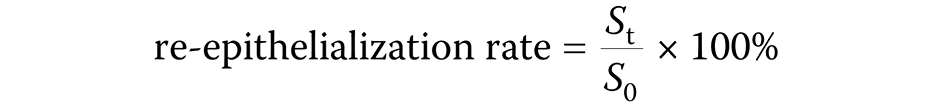Abstract
Background. Probiotics, known for their anti-inflammatory properties and ability to balance the oral microbiome, show promise in enhancing wound healing, particularly through topical application, in oral healthcare.
Objectives. The main objective of the present study was to investigate the topical application of probiotics to accelerate oral wound healing, focusing on key indicators, like collagen density, angiogenesis, the re-epithelization rate, the wound area, and the wound length.
Material and methods. Palatal wounds were induced in 60 male Sprague–Dawley® rats, which were divided into 4 groups. Probiotics, including Streptococcus salivarius K12 (BLIS K12®) and Lactobacillus reuteri (Interlac® Pro-D and Interlac®), were topically administered. The rates were sacrificed on days 3, 7 and 14 to evaluate the early, middle and late proliferation phases. Histopathological examinations assessed collagen density, angiogenesis, the re-epithelialization rate, the wound area, and the wound length.
Results. Probiotics showed beneficial effects on the oral wound healing indicators examined in this study. This study demonstrates the significant benefits of applying probiotics in enhancing wound healing throughout various proliferation stages. Our findings consistently highlight their positive impact across key indicators. With 3 different probiotic types, we observed improvement in all aspects of wound healing, from early to late stages.
Conclusions. The study underscores the potential of probiotics as effective agents in promoting wound repair and regeneration, offering promising avenues for enhanced clinical outcomes.
Keywords: Streptococcus salivarius, angiogenesis, probiotics, wound healing, Limosilactobacillus reuteri
Introduction
Oral and maxillofacial surgeons deal with surgeries in the mouth, jaw and face, which involves the intricate process of oral wound healing.1, 2 Oral wounds pose unique challenges due to the warm oral environment and the presence of abundant microorganisms. Wound healing, a complex physiological process involving various cell types, can be influenced by multiple factors. Understanding these intricacies is crucial for addressing the complexities of healing oral wounds within this surgical domain.3
The wound healing process in the oral cavity, especially in the proliferation phase, relies on several critical factors. In this phase, which is part of the 4 accurately programmed stages of wound healing – hemostasis, inflammation, proliferation, and remodeling or maturation, specific biophysiological mechanisms are activated.4 These include collagen production, the development of new blood vessels through angiogenesis, and the restoration of the protective epithelial layer via re-epithelialization. The interactions between these elements are intricately tied to the size and morphology of the oral wound, amplifying the complexity of the overall healing process.3
Advancement in wound care has led to the development of materials specifically designed for wound dressing. These materials not only aim to expedite the healing process, but also address the unique challenges posed by mucosal wounds within the oral cavity. Such advances offer promising avenues for enhancing the management and treatment strategies for oral wounds, marking a significant progression in oral cavity care and wound management.5
In recent years, research delving into the potential of probiotic agents in oral healthcare has seen a significant surge. Probiotics, renowned for their role in fostering a balanced and healthy oral microbiome, have demonstrated considerable promise due to their notable anti-inflammatory properties. This particular attribute presents an opportunity to potentially alleviate inflammatory responses, thereby potentially enhancing the subsequent stages of wound healing. Studies conducted in the field have validated that both the oral administration and injection of probiotics can yield benefits in wound healing, particularly observed in rat models, where these interventions have stimulated the inflammatory processes, aiding in the healing of wounds.6, 7 This revelation has sparked interest and optimism in leveraging probiotics as a therapeutic approach for wound management in oral healthcare. Furthermore, investigations have highlighted the superiority of topical treatment over systemic approaches in addressing the physical traumas and chemical injuries of the oral mucosa.8 This finding emphasizes the potential efficacy of localized application in the wound healing process within the oral cavity.
A deliberate push for further research in oral healthcare revolves around the topical application of probiotics to enhance the healing of oral wounds. This investigation aims to unravel the specific mechanisms through which topically applied probiotics can positively impact and expedite oral wound healing. By focusing on indicators like collagen density, angiogenesis and re-epithelialization, this research endeavors to deeply explore the interplay between probiotics and the intricate mechanisms governing oral wound healing. The ultimate goal is to develop innovative strategies that could revolutionize the management and treatment of oral wounds, offering promising new avenues in oral healthcare.
Material and methods
This research was conducted at the Veterinary and Biomedical Sciences Hospital and the Faculty of Veterinary Medicine and Biomedical Sciences of IPB University (Institut Pertanian Bogor), Bogor, Indonesia, from May to July 2023.
Probiotics
In this research study, 3 commercially available probiotics were employed: the Streptococcus salivarius K12 lozenge containing 1 billion colony-forming units (CFU) (BLIS K12®); Lactobacillus reuteri ATCC PTA 5289 at 1 × 108 CFU (Interlac® Pro-D) in lozenge form; and Lactobacillus reuteri DSM 17938 at 1 × 108 CFU provided in powder sachet form. It is worth noting that the preparation of BLIS K12 and Interlac Pro-D formulations involved grinding down in a mortar, leading to the transformation of all tested probiotics into a powdered state, which facilitated the experimental procedures.
Rats
Based on the Federer formula, it was determined that the minimum sample size per intervention is 3. In this study, 5 rats were used per intervention; therefore sixty 8-week-old Sprague–Dawley® male rats weighing between 200–300 g were utilized. The rats were randomly assigned to 4 distinct groups, with each group consisting of 15 rats: group 1, receiving treatment with BLIS K12; group 2, treated with Interlac Pro-D; group 3, administered with Interlac; and finally, group 4 as the control group. During the adaptation period lasting 7 days, the rats were given the prophylactic deworming medication albendazole at a dosage of 30 mg/kg body weight (b.w.) orally. This was aimed at ensuring the health of the rats and eliminating any parasitic infestation before commencing the research. The 60 rats were housed in 30 cages, with each cage accommodating 2 rats. The cages were plastic boxes measuring 30 × 40 cm, equipped with wood shavings as bedding. The rat housing was situated within a specially regulated room maintained at a temperature of 25 ±2°C, with humidity levels set at 55 ±10% and a lighting cycle of 12 h of bright light, followed by 12 h of darkness.
Wound model
Under sterile conditions, surgical wounds were created on the rats’ palates. Anesthesia for the experimental animals was induced by an intraperitoneal (i.p.) injection of Ketamine-Hameln® as the primary anesthetic, dosed at 95 mg/kg b.w. Additionally, xylazine was administered at 5 mg/kg b.w. to promote muscle relaxation and prolong the anesthesia. Both medications were injected into the rat’s abdominal area, using a 1-cc syringe equipped with a 30G needle, allowing approx. 5 min for the drugs to take effect. Following successful anesthesia, the rat’s palate was sterilized using gauze, forceps and 70% alcohol. A 5-mm diameter punch biopsy was then performed at the midline of the rat’s hard palate to create a wound, followed by swabbing the wound site until bleeding ceased. Notably, no analgesics or antibiotics were administered to the rats after the creation of the wound.
Application of treatment
After creating the wounds on the rats’ palates, the test groups were administered probiotics in powdered form, applied evenly to the wounds at a dosage of 4 mg, using a cotton pellet. Subsequently, a mucosal patch, Curatick™, was placed over the wounds. In the control group, only the mucosal patch was applied directly onto the wounds. This treatment was administered daily throughout the experimental period, and the mucosal patch was replaced daily. Then, 5 rats from each group were anesthetized and sacrificed on days 3, 7 and 14, using the exsanguination technique.
Observation of wound healing
Tissue samples were excised and fixed in Bouin’s fixative solution for 24 h. Subsequently, they were sectioned into pieces measuring 2 × 1 mm with a thickness of 2 mm, using a No. 12 surgical blade knife. Dehydration followed using 100% alcohol before embedding the tissue blocks in paraffin of a temperature of 70°C within a base mold. After setting, the tissues underwent cryotome sectioning and were placed on glass slides for subsequent staining. The excised wound areas were then fixed and subjected to histopathological examination involving hematoxylin and eosin (H&E) staining, as well as Masson’s trichrome staining. Quantitative analysis of collagen density, angiogenesis, re-epithelialization, the wound area, and the wound length was performed utilizing the ImageJ software (https://imagej.net/ij/index.html).
The method employed for assessing collagen density areas involved Masson’s trichrome staining, with the areas being identified by distinctive bluish coloration, and characterized by thick, wavy cytoplasm with transverse fibers and the absence of a nucleus. Quantification of the collagen density area [mm2] was accomplished by determining the total collagen area relative to the overall wound area in 6 distinct regions. Angiogenesis, visualized through H&E staining, showcased purple endothelial cells and red-colored erythrocytes. The measurement of angiogenesis was based on the total count of blood vessels identified in the 6 specified regions. The microscopic wound length was measured using the H&E-stained slides by calculating the distance between both wound edges, relying on the examination of the epithelial tissue under a microscope with ×4 magnification. The wound area was measured macroscopically by calculating the clinical wound area based on the clinical photographs of the rat wounds. A ruler was placed next to the wound in the photographs as a size reference, which was then used to measure the wound area accurately. Finally, the re-epithelialization rate was computed using a specific formula (Equation 1):
where:
St – residual wound area at the indicated time; and
S0 – initial wound area.
Blinding
Blinding was implemented during the allocation, outcome assessment and data analysis phases of the study. This approach was used to minimize bias, and ensure that the allocation of participants, the evaluation of outcomes and the interpretation of data remained objective and uninfluenced by the knowledge of the treatment groups.
Statistical analysis
The data was statistically analyzed using the IBM SPSS Statistics for Windows, v. 26.0 (IBM Crop., Armonk, USA). The normality of data distribution was tested using the Shapiro–Wilk test (p > 0.05), and the multivariate analysis of variance (MANOVA) with Tukey’s post hoc honestly significant difference (HSD) was performed to compare differences between the groups and across the necropsy days.
Results
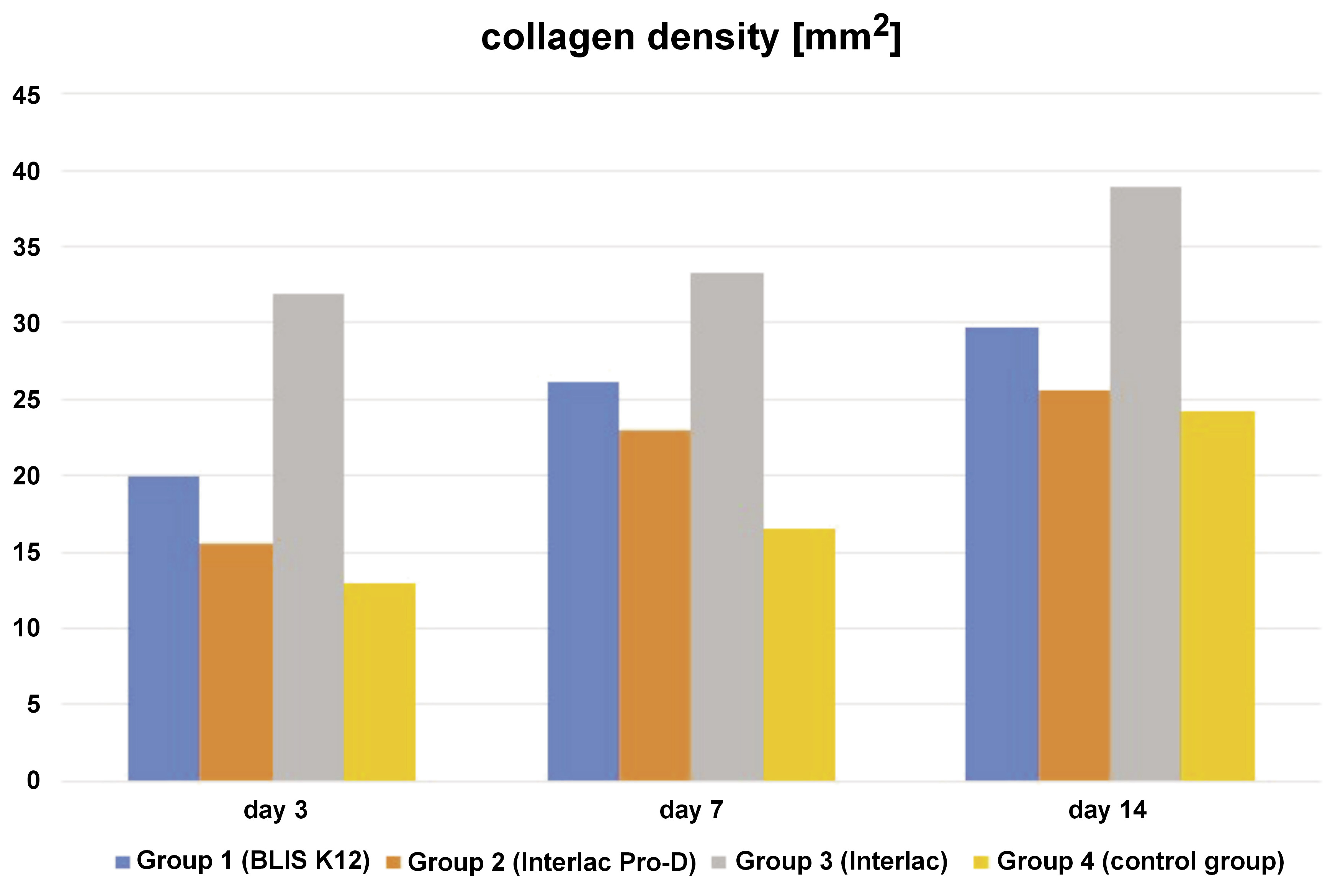
The data pertaining to collagen density, angiogenesis, the re-epithelialization rate, the wound area, and the wound length is displayed in Table 1. In a descriptive analysis of the collagen density variable, it was observed that there was an increase in collagen density across all groups, corresponding directly with the necropsy/observation days. The group of rats serving as controls and necropsied on the 3rd day exhibited the lowest collagen density value, whereas the group treated with the probiotic Interlac and necropsied on the 14th day showed the highest collagen density value, suggesting enhanced wound healing over time (Figure 1). This increase in collagen density corresponds with the body’s natural healing process, where collagen deposition plays a critical role in strengthening the wound.
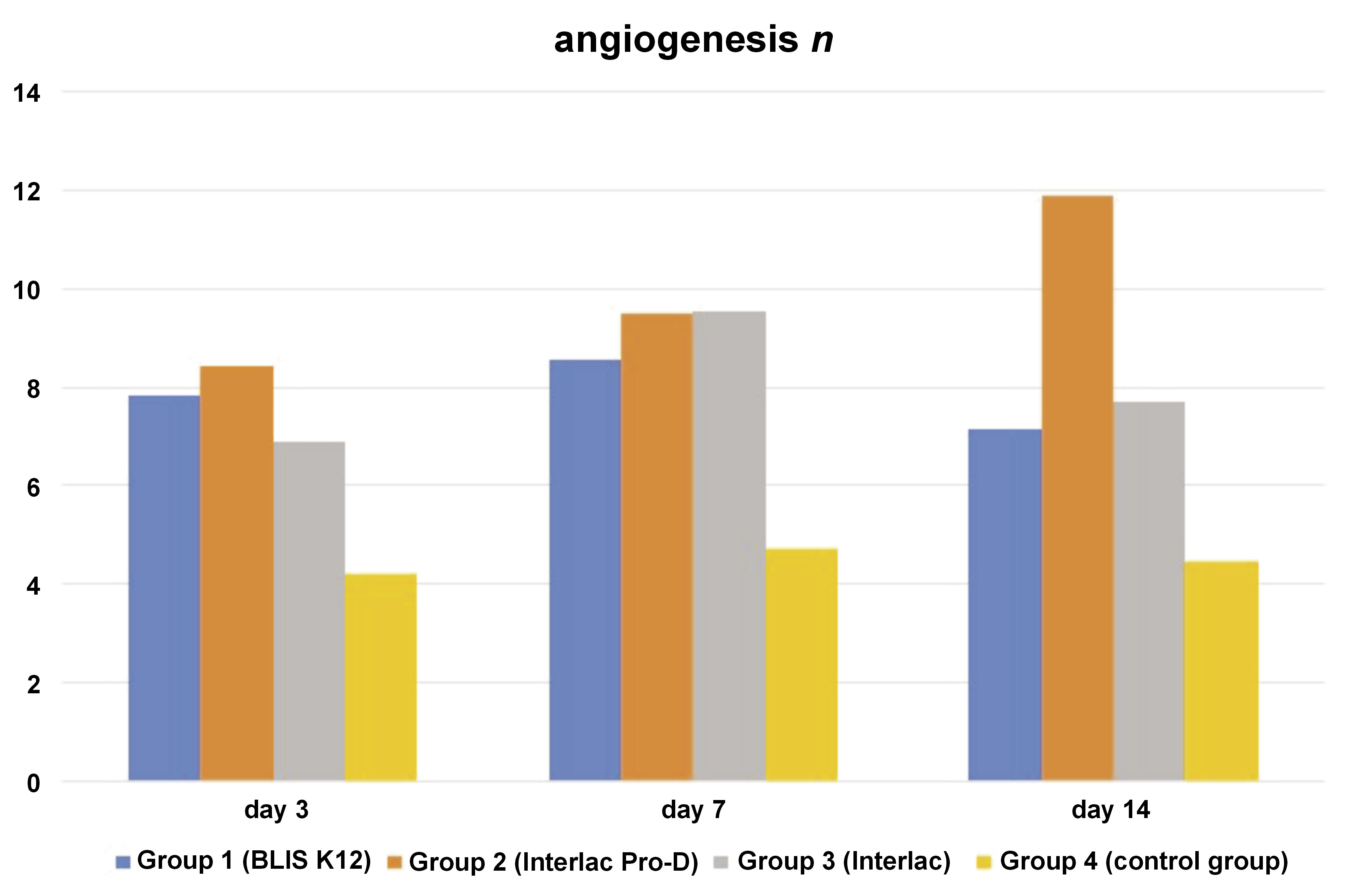
Regarding the dependent variable angiogenesis, there is a pattern of an initial increase followed by a slight decline; the lowest angiogenesis values appeared on day 3, gradually increasing by day 7, and slightly decreasing by day 14 (Figure 2). This trend likely reflects the peak of new blood vessel formation necessary for tissue repair, and subsequent stabilization as healing progresses. The Interlac Pro-D group exhibited the highest angiogenesis by day 14, indicating a robust vascular response that supports tissue regeneration.
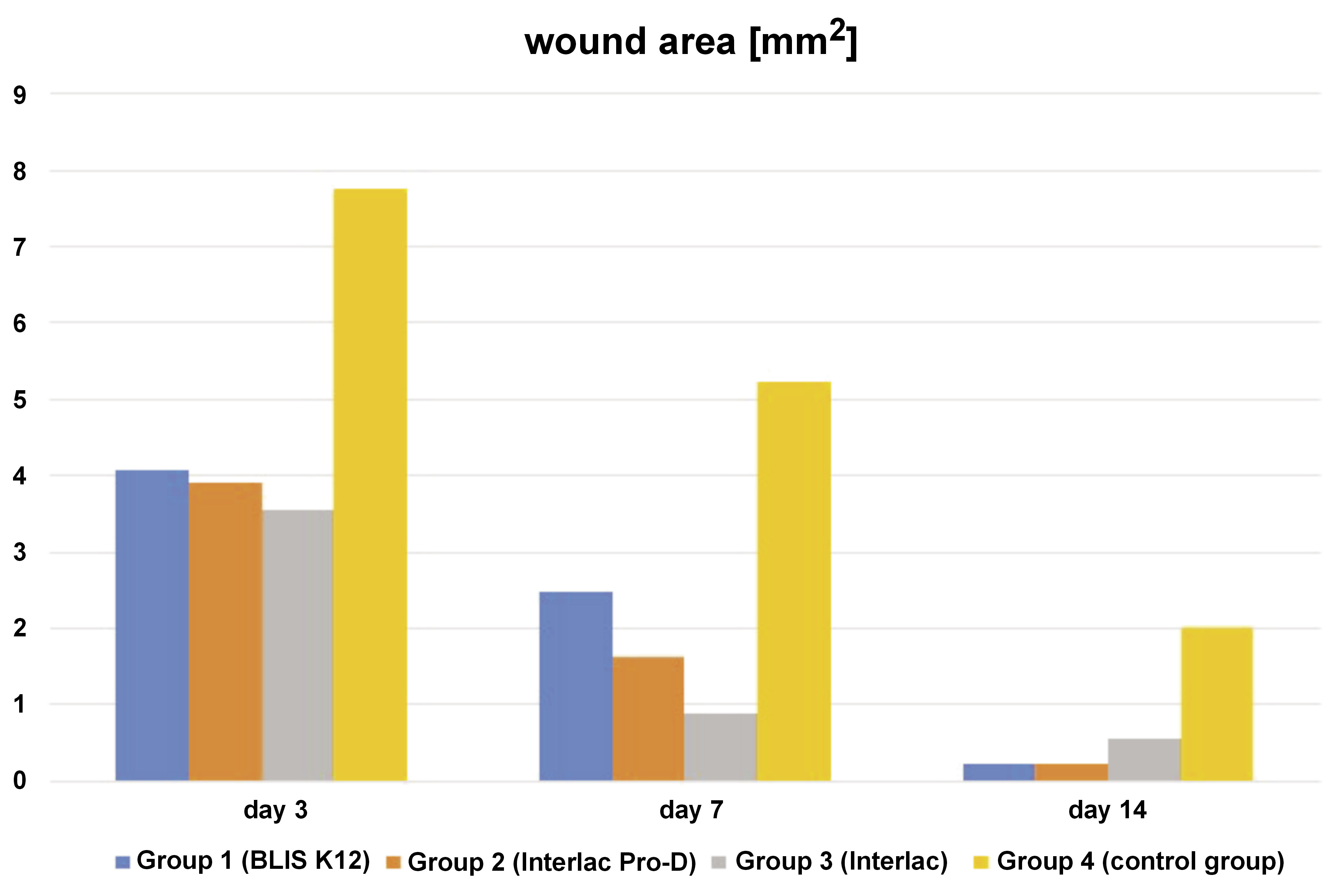
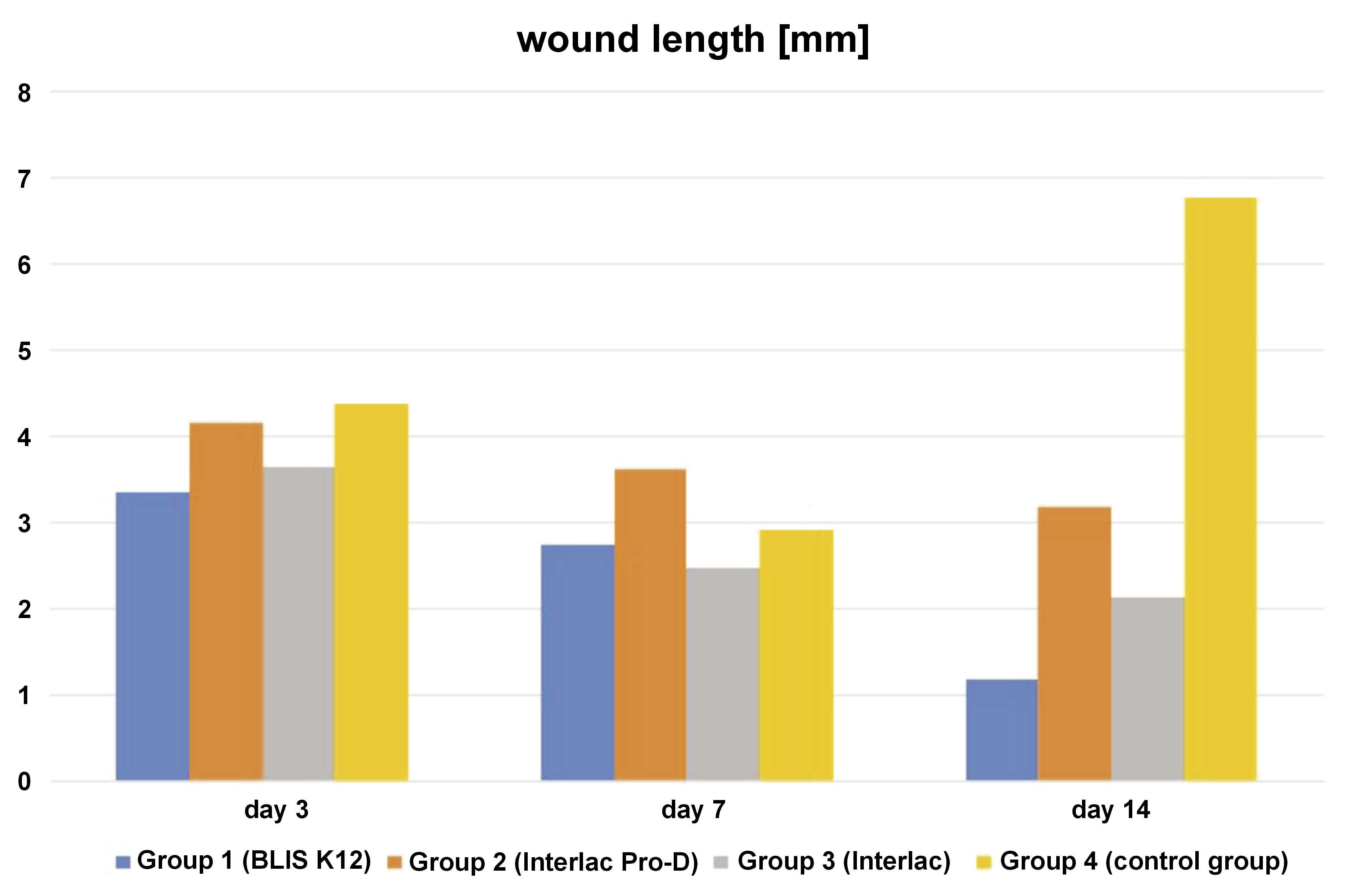
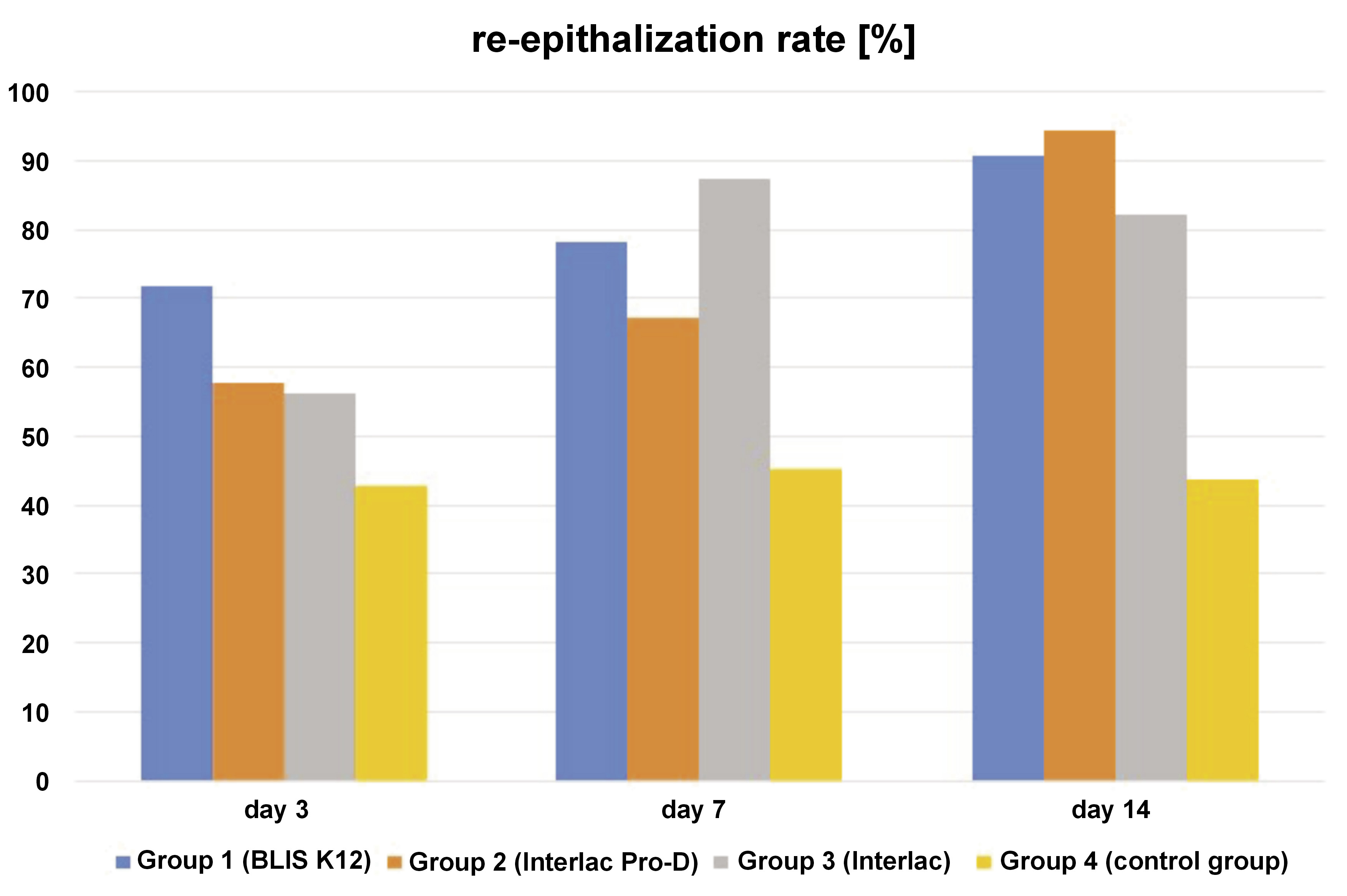
The dependent variable re-epithelialization rate logically shows an inverse relationship with the dependent variables wound area and wound length. Initially, a low re-epithelialization rate is expected, increasing gradually in subsequent days (Figure 3). Conversely, in the early days, the wound area and the wound length are expected to be large, gradually decreasing in the following days (Figure 4 and Figure 5). For the re-epithelialization rate, the lowest value was in the control group necropsied on the 3rd day, while the highest was in the group of rats treated with the probiotic Interlac Pro-D and necropsied on the 14th day. Regarding the wound area, the lowest value was in the group of rats treated with the probiotic Interlac Pro-D and necropsied on the 14th day, while the highest was in the control group necropsied on the 3rd day. As for the wound length, the lowest value was in the group of rats treated with the probiotic BLIS K12 and necropsied on the 14th day, while the highest was in the control group necropsied on the 14th day.
The Shapiro–Wilk test indicated that the data was normally distributed (p > 0.05). To compare the wound healing variables (collagen density, angiogenesis, the rate of re-epithelialization, the wound area, and the wound length) between the groups and across the necropsy days, MANOVA with Tukey’s post hoc HSD test was employed. The results of these analyses are presented in Table 2, Table 3, Table 4.
Table 2 presents the results of the MANOVA test aimed at evaluating the effects of 2 independent variables, namely the groups and the necropsy days, on 5 dependent variables: collagen density; angiogenesis; the re-epithelialization rate; the wound area; and the wound length. The analysis also assessed the interaction between the independent variables and their impact on the dependent variables. The MANOVA test revealed that all the dependent variables were significantly affected by different groups. Additionally, with the exception of angiogenesis, all the dependent variables show significant differences across different necropsy days. However, the interaction between the groups and the necropsy day did not have a significant effect on any of the dependent variables. The lack of a significant interaction between the groups and the necropsy days suggests that while each factor individually affected the healing outcomes, their combined influence did not further alter the patterns observed.
Table 3 shows the results of Tukey’s post hoc HSD test for intergroup comparisons. It reveals significant differences in all the dependent variables when comparing group 4 (the control group) with the other treated groups (groups 1–3), except for collagen density when comparing group 4 (control) with group 2 (Interlac Pro-D). Interestingly, when comparing between the treated groups, most of the dependent variables showed no significant differences. However, there are exceptions: collagen density showed significant differences when comparing group 1 (BLIS K12) with group 3 (Interlac), as well as group 2 (Interlac Pro-D) with group 3 (Interlac). Additionally, the wound length exhibited significant differences when comparing group 1 (BLIS K12) with group 2 (Interlac Pro-D), and when comparing group 2 (Interlac Pro-D) with group 3 (Interlac).
Table 4 displays the outcomes of Tukey’s post hoc HSD test for comparisons among the necropsy days. The analysis indicated significant differences in collagen density between each pair of necropsy days (day 3 and day 7, day 3 and day 14, and day 7 and day 14). However, angiogenesis did not exhibit any significant differences between the various necropsy day pairs. Notably, the re-epithelialization rate and the wound area demonstrated significant differences between necropsy day 3 and day 7, as well as between necropsy day 3 and day 14, but not between necropsy day 7 and day 14. Similarly, the wound length displayed a significant difference between necropsy day 3 and day 7, but no significant differences were observed between necropsy day 3 and day 14, or between necropsy day 7 and day 14.
Discussion
Intraoral wounds, prone to bacterial contamination, often result in postoperative complications, such as wound dehiscence, infection and pain.1 However, effectively managing these problems remains a challenge. While conventional treatment, like silver dressings, have been historically used, recent research questions their effectiveness in promoting wound healing. Topical antibiotics, though frequently prescribed, can lead to resistance and skin irritation. Similarly, iodine has been associated with cellular toxicity. Conversely, topical probiotics offer broad antimicrobial activity with minimal systemic side effects and effectively inhibit biofilm formation. Both human and animal studies have shown promising results in using probiotics to enhance wound healing.9
Probiotics and their byproducts are gaining attention for their ability to help the body heal by regulating vital biological processes. Probiotics can be applied directly to the wound or taken orally.6 However, using active ingredients systemically, either through the mouth or by injection, has drawbacks, like needing higher doses for effectiveness.10 Recent studies show that applying probiotics directly to wounds can reduce bacterial growth and speed up tissue repair.6 Based on these reasons, this study was undertaken utilizing probiotics applied topically.
The hypothesis that probiotics L. reuteri and S. salivarius K12 can enhance the wound healing process is based on their ability to inhibit the formation of pathogenic bacterial biofilms.11, 12 Biofilms often act as a physical barrier that hinders the migration of epithelial cells, which is essential for wound closure. This delayed epithelialization is a hallmark of chronic wounds, contributing to slower healing.13 Additionally, the presence of biofilms can trigger an excessive inflammatory response, leading to prolonged inflammation and subsequent tissue damage. Chronic inflammation further impedes the wound healing process.14 By inhibiting biofilm formation, L.reuteri and S. salivarius K12 may help reduce inflammation and promote faster epithelialization, ultimately enhancing the overall wound healing process.13, 14
The use of antibiotics and analgesics can significantly influence the wound healing process. Antibiotics have been shown to suppress key inflammatory mediators, such as interleukin 1 beta (IL-1β), C-C motif chemokine ligand 2 (CCL2) and interferon alpha/beta (IFN-α/β), potentially leading to delayed wound healing. Moreover, although studies on non-steroidal anti-inflammatory drugs (NSAIDs) have yielded mixed results, it is well-established that NSAIDs inhibit the COX pathway, which plays a critical role in the proliferation phase of wound healing. To avoid potential bias, no antibiotics or analgesics were used in this study. The absence of these medications did not affect the wound healing process, as normal wound healing morphology was observed in both groups, and none of the rats exhibited signs of secondary infection. Ethical considerations were strictly adhered to, ensuring that the well-being of the rats was monitored closely throughout the study.10
One notable limitation of this study is its reliance on Sprague–Dawley rats as the experimental subjects, which may not fully reflect the physiological responses of humans. Despite the differences between humans and Sprague–Dawley rats in oral wound healing, this rat strain remains a valuable model for pilot studies. Their biological processes, particularly the fibroblast growth factor (FGF) pathways, closely resemble those of humans and provide useful insights into oral wound repair.15, 16 However, the inherent differences in metabolism, immune responses and the speed of wound healing between rats and humans can influence the outcomes, and these variations may affect the generalizability of findings to human subjects. While Sprague–Dawley rats are practical for understanding the basic mechanisms of tissue regeneration and wound contraction, further research on human subjects is crucial to confirm the applicability and clinical relevance of these results.17
Additionally, the presence of outliers in the data introduces the potential for bias in the analysis of certain variables, which could influence the interpretation of results. However, it is essential to emphasize our commitment to transparently and honestly reporting the data within this study, ensuring that the findings presented reflect the genuine observations made during the research process. Despite these limitations, this study lays valuable groundwork for future investigations aimed at understanding the potential effects of probiotics in wound healing.
Given the potential of probiotics and notwithstanding the aforementioned limitations, further research into probiotics as alternative, safe antimicrobial agents for wound care is still imperative. This study aims to explore the effects of commercially available probiotics on wound healing, particularly focusing on the proliferation phase. Assessment at the 3rd, 7th and 14th days post-application provides insight into their impact during the early, mid and late stages of proliferation, respectively.
The statistical analysis demonstrated that the interaction between the treatment groups and the necropsy days did not exert a significant effect on any of the dependent variables, encompassing collagen density, angiogenesis, the re-epithelialization rate, the wound area, and the wound length. This suggests that the influence of different treatment groups remained consistent throughout the evaluation days, indicating stable effects regardless of the assessment time. This underscores the reliability and consistency of the observed outcomes across the study timeline. However, the application of probiotics still significantly impacted these variables, as evidenced by the findings. Therefore, each variable will be discussed in detail in the following subsections.
Collagen density
Notably, group 4 (control group) exhibited the lowest collagen density across all evaluation days, suggesting a discernible effect of probiotics on this metric. Statistical analysis revealed significant differences between the control group and both group 1 (BLIS K12) and group 3 (Interlac), indicating their effectiveness in enhancing collagen density. Surprisingly, while group 2 (Interlac Pro-D) demonstrated a tendency toward higher collagen density as compared to the control group, the difference was not statistically significant, suggesting limited efficacy in this context. Of particular interest is the observation that group 3 (Interlac) displayed the highest collagen density among all groups, with a statistically significant difference as compared to other groups. This underscores the superior effectiveness of Interlac in promoting collagen density during wound healing within the scope of this study.
A previous study conducted by Moraes et al. investigated the effects of both live probiotic and paraprobiotic forms of Interlac Pro-D on the collagen levels in periodontitis-induced rats.18 Their findings indicated that the live probiotic Interlac Pro-D did not significantly alter collagen amounts, while the paraprobiotic forms containing non-viable L reuteri DSM 17938 and ATCC PTA 5289 showed a significant increase in collagen production.18 This aligns with the results of our study, which also found that Interlac Pro-D did not significantly affect collagen density. Furthermore, previous research by Garcia et al. demonstrated improvement in the collagen levels in the probiotic-treated group using Interlac as compared to both the control group and those treated with systemic saline solution.19 These findings parallel our own, highlighting the potential of Interlac in enhancing collagen production. Our research revealed that the topical application of L. reuteri DSM 17938 can enhance collagen production, although combining this strain with others, such as ATCC PTA 5289, may yield different results. Additionally, the potential influence of S. salivarius K12 on collagen deposition remains largely unexplored. However, the results of this study serve as a foundational or pilot project, providing insight into the notion that the probiotic S. salivarius K12 may indeed enhance collagen production in oral wound healing.
The findings of this study indicate a progressive increase in collagen density over time, particularly in the early, middle and late proliferation phases. Statistical analysis further corroborates these results, demonstrating significant differences in collagen density among all evaluation days. This observed temporal variation suggests a dynamic process of collagen deposition during the course of wound healing. The underlying mechanism driving this phenomenon can be attributed to the heightened fibroblast activity essential for tissue repair, particularly during the granulation phase.20
Angiogenesis
The benefits of probiotic bacteria for wound healing, including their potential role in promoting angiogenesis, have been suggested in previous research.20 Angiogenesis, a crucial aspect of the wound healing process, involves a meticulously orchestrated series of biological events. These events facilitate the recruitment of inflammatory cells and the production of cytokines, matrix-degrading enzymes, and chemokines, ultimately leading to the formation of new capillaries from the existing ones.21 This research provided a valuable perspective on the impact of various probiotics on angiogenesis during the proliferation phase of wound healing. The findings indicate that the angiogenesis levels in the control group were consistently lower as compared to all other groups across all evaluation days.
Statistical analysis further confirms substantial differences in angiogenesis between the control group and all other groups, suggesting that the topical application of the 3 types of probiotics investigated in this study does indeed influence angiogenesis. However, intriguingly, there were no statistically significant differences observed among the probiotic groups themselves. This implies that each of the 3 probiotics utilized in this research yields similar outcomes in enhancing angiogenesis during the proliferation phase of wound healing.
Consistent with the findings of this study, Zhou et al. reported in their research that the utilization of the probiotic L. reuteri led to an increase in the CD31 and vascular endothelial growth factor (VEGF) levels, both of which are proteins associated with angiogenesis.22 Moreover, their study revealed that the application of metal-phenolic self-assembly shielded L. reuteri in a reinforced hydrogel further enhanced the expression of these proteins as compared to using L. reuteri alone. These results suggest that the incorporation of probiotics, particularly in novel delivery systems such as hydrogels, holds promise for augmenting angiogenesis and potentially improving wound healing outcomes.22
Limited in vivo and in vitro studies have suggested that certain probiotics, whether in live form or in a bacterial culture supernatant, can locally stimulate angiogenesis. They have been shown to induce the production of VEGF, thereby promoting endothelial cell growth and migration.23 However, no study on the influence of S. salivarius K12 on angiogenesis has been found. This research can provide foundational knowledge that the effect of enhancing angiogenesis by S. salivarius K12 is comparable to that of L. reuteri, which has been previously researched.
Moreover, the lack of significant differences in angiogenesis across time points may be due to the nature of angiogenesis itself, which typically peaks early in the wound healing process, around days 3 to 7, when new blood vessels are formed to support tissue regeneration. By day 14, angiogenesis may have already stabilized, meaning that further changes in blood vessel formation are minimal, which could explain why no significant differences were observed at the later necropsy days.10
Re-epithelialization rate, wound area and wound length
The wound area and length exhibit an inverse relationship with the level of re-epithelialization and fibroblast activity. Therefore, it is unsurprising to observe in this research that there is an inverse correlation between the re-epithelialization rate and both the wound area and the wound length. This is because a higher rate of re-epithelialization leads to smaller wound areas and lengths.10 In this study, statistically significant differences were found in the re-epithelialization rate, the wound area and the wound length between the control group and the 3 treatment groups receiving different types of probiotics. This indicates that the topical application of the 3 types of probiotics on oral wounds can enhance re-epithelialization and expedite wound closure, as evidenced by the reduced wound area and shorter wound length over time.
This study revealed that all 3 types of probiotics showed no significant differences in enhancing re-epithelialization and reducing the wound area. However, notable variations emerged regarding the wound length, with significant differences observed between group 2 (Interlac Pro-D) and both group 1 (BLIS K12) and group 3 (Interlac). This suggests that Interlac Pro-D may be less effective in reducing wound length as compared to BLIS K12 and Interlac. Conversely, no statistical differences were observed in the effect of reducing the wound length between group 1 (BLIS K12) and group 3 (Interlac), indicating comparable effects for both these probiotics.
The difference in outcomes between the wound area and length measurements could be due to the methodology employed for wound assessment. The wound length is determined by measuring the longest linear dimension within the wound, which may not offer such a precise measurement as in the case of the wound area in fully capturing the overall dimensions of the wound. This discrepancy arises because, even if the wound area seems small, variations in the wound width could result in a greater length measurement.24
Moysidis et al. in their study concluded that their experimental findings support the notion that the manipulation of the wound environment using beneficial bacteria, such as probiotics, positively impacts the healing process.25 In their research, they utilized Lactobacillus plantarum UBLP-40, as well as a combination of Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64. The authors further stated that probiotics operated through various, potentially unique mechanisms that are specific to each strain, yet collectively lead to faster wound healing.25 These findings align with our own research, which demonstrates that the topical application of 3 probiotic strains on oral wounds enhances re-epithelialization and expedites wound closure.
On the other hand, Öhnstedt et al. demonstrated the efficacy and tolerability of the novel drug candidate resulting from the significant development of Limosilactobacillus reuteri R2LC.26 This strain has been genetically modified to encode human CXCL12 1 alpha, leading to the creation of the drug candidate ILP100. This innovative approach has facilitated the development of ILP100 in a freeze-dried formulation. Their research revealed that topical treatment with ILP100 significantly accelerated the healing process of full-thickness wounds in minipigs.26
Conclusions
In conclusion, the present study demonstrates the significant benefits of probiotics in enhancing wound healing across various timing of the proliferation phase. Through the analysis of 5 key aspects, including collagen density, angiogenesis, the re-epithelialization rate, the wound area, and wound length, our findings reveal a consistent positive impact of probiotic use. Utilizing 3 distinct types of probiotics, we observed improvement in all aspects of wound healing, from the early to late stages of proliferation. This underscores the potential of probiotics as effective agents in promoting wound repair and regeneration, offering promising avenues for enhancing clinical outcomes in wound management. Further research and clinical trials are warranted to elucidate the specific mechanisms underlying the therapeutic effects of probiotics, and to optimize their utilization in clinical practice.
Ethics approval and consent to participate
The study protocol was approved by the Animal Ethics Committee at the Faculty of Veterinary Medicine and Biomedical Sciences of IPB University (Institut Pertanian Bogor), Bogor, Indonesia (approval No. 108/KEH/SKE/IX/2023).
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.