized as a promising preventive tool. Several types of lasers are now being studied and applied in dental practices, often in combination with other methods like fluoride treatment, fissure sealing and enhanced oral hygiene. This integrated approach aims to strengthen the natural protection of the teeth, thus reducing the risk of demineralization and cavity formation.
Several procedures are cited in the literature to increase the enamel resistance to caries. Below we summarize the main methods playing a key role in preventing tooth decay.
Enamel modification for acid resistance
Laser-assisted caries prevention strategies increasingly focus on enhancing the acid resistance of enamel – a critical barrier against demineralization and caries formation.27 By altering both the physical and chemical properties of enamel, laser irradiation can strengthen its structure and improve its protective capabilities.7 Depending on the energy level, wavelength and type of laser used, these effects range from superficial melting and prism disorganization to improved uptake of topical fluoride. While high-power lasers, such as Er:YAG and Er,Cr:YSGG, enable enamel fusion and surface glazing, lower-energy systems, such as argon or diode lasers, are employed to enhance fluoride retention without damaging the tissue. Recent clinical studies suggest that combining subablative laser protocols with fluoride application offers a synergistic approach, reinforcing enamel resistance to acid attacks in a minimally invasive manner.
On the other hand, recent in vitro studies indicate that CO2 laser irradiation (commonly at ~10.6 μm) can improve the acid resistance of tooth enamel, particularly when combined with fluoride treatment. For example, human premolar enamel surfaces irradiated with a pulsed CO2 laser showed significantly lower calcium (Ca) release and shallower erosion depths as compared to controls and those treated with Nd:YAG lasers. However, these benefit effects diminished over extended acid exposure times and did not affect subsurface enamel layers.28 Moreover, the application of CO2 laser through a topical amine fluoride solution enhanced fluoride uptake and increased surface acid resistance in both sound and demineralized enamel, with fewer surface alterations observed via scanning electron microscopy (SEM) than after laser alone.24, 29, 30 Similarly, combining CO2 laser irradiation with acidulated phosphate fluoride (APF) demonstrated a synergistic effect, further reducing demineralization as compared to either treatment alone.30, 31 One study revealed that while CO2 laser alone slightly prevented enamel erosion, it provided the most effective protection when paired with a stannous fluoride (AmF/NaF/SnCl2) solution, particularly in human enamel models.32 Together, these findings suggest that CO2 laser treatment, especially when used with fluoride agents, can enhance enamel resistance to acid challenges, though effects may be limited to superficial layers and are most pronounced when complemented by fluoride therapy.
Structural fusion and laser surface effects on enamel
Mature dental enamel is composed of a predominantly Ca-based material, primarily in the form of HAP, which constitutes approximately 96% of its composition. During laser irradiation, the generated heat can induce the dehydration of enamel, potentially leading to the formation of surface microfissures. The literature indicates that laser-induced melting and glazing of dental enamel can result in the development of these microcracks on the irradiated surface. Such fissures may, over time, contribute to the structural failure of enamel within the oral cavity, culminating in the physical destruction of the tooth. Considering these detrimental effects, recent research has focused on the application of low-power lasers that do not cause significant heating or fusion of enamel, thus minimizing the risk of damage.27, 30, 33
When interacting with the structure of enamel, lasers induce its fusion through heat, followed by rapid re-solidification. This fusion process alters the structural integrity of enamel by disrupting the prism (rod) structure, which results in the loss of the characteristic orientation of HAP crystallites. Additionally, the organic interrod matrix undergoes disintegration. These physical alterations hinder and slow down the progression of acid attacks within enamel by disrupting the preferred pathways (inter-prismatic and under-crystallite spaces) for acid infiltration. Furthermore, heating and subsequent melting of enamel trigger chemical changes in its constituent phases, which can further enhance enamel resistance to acidic degradation.
By modifying the structure of enamel through melting it superficially, lasers can make it more resistant to acid attacks responsible for cavities.
Physicochemical alterations of enamel through laser irradiation
The effects of various types of lasers on dental hard tissues are being thoroughly investigated. These effects are highly dependent on laser-specific parameters, including wavelength, energy output and the technique of irradiation.34 Among laser devices, erbium lasers (Er:YAG and Er,Cr:YSGG) are especially relevant in dentistry due to their high absorption by water. This property enables their safe application with water spray, effectively reducing thermal damage during the ablation of hard tissues.34, 35, 36, 37
In enamel, which has a lower water content than dentin, the action of erbium lasers at low energy densities primarily removes organic materials, such as proteins and lipids, located between enamel rods. This results in the creation of a micro-roughened surface, which can enhance adhesion in restorative procedures.38 However, it is important to note that enamel cutting generally requires significantly higher power settings as compared to dentin. For instance, the effective ablation of enamel may necessitate power levels up to 2 W and energy densities around 31.44 J/cm2 at a frequency of 10 Hz.39
For the preparation of dentin, which is more sensitive to thermal effects due to its organic matrix and proximity to pulp, Nahas et al. recommend lower energy settings – specifically, 60 mJ at 10 Hz in the micro-short pulse (MSP) mode – in combination with water irrigation and air cooling to minimize the risk of pulpal damage.40
Cracks on the surface of enamel can occur as a result of laser irradiation due to the rapid thermal changes induced by laser energy. When enamel is exposed to high-intensity laser beams, as in the case of Er:YAG lasers, the abrupt rise and fall in temperature may generate thermal stress within the structure of enamel, exceeding the tensile strength of the material, and resulting in microcracks or fissures on the surface. Early experimental investigations had already documented such morphological changes: Hibst and Keller demonstrated that Er:YAG laser irradiation could cause microexplosions and crack formation in enamel and dentin,41 while Fried et al.42 and McCormack et al.43 confirmed that rapid thermal expansion, under insufficient cooling conditions, promoted surface fissures and irregularities visible under SEM.42, 43, 44 Similarly, White et al. reported microcracks in the enamel irradiated by CO2 lasers, emphasizing that energy density and the pulse mode are the critical determinants of surface safety.45 Subsequent investigations on CO2 lasers confirmed that even at subablative fluences, enamel exhibited subsurface cracking and carbonization, especially in the absence of water cooling.46
Comparable structural alterations have been described with other wavelengths. Nd:YAG irradiation has been associated with crater formation, localized melting and subsurface cracks in enamel due to its deeper penetration and high thermal load.47, 48 In a comparative study, Nd:YAG-treated enamel exhibited a higher frequency of fissures than Er:YAG-irradiated samples, particularly under dry conditions.49 Argon lasers, although primarily used for curing and caries prevention, have also been shown to induce localized thermal stress and fissures in enamel prisms when applied at high energy densities.50 These cross-wavelength findings consistently point to the risk of thermomechanical stress concentration whenever energy delivery exceeds the capacity of the tissue for rapid heat dissipation.
More recent studies have refined these observations with advanced imaging. For instance, using atomic force microscopy (AFM), SEM and energy-dispersive X-ray spectroscopy (EDS), Rodríguez Vilchis et al. observed triangular cracks in enamel, with depths ranging from approx. 250 nm to 750 nm and widths between 2.58 μm and 5.37 μm, along with the accompanying craters and irregular surfaces.51 Likewise, nanoscale X-ray computed tomography (nano-CT) confirmed that, while glazing and surface roughness occurred on the irradiated enamel, subsurface structural damage was minimal and prismatic enamel architecture largely remained intact, suggesting that surface alterations may not always translate into deeper compromise.52
These thermally induced modifications could nevertheless act as stress concentrators, weakening enamel and potentially jeopardizing adhesive performance or mechanical integrity. Despite the expanding clinical adoption of lasers in dentistry, there remains a pronounced gap in research regarding the long-term mechanical quality of laser-treated enamel, particularly concerning the risk of tooth fracture. Most mechanical investigations have focused on dentin rather than enamel. For example, the dentin beams irradiated without water cooling, using Er:YAG or Q-switched Er:YSGG lasers, exhibited visible surface cracks and a significant reduction in bending strength – from approx. 142 MPa in controls to 91 MPa in the dry-irradiated group.53 By contrast, comparable tests on enamel, such as the assessments of fracture toughness, load-bearing capacity or crack propagation under functional conditions, are notably scarce. Earlier descriptive studies largely focused on identifying surface fissures,42, 45, 46, 47, 50 but they did not extend to quantitative biomechanical evaluations.
This lacuna in the literature underscores a critical oversight: the risk of tooth fracture due to laser-induced structural alterations in enamel remains insufficiently studied, with scant mechanical testing performed thus far. Rigorous biomechanical evaluations of laser-treated enamel are urgently needed to ensure both safety and efficacy in clinical applications.
To summarize, erbium lasers provide a versatile tool for the modifications of hard tissues. High energy levels are recommended for efficient enamel cutting, while dentin benefits from lower energy parameters to preserve its structural and biological integrity. This differentiation is essential when preparing enamel and dentin surfaces for bonded restorations (Table 1).34, 38, 39, 40 However, the irradiation conditions must be reduced for dentin conditioning before bonded fillings (Table 2).
Combined use of laser and fluoride in restorative dentistry
The combination of laser irradiation and topical fluoride application has emerged as a promising strategy to enhance enamel resistance against acid demineralization. Fluoridation, a traditional yet highly effective method, involves applying fluoride in the form of varnish or gel to strengthen enamel and increase its acid resistance by promoting remineralization.54, 55, 56, 57 Low-energy laser treatment can further improve fluoride uptake and retention by modifying enamel chemistry and microstructure without causing thermal damage. This synergistic interaction is being investigated both in preventive and restorative dentistry, with reported benefits including enhanced enamel protection, as well as improved adhesion and sealing performance of restorative materials.
One of promising adjunctive strategies in laser-assisted caries prevention involves the combination of low-energy laser irradiation with topical fluoride application.58 The argon laser, operating at low power – typically in the milliwatt range – and emitting in the blue visible spectrum, has been widely studied in this context.17, 58 Due to its interaction at the electronic level of atoms, visible light from such lasers is hypothesized to induce chemical changes in the HAP structure of enamel. Specifically, this interaction may facilitate the substitution of hydroxyl ions (OH−) with fluoride ions (F−), which are more electronegative. This chemical alteration enhances fluoride uptake and contributes to greater acid resistance of the enamel surface.17, 19, 20, 58, 59, 60, 61, 62, 63, 64, 65
Supporting this hypothesis, an in vivo clinical study assessed fluoride retention following laser irradiation under subablative settings, i.e., low energy levels insufficient to cause morphological changes or damage to enamel.66 The results confirmed that laser application after fluoride treatment significantly improved fluoride retention in dental enamel without compromising structural integrity.66 These findings underscore the clinical potential of low-energy lasers as safe and effective adjuncts to conventional fluoride-based preventive strategies.
Laser preconditioning for improved adhesion and sealing
Laser surface conditioning for fissure sealants
Pit and fissure areas – narrow grooves typically found on the occlusal surfaces of molars and premolars – are highly susceptible to dental caries.67 These regions tend to trap plaque and debris, and are difficult to clean effectively with a toothbrush, particularly in children and adolescents. To counter this risk, fissure sealants are widely used as a preventive measure. The application of fluid resin into pits and fissures creates a physical barrier, which blocks the entry of food particles and bacteria, thereby reducing caries incidence in these vulnerable zones.67 The procedure is simple, minimally invasive, and provides long-term protection.
Recent advances in preventive dentistry have introduced laser irradiation as a surface preconditioning tool to improve the adhesion of sealants.68 By modifying the surface of enamel – without the need for acid etching – lasers can enhance resin retention and sealant longevity. In particular, laser preparation has shown potential for increasing micromechanical interlocking and reducing microleakage. Furthermore, laser use extends beyond the application of sealants. In modern caries prevention strategies, lasers play a growing role in enhancing enamel resistance by promoting fluoride incorporation into the HAP structure. This synergistic effect contributes to increased acid resistance and long-term protection against demineralization. Nonetheless, these technological approaches should always be integrated with fundamental oral hygiene practices and a balanced diet. Laser-based preventive strategies are most effective when used as part of a comprehensive caries management protocol.67, 68
Laser-assisted adhesion strategies in restorative dentistry
Bonded restorations in dentistry rely essentially on resin micromechanical interlock with the bonded surfaces.
Among the lasers commonly used in dentistry are erbium lasers, such Er:YAG and Er,Cr:YSSG, which are particularly effective for cutting and preparing dental hard tissues. The effects of various laser types on hard tissues can be summarized as follows:
The CO2 laser enhances dentin resistance to caries by melting dentin and obliterating dentinal tubules.24 However, overheating of the surface in a localized area may modify the chemical composition of dentin due to melting and vaporization of certain components.69 Moreover, such irradiation negatively affects the shear bond strength (SBS) of the composite materials bonded to dentin.69
Diode lasers are not typically used for the ablation of dental hard tissues. However, when used to irradiate bonding systems at low energy levels prior to photo-polymerization, they can increase SBS to enamel. Furthermore, this enhancement in bond strength is not affected by thermal aging.70 In addition, the diode laser pre-treatment of non-carious cervical lesions acts better against bacterial microinfiltration as compared to chemical surface treatment.71 Moreover, diode laser irradiation does not affect the microleakage of the restored materials, whether it is resin composite, glass ionomer or resin-modified glass ionomer.72
The Nd:YAG laser is commonly used in dentinal treatment. Controversial results can be found in the literature. Studies by Sun et al.73 and Landmayer et al.74 showed that bonding to dentin after being irradiated with Nd:YAG at 124.4 mJ/cm2 enhanced the shear and microtensile bond strength of the composite, even after thermocycling. Although the composition of dentin was modified and some of the collagen fibers were removed, the hardness and roughness of dentin surface were equivalent to those of the unirradiated dentin.73, 74 On the other hand, Hamidi et al. were totally against the use of Nd:YAG before bonding to dentin.10 The reported microtensile bond strength to the dentin irradiated with 50 mJ was lower as compared to the unirradiated dentin.10 Even though, using Nd:YAG at 60 mJ does not affect the collagen layer needed for the hybrid layer.75 Low SBS to Nd:YAG-irradiated dentin was also reported by Al Ahdal et al.22
Erbium laser (Er:YAG and Er,Cr:YSGG) beams are highly absorbed by water. They can be employed under water spray, thus reducing thermal damage during the ablation of hard tissues.34 The ablation effect of erbium lasers is more pronounced on dentin than enamel due to the difference in water content. At low energy densities, they act on enamel through removing organic particles between enamel rods, thus creating a rough surface.38 However, the conventional etching of enamel is still giving better surface preparation for composite bonding than laser alone. Moreover, combining acid etching with laser increases bond strength when compared to the conventional etching of enamel.76
Latest studies have shown successful bond strength of the composite to the dentin irradiated with erbium lasers, and laser use was recommended as an alternative technique to the conventional one.16, 77 It has been demonstrated that the irradiation of dentin modifies its surface by eliminating the smear layer and exposing dentinal tubules for bonding infiltration.23 Caries removal and cavity preparation with the use of erbium lasers are less invasive procedures as compared to those performed with rotary burs or chemical agents.23 Unfortunately, the results published by Karatas et al. prove the contrary, but only for the Er,Cr:YSGG laser, which negatively affected SBS to the irradiated dentin by altering the structure of the hybrid layer.78 Cutting enamel and dentin requires high power, especially for enamel (up to 2 W), and energy density of 31.44 J/cm2 at a frequency of 10 Hz.39 Nahas et al. recommended conditioning for dentin preparation to receive a bonded restoration with a decrease in the level of the delivered energy to 60 mJ, with a frequency of 10 Hz, the MSP mode under water irrigation and air cooling.40 Another study by Demir et al. used 200 mJ, 20 Hz and pulse duration of 50 µs; that way, the laser beam eliminated the smear layer, opened dentinal tubules and prepared the surface of dentin for the bonding composite.79
Laser-assisted caries removal and treatment
Laser-assisted caries removal is a minimally invasive approach that exploits selective ablation mechanisms, where laser energy specifically targets carious tissues due to the differences in water and mineral content, allowing the preservation of healthy enamel and dentin. Er:YAG and Er,Cr:YSGG lasers are highly absorbed by water and HAP, efficiently ablating carious dentin through microexplosive effects and rapid vaporization. The water/air spray assures minimal thermal diffusion and prevents collateral pulp damage.47 Certain laser wavelengths exhibit bactericidal properties, making lasers capable of reducing the microbial load in carious lesions via thermal and photomechanical disruption, contributing to improved clinical outcomes.9, 80, 81 Laser irradiation induces physicochemical modifications in enamel, such as superficial melting and recrystallization, thus increasing resistance to acid attacks responsible for caries initiation.82, 83 Prior to the placement of fissure sealants, laser conditioning modifies enamel morphology, creating microroughness and improving surface energy, which significantly enhances resin adhesion and sealant durability, reducing microleakage.84, 85, 86
Photobiomodulation facilitates remineralization in combination with remineralizing agents, promoting mineral deposition and accelerating repair mechanisms in early carious lesions.87, 88
Optimal laser treatment outcomes depend on precise control of parameters, such as wavelength, energy density, pulse duration, and cooling mechanisms to balance effective ablation with the preservation of structural integrity and biological function. Continuous investigation into the mechanisms and optimization of laser-based caries treatment is critical for standardizing protocols, and ensuring their effectiveness and safety for widespread clinical adoption.
Enhancing enamel resistance and remineralization: The role of Er,Cr:YSGG and Er:YAG lasers
Effect on sound enamel
Multiple in vitro studies have demonstrated that Er,Cr:YSGG laser irradiation enhances the acid resistance of sound enamel. Ulusoy et al. used SEM and EDS to show decreased solubility of both primary and permanent enamel after irradiation.89 Serdar-Eymirli et al. confirmed that combining laser with fluoride or CPP–ACP significantly increased enamel microhardness.90 Mahdi and Hussein validated this synergistic effect in a randomized clinical trial on primary teeth, using APF gel.91
Effect on enamel erosion
Studies confirm that the Er,Cr:YSGG laser helps protect enamel against acid erosion. Hadi and Ali found increased enamel microhardness and reduced mineral loss after combining laser with APF.92 AlShamrani et al. showed superior protection against acid challenges with the same combination.93 Fornaini et al. emphasized that subablative parameters create structural changes that enhance acid resistance without damaging enamel.94
As for the Er:YAG laser, several studies have evaluated its preventive effect with regard to enamel acid demineralization, yielding mixed results. A systematic review and meta-analysis by Lombardo et al. summarized findings from 6 such studies comparing laser to no treatment.8 Four of these studies found that the Er:YAG laser did not significantly improve enamel resistance, as measured by parameters such as microhardness, lesion depth and Ca dissolution. However, 2 studies reported a positive effect: one showed a 41% reduction in mineral loss, and another found a significant decrease in the lesion depth when the laser was applied at a 4-millimeter distance with water cooling at 2 mL/s. When combined with other interventions, the Er:YAG laser demonstrated enhanced efficacy. It significantly increased enamel microhardness when used with 5% fluoride varnish and it reduced Ca dissolution when combined with 1.23% APF gel. Furthermore, the combination of the laser with 2% NaF gel resulted in a 54% reduction in acid-induced mineral loss as compared to only 24% with the gel alone.8
Remineralization of initial carious lesions
The Er,Cr:YSGG laser enhances the efficacy of remineralizing agents in treating initial enamel lesions. Cheng et al. concluded that laser with CPP–ACP significantly improved enamel microhardness and reduced the lesion depth.95 Damar et al. observed improved outcomes with theobromine-containing products.96 Yilmaz et al. confirmed similar effects in primary enamel.97
One in vitro study assessed the effect of the Er:YAG laser irradiation combined with APF therapy on the remineralization of white spot lesions (WSLs).98 The study found that the combination treatment significantly enhanced enamel resistance to acid attacks as compared to either treatment alone. Laser irradiation was shown to increase the size of HAP crystallites by melting, causing the recrystallization of enamel, and subsequently decreasing the permeability of enamel and enhancing its resistance to acid attacks.98
Laser parameters and protocols
The most common parameters used in caries-related studies include: Er,Cr:YSGG laser at 2,780 nm; power 0.25–0.50 W; energy density 4.5–9.0 J/cm2; frequency 20 Hz; and pulse duration 140 µs. The water/air spray (20/20% or modified) is used to limit thermal effects. Subablative settings are the key to ensuring enamel integrity while promoting mineral uptake.
Pulse energies between 100 and 200 mJ and fluence values ranging from 12.7 to 44.4 J/cm2 are effective in reducing enamel demineralization. A frequency of 10 Hz and pulse duration between 250 and 400 µs are commonly used settings in the protocols aimed at improving enamel acid resistance.
Carefully selected parameters, such as moderate pulse energies, appropriate fluence, and controlled frequency and pulse duration, are critical for achieving optimal results without compromising enamel integrity. As research progresses, laser-based treatment is poised to become a valuable adjunct in minimally invasive, preventive dental care protocols (Table 3).
Marginal integrity and microleakage in Class V restorations
Class V restorations, typically found at the cervical third of the tooth, present challenges due to the mixed substrates of enamel, dentin and cementum, as well as their susceptibility to microleakage. Laser-based cavity preparation, particularly using Er:YAG and Er:Cr:YSGG lasers, has been explored as an alternative to traditional burs to improve surface quality and bond strength at the enamel and cementum margins. However, several studies have demonstrated that microleakage at the cementum margin of the restoration, in particular, with composite resin, was greater than the cervical margin at some laser parameters.104, 105, 106, 107 Consequently, the parameter settings for laser devices for the cervical margin of Class V preparation comprising cementum need to be thoroughly investigated and should differ from the standard recommendations typically applied for enamel or dentin. No clinical trials were identified; only in vitro studies were found, as microleakage must be examined in the extracted teeth.
The Er:YAG laser (2,940 nm) was used under variable energy and frequency settings. Low-energy settings ranging from 75 to 200 mJ at 2–20 Hz proved to be effective in comparison with traditional bur preparation methods; the Er,Cr:YSGG laser (2,780 nm), set at 175 mJ and 20 Hz, also demonstrated effective sealing of restorations when acid etching was used.108 The comparison of total-etching and self-etching adhesives and selective etching of enamel (with no etching for cementum) showed no significant differences in microleakage when using the Er:YAG laser at 200 mJ and 20 Hz.109 Other studies demonstrated comparable or less microleakage when using the Er:YAG and Er,Cr:YSGG lasers vs. bur preparation in the groups that utilized a primer with glass ionomer cement or a self-etching adhesive with a composite resin restoration.106, 107, 110 Based on a scoping review, the following recommendations for minimizing microleakage are made for the cementum margin of Class V restorations, which can also be applied to the enamel margins (Table 4).
Recommended laser parameters
Laser type: Er:YAG (2,940 nm) or Er,Cr:YSGG (2,780 nm).
Energy per pulse: 100–200 mJ.
Frequency: 10–20 Hz.
Pulse duration: 100–200 µs.
Water spray: ≥5 mL/min.
Conditioning recommendations by margin type
Enamel margins
Preparation: laser or a bur
Conditioning: 3 steps of acid etching, primer and bonding, or a self-etching adhesive
Material: a resin composite with good enamel adhesion
Cementum (cervical) margins
Preparation: laser or a bur.
Conditioning: 3 steps of acid etching, primer and bonding, or a self-etching adhesive.
Avoid: laser-only surface conditioning.
Material: a resin composite or glass ionomer.
Adjunctive benefits of laser use in preventive dentistry: Management of tooth hypersensitivity
Although dentin hypersensitivity (DH) is not a direct outcome of carious lesions, it is frequently encountered in clinical practice as a comorbidity of non-carious cervical lesions, enamel erosion or preventive interventions, such as scaling and polishing, orthodontic movement, or even conservative laser treatment. Therefore, its effective management is essential to ensure patient comfort and the long-term success of preventive care strategies.
Laser-assisted therapies, especially using high- and low-level laser systems, have emerged as valuable tools for managing DH. These approaches complement preventive and restorative procedures by reducing pain, sealing the exposed dentinal tubules and improving patient compliance with minimally invasive dental protocols.
Dentin hypersensitivity is defined as a short, sharp pain arising from the exposed dentin in response to thermal, tactile, osmotic, or chemical stimuli, and is not attributable to any other form of dental pathology.112, 113 It significantly affects patient quality of life, and is commonly associated with gingival recession and enamel or cementum loss, which expose dentinal tubules to external stimuli.21, 114
Role of photobiomodulation
Photobiomodulation has emerged as a non-invasive, effective adjunct in the management of DH, owing to its dual mechanism – the occlusion of dentinal tubules and the modulation of nerve activity.113, 115 The lasers used in DH treatment are broadly categorized by power and mechanism:
– high-power lasers (e.g., Nd:YAG, Er:YAG, Er,Cr:YSGG) deliver photothermal energy, melting and recrystallizing dentin to physically seal dentinal tubules (Table 5)116, 117, 118;
– low-level lasers (e.g., diode 660–830 nm) induce PBM, altering pain perception via anti-inflammatory and neural mechanisms (Table 6).119, 120, 121
Scanning electron microscopy has confirmed that laser-treated surfaces show occluded or narrowed dentinal tubules, reducing fluid flow and the transmission of stimuli.113
Clinical performance
High-power lasers like Nd:YAG and neodymium-doped yttrium aluminum perovskite (Nd:YAP) have shown rapid desensitization with protocols such as 0.5 W at 10 Hz for Nd:YAG, producing a significant and sustained reduction in DH.114, 118 The Er,Cr:YSGG laser has similarly demonstrated safe and effective outcomes when using subablative protocols.115
Photobiomodulation, particularly with diode lasers (660–1,064 nm), has proven effective in neuromodulation and dentin repair. Studies report reduced VAS scores sustained over months after 6 sessions using 50–100 mW for 50–120 s per point.121, 123, 128
Combination therapies
Synergistic effects are observed when laser therapy is combined with desensitizing agents, such as KNO3, fluoride gels or CPP–ACP pastes.
For example, Guanipa-Ortiz et al. demonstrated a 77% reduction in DH with diode laser and casein phosphopeptide–amorphous calcium phosphate fluoride (CPP–ACPF) paste,129 while Jomaa et al. reported 9-month relief when combining diode laser with 1.23% NaF gel (Table 7).130
Comparative efficacy
Several systematic reviews affirm the superiority or at least equivalence of laser therapy in comparison with conventional agents.132, 133 However, the Cochrane review by Mahdian et al. emphasized the need for high-quality, standardized trials due to heterogeneous methodologies and short follow-ups.113 Laser therapy, particularly when individualized by wavelength, energy and treatment protocol, offers a valuable tool in the management of DH. The promising outcomes are observed in combination strategies that integrate lasers with topical agents, simultaneously targeting neural modulation and dentinal tubule sealing.116, 117, 128
Photodynamic therapy in carious lesions
Scientific publications support the idea that photodynamic therapy (PDT) can play a beneficial role in caries treatment, predominantly by reducing the microbial load in deep lesions and supporting conservative, minimally invasive dentistry approaches (Table 8).134, 135, 136, 137, 138, 139, 140, 141, 142
Literature reports
Photodynamic therapy is gaining attention as an adjunctive method for managing carious lesions, mainly because of its antimicrobial activity and its compatibility with minimally invasive treatment strategies. A growing number of laboratory and clinical studies demonstrate that antimicrobial photodynamic therapy (aPDT) can reduce cariogenic microorganisms, such as Streptococcus mutans and Lactobacillus spp., in infected dentin.134, 135, 136 Although the degree of this reduction varies across studies, aPDT generally enhances disinfection when combined with the selective removal of carious tissue.135
In pediatric dentistry, aPDT has shown particularly encouraging results. Randomized clinical trials on primary molars indicate that aPDT used alongside selective caries removal improves microbial control and may support better restoration performance during follow-up.134, 137, 138, 139 One 12-month clinical study reported favorable marginal integrity of the composite restorations placed after aPDT, suggesting a potential clinical benefit beyond microbial reduction.139 Other investigations similarly show reduced bacterial load in deep lesions and good short-term clinical outcomes, including maintained pulp vitality.138 In vitro research confirms that aPDT can be effective against cariogenic biofilms, although the outcome is strongly dependent on the photosensitizer concentration, wavelength and irradiation time.140, 141 Systematic reviews emphasize the promise of PDT, while highlighting that inconsistent protocols and relatively few high-quality trials still limit firm clinical recommendations.141, 142 Given the current evidence, PDT should be considered an adjunctive approach rather than a replacement for conventional caries management. When used together with selective excavation and standard restorative procedures, PDT may enhance dentin disinfection and support improved treatment outcomes.134, 135, 136, 137, 138, 139, 140, 141, 142
Conclusion
There is scientific evidence that PDT can play a beneficial role in caries treatment, primarily by reducing the microbial load in deep lesions and supporting conservative, minimally invasive dentistry approaches. Additionally, PDT has been associated with clinical improvement in restoration margins over time. However, the effectiveness of the therapy remains highly dependent on protocol variables (photosensitizer choice, concentration, light wavelength/intensity, and exposure duration), and long-term outcomes and standardization are lacking.
Currently, PDT should be used as an adjunct to conventional caries treatment, rather than as a standalone approach.
Economic aspect of integrating lasers into routine dental care
Laser treatment, like many other dental procedures, requires special equipment and trained practitioners.143 Depending on the specificity of the treatment, different types of laser might be recommended. Unfortunately, the effective cost of a laser machine ranges from a low price, as in the case of diode lasers,144, 145 and increases with the complexity of manufacturing.146 Not only is the cost of a laser machine important, but also the accessories used could affect the final price of the treatment. An example of these accessories are delivery tips of different diameters, where a simple intervention at different areas might require more than one tip.143 Although some tips can last for more than one procedure, these accessories, as well as handpieces with the embedded mirrors that degrade with time, will eventually need maintenance or replacement.143 Therefore, the cost of laser treatment could be high, and thus may create a burden in some economically affected countries, since increasing the fee for dental treatment to reflect the cost of the machine, the tips, or any other accessories is difficult to justify. Consequently, in these countries, laser educational programs in universities should be the first step toward the integration of laser concepts, preparing practitioners to acquire laser practice in their dental cabinet, starting from low-cost diode lasers.144, 145 Moreover, manufacturers should increase their efforts and assure special prices for newly interested societies in laser treatment or through government subsidies, and couple their machines with long-term warranty, as well as enlarge their infrastructure for proper maintenance and repair to support dentists continuing laser treatment within their dental procedures.
Discussion with a global conclusion
This consensus report provides comprehensive and evidence-based guidance on the use of laser technologies in caries prevention and management. The integration of lasers, ranging from erbium and diode systems to argon and CO2 devices, demonstrates a significant promise in enhancing enamel acid resistance, improving fluoride uptake, facilitating minimally invasive caries removal, and optimizing restorative outcomes. Laser-assisted strategies also offer adjunctive benefits in treating DH, and preparing tooth surfaces for sealants and bonding agents.
While diverse protocols and device types are currently in use, their clinical success hinges on precise parameter control and proper technique selection. Synergistic approaches combining lasers with conventional agents such as fluoride varnishes, sealants or desensitizers show the greatest potential for durable, patient-centered outcomes. Despite the encouraging data, gaps remain in standardization and long-term validation.
The WFLD endorses continuous clinical education, research and collaboration to refine laser protocols, promote safety and expand global accessibility. As part of an integrated preventive model, laser-assisted dentistry represents a valuable complement to traditional practices, reinforcing the goal of preserving healthy tooth structure and improving overall oral health.
Although numerous in vitro and laboratory studies demonstrate promising effects of laser-assisted protocols in enhancing enamel resistance, facilitating remineralization and improving restorative outcomes, the number of well-designed clinical trials remains limited. Consequently, the current evidence base is stronger in experimental settings than in real-world clinical applications, which represents a significant limitation of this consensus report.
The adoption of laser-assisted dentistry is limited by the high cost of devices, variability between systems, and the operator learning curve that requires specific training. The consensus was developed using a modified Delphi process, which ensured transparency, but did not include multiple structured voting rounds. Furthermore, much of the supporting evidence is derived from laboratory studies, while high-quality randomized controlled clinical trials remain scarce. Despite these limitations, this report provides the most comprehensive synthesis to date, and offers a valuable framework for future research and clinical guidelines.
While laboratory data provides encouraging support for the potential of laser-assisted caries prevention and management, robust clinical trials and long-term follow-up studies are still required to confirm these benefits and to establish standardized, evidence-based protocols for daily practice.
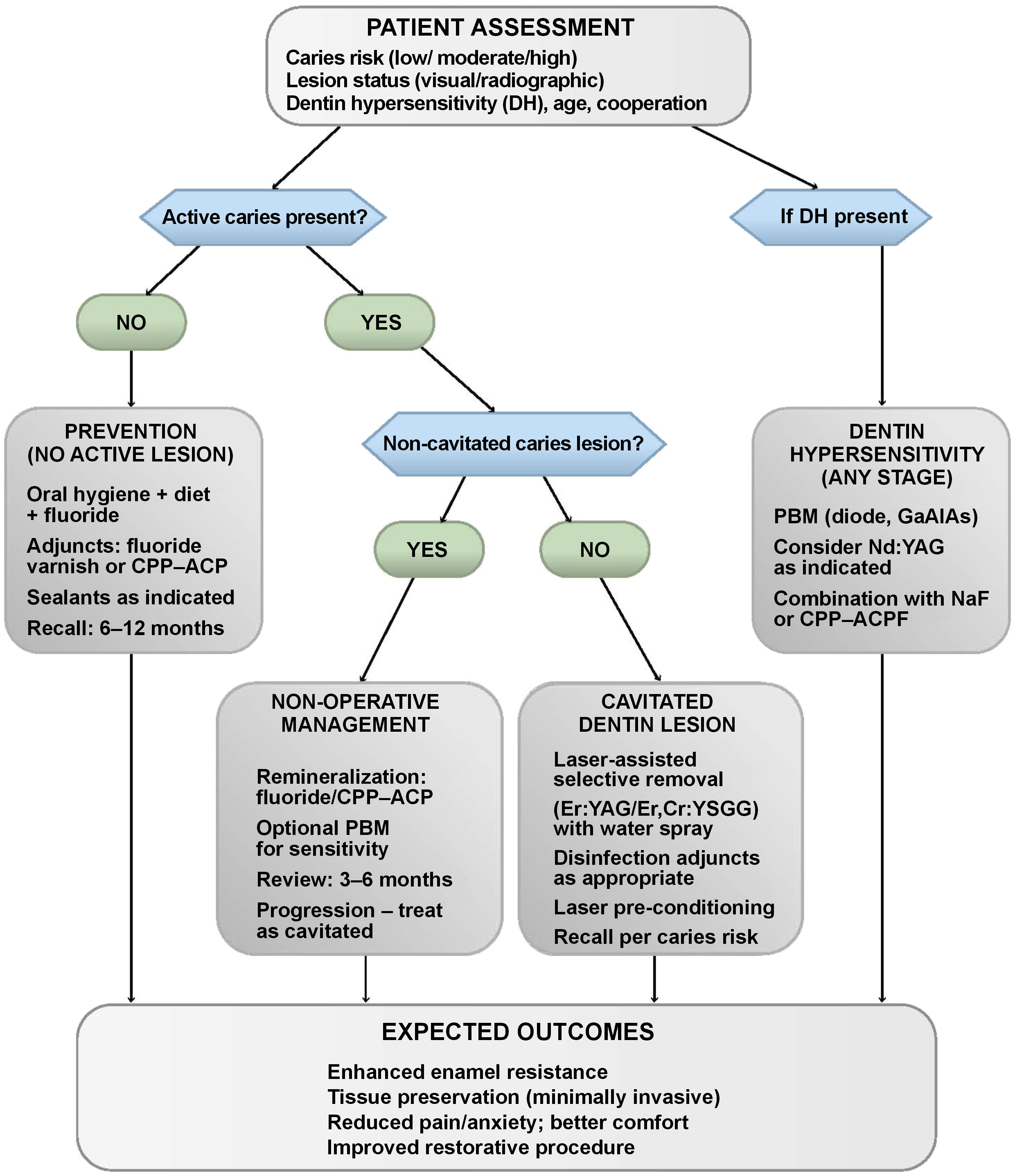
Figure 1 presents a flow diagram of the clinical decision-making process for laser-assisted caries treatment and prevention.
Ethics approval and consent to participate
Not applicable.
Data availability
Not applicable.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.