Abstract
Background. Nasal obstruction in pediatric patients can lead to serious issues, such as facial growth alterations, otitis media with effusion, and sleep disorders. Diagnosing nasal obstruction is challenging because subjective evaluations are often inaccurate, and objective measures like rhinomanometry are difficult to perform in children. This study proposes using an oral screen test as a rapid and cost-effective diagnostic method.
Objectives. The aim of the study is to validate the oral screen test as a method for diagnosing nasal obstruction in children. This objective is based on the observation that children with nasal obstruction do not tolerate the oral screen test well.
Material and methods. The validation of the diagnostic test was assessed based on the results of 104 children aged 4–15 years undergoing rhinomanometry. A silicone oral screen (Forwardontics®) and the Spanish version of the Sinus and Nasal Quality of Life Survey (SN-5) were used.
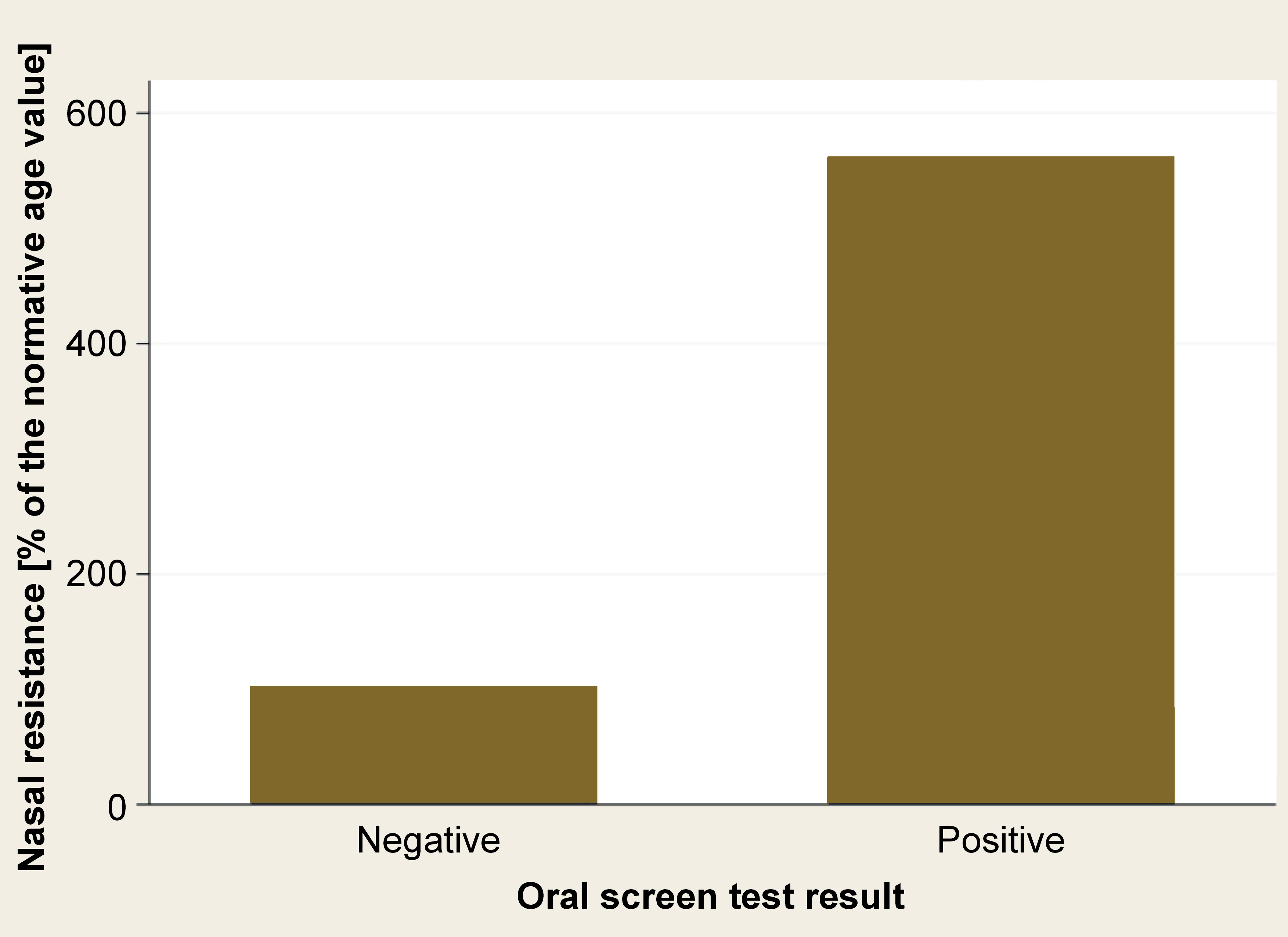
Results. The oral screen test yielded a positive result in 78 participants (75%). The children with a positive test result exhibited higher nasal resistance (561.3 ±140.5%) than those with a negative test result (102.0 ±3.4%), with an odds ratio (OR) of 18.5 (95% confidence interval (CI): 5.5–64.1).
Conclusions. The oral screen test is a highly sensitive and moderately specific method for diagnosing nasal obstruction in children, making it a useful screening tool in clinical practice.
Keywords: rhinomanometry, nasal obstruction, rhinitis, oral screen, SN-5
Introduction
Nasal obstruction is a common complaint in general pediatric consultations, otolaryngology, as well as pediatric odontology, either directly or indirectly due to its consequences, such as facial growth alterations,1 otitis media with effusion,2 and sleep disturbances.3
However, nasal obstruction is challenging to diagnose.4 Subjective assessment is not enough, as children and their parents often lack the capacity to accurately self-diagnose nasal patency,5 and symptoms are not always related to objective nasal obstruction.6 Objective measures such as rhinomanometry, which is considered the gold standard, are not universally feasible due to the need for child collaboration and the time-consuming nature of the procedure.4
As children with nasal obstruction are forced to mouth breathe, it is supposed that they will not tolerate mouth taping. A simple method to force nasal breathing is the use of oral screens. Oral screens are common instruments in orthodontic consultations. These silicone pieces are designed to be placed between the cheeks, the lips and the teeth.7 Oral screens are mainly used to prevent the perioral muscles from exerting forces on the teeth. The instruments block the oral air passage and force nasal breathing. Based on the observations from daily consultations, children with nasal obstruction do not tolerate oral screens and dismantle them. In consequence, it is hypothesized that the oral screen test may serve as a fast screening test to diagnose nasal obstruction.
The present research is designed with the aim of assessing the oral screen test as a diagnostic method of nasal obstruction.
Material and methods
Study sample
The diagnostic test validation method was followed. Before performing the physical examination, the parents were requested to provide informed consent for their children’s participation in the study. The data collection process was planned before the index test and reference standard were performed.
Inclusion criteria
The inclusion criteria were as follows: 4–15 years of age; children attending the pediatric otolaryngology unit at the University Hospital Complex of Santiago de Compostela, Spain, and undergoing rhinomanometry.
The participants were selected consecutively from January 2022 to January 2023. All subjects undergoing rhinomanometry were included, irrespective of the indication for the procedure.
Exclusion criteria
The exclusion criteria encompassed children whose parents declined to participate in the study, subjects not collaborating during rhinomanometry, and those for whom rhinomanometry could not be performed (e.g., due to complete nasal obstruction, septal perforation).
The age limit was set at 4 years, as children under this age usually do not collaborate during rhinomanometry. The superior age limit was established at 15 years, as in Spain, this is the maximum age at which a patient may be considered pediatric.
The sample included healthy children undergoing a health-related consultation prior to the initiation of pediatric orthodontics, postoperative controls, as well as children with symptoms of nasal obstruction due to nasal septum deviation, adenoid hypertrophy, or rhinitis. The study population comprised healthy children in order to encompass the entire spectrum of nasal patency for the external validation of the diagnostic test.
Validation
The oral screen test was the primary evaluation, with rhinomanometry established as the gold standard. True positives were defined as cases where nasal resistance exceeded 100% of the normative data for the child’s age. True negatives (controls) were defined as children with an unobstructed nose, showing nasal resistance below 100% of the normative data for their age. For the external validation process, a contingency table was used to assess sensitivity, specificity, as well as positive and negative predictive values.
Oral screen test
A silicone oral screen (Up-Locker Vacuum Activator; Forwardontics®, San Mateo, USA) was used (Figure 1). It was recommended that children keep the oral screen in their mouth for 2 min, ensuring that their lips were firmly closed. The examiner observed the children, as some subjects may leave their lips apart and breathe through the mouth even with the oral screen. The participants were informed that they can stop the test if they experienced difficulty breathing through their nose. If a child tolerated the test, it was marked as negative. If the test was not tolerated, it was recorded as positive.
The test was performed before rhinomanometry to prevent observation bias.
Physical examination
The direct examination by nasofibroscope is currently considered the gold standard for the evaluation of adenoid and turbinate hypertrophy.8 Turbinates were classified based on the study by Camacho et al.,6 while adenoids were classified according to the study by Cassano et al.7 The assessment of septal deviation was determined based on the study by Mariño-Sánchez et al.8
Adenoid hypertrophy was defined as a Cassano score greater than 2,9 turbinate hypertrophy as a full Camacho score greater than 4,10 and obstructive septal deviation as a Mariño-Sánchez score of 2.11
Sinus and Nasal Quality of Life Survey (SN-5)
In this study, the Spanish version of the Sinus and Nasal Quality of Life Survey (SN-5) was used.12 The survey evaluates 5 clusters of symptoms, namely sinus infection, nasal obstruction, allergy, emotional distress, and activity limitations.13 Each cluster contains symptoms that have been selected to assist parents in comprehending the nature of the assessment. Each cluster is rated on a 7-point Likert scale, ranging from 0 (never) to 6 (all the time). In addition to the symptomatic evaluation, caregivers were instructed to evaluate the child’s overall quality of life on a visual analog scale (VAS) ranging from 0 (worst possible) to 10 (best possible).
Rhinomanometry
The recommendations of the International Committee on Standardization of Rhinomanometry were followed throughout the study.11 Rhinomanometry was performed after 30 min of acclimatization, in a room where humidity was constant and the temperature regulated with a thermostat. The results were assessed using a reference pressure gradient across the nose of 150 Pa.14
Following the recommendations outlined in previous reports,15 the results of nasal resistance were standardized according to pediatric reference values for each age subgroup.16 The nasal resistance value that corresponded to the normative value of an age subgroup was designated as 100%. The results falling below 100% corresponded to children with unobstructed nasal passages, while the results exceeding 100% corresponded to subjects with increased levels of resistance. For the contingency table, 110% was selected as the resistance value of a patient with nasal obstruction.
In cases where rhinomanometry could not be performed due to severe obstruction, the data was designated as missing.
Statistical analysis
The normality of the quantitative variables was assessed through the implementation of the Shapiro–Wilk test. A comparison between quantitative and dichotomic variables was performed using the t-test for a normal distribution or the non-parametric variation rank sum test for a non-normal distribution. The statistical significance was set at p < 0.05. The statistical analysis was conducted using Stata 17 (StataCorp LLC, College Station, USA).
Results
Description of the study sample
After the selection process, a total of 104 participants were included in the study: 75 individuals with known obstructive nasal disorders; and 29 healthy participants. Diagnosed obstructive nasal disorders included hypertrophic rhinitis (n = 68), adenoid hypertrophy (n = 46) and septal deviation (n = 5).
The results pertaining to age, sex, nasal resistance, and SN-5 are summarized in Table 1.
Oral screen test results
All the initially included participants underwent the oral screen test. The test yielded a positive result in 78 participants (75%).
Nasal resistance was higher in patients with a positive test result (561.3 ±140.5%) than those with a negative result (102.0 ±3.4%), with an odds ratio (OR) of 18.5 (95% confidence interval (CI): 5.5–64.1) (Table 2) (Figure 2).
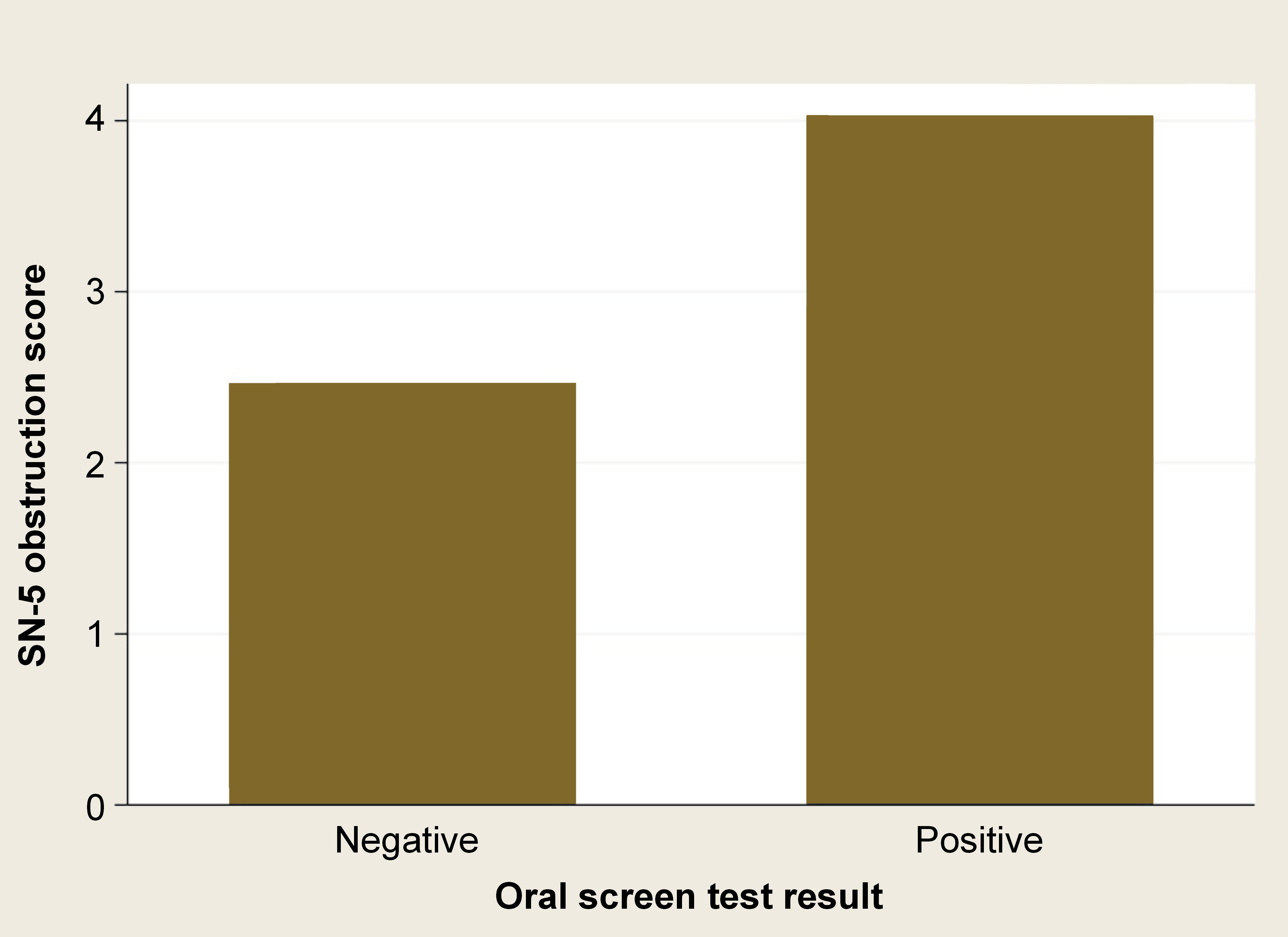
The SN-5 subdomain of nasal obstruction was higher in subjects with a positive test result (z = −4.38; p < 0.001) (Table 3) (Figure 3).
Discussion
The present study validated the oral screen test as an effective tool for diagnosing nasal obstruction in children, obtaining high sensitivity and a moderate to high specificity. The children with a positive result were found to be 18.5 times more likely to have nasal obstruction.
The oral screen test is not intended to substitute any other objective test. It has been developed as a fast, inexpensive and innocuous method to identify children at risk of nasal obstruction. If nasal obstruction is suspected, a specialist must be consulted to confirm the diagnosis and determine its exact cause.
For a diagnostic test to be considered suitable for screening purposes, it must meet specific criteria. Firstly, the evaluation must be affordable, easy to perform, innocuous, and have high sensitivity.17 Secondly, the illness or condition under investigation must be highly prevalent, with early treatment altering the course of the disease. The oral screen test fulfills all these characteristics.
Pediatric nasal obstruction is a prevalent symptom that merits attention and prompt diagnosis. The exact prevalence of pediatric nasal obstruction remains unknown. However, some of its most common causes have been well studied. In Spain, for instance, rhinitis has been documented in 39% of the population,18 while adenoid hypertrophy has been diagnosed in 42%.19
Nasal obstruction has been associated with several conditions, including caries,20 middle ear disease, sinusitis, and alterations in facial growth. The early treatment of nasal obstruction has been related to the improvement in facial growth,21 middle ear ventilation,22 and the reduction of otitis media23 and sinusitis.24
Despite its moderate specificity, the current study demonstrated a satisfactory level of sensitivity. This finding indicates that the test accurately identified the majority of children with nasal obstruction; however, several healthy children were misdiagnosed as obstructed. This phenomenon is presumably attributed to the fact that some children were restless during the test, stating that they could not appropriately breathe only to be authorized to remove the oral screen.
Regarding the cost of the procedure, it must be noted that, in our practice, we sterilize the oral screens. However, the utilization of single-use screens has the potential to result in an unacceptable level of plastic waste.
It was challenging to choose the gold standard test to compare against the oral screen test. Physical examination is widely regarded as the preferred method for diagnosing nasal obstruction. However, this technique can diagnose causes, but not the obstruction itself. The diagnosis of nasal obstruction can be made on the basis of either a subjective complaint, or an objective method. The subjective assessment by parents or children has been proven to be inadequate, as children and their parents often misdiagnose their own symptoms.22 Several objective tests are available, each with its own advantages and limitations, such as rhinomanometry, acoustic rhinometry, rhinohigrometry, and nasal peak flow. Despite its limitations, the most widely accepted gold standard procedure is rhinomanometry.4
Rhinomanometry is criticized due to the low rate of collaboration exhibited by pediatric patients. Despite being true in some cases, most patients over 4 years of age can cooperate during rhinomanometry. Rhinomanometry is a time-consuming test. Both of these reasons justify using the oral screen test.
Another critique of rhinomanometry is that its reference values vary with age. Therefore, we have used normative data for each age subgroup in order to use relative values. This approach enabled the comparison of measurements of all the children despite their different ages.
The third limitation asserts that there is a low correlation between subjective measurements and rhinomanometric values. This claim is generally valid. However, in this study, we have assessed the quality of life via the SN-5. The study found that children with a positive oral screen test exhibited worse results in the SN-5 and the nasal obstruction subdomain of SN-5 (Table 3). Despite these outcomes, the overall quality of life assessed using the VAS score did not reach statistical significance. This is in contrast with previous studies, as most available evidence reported a low correlation between both measures. This phenomenon can be attributed, at least in part, to the fact that a number of children under study were regular patients and were more aware of their own bodies and illnesses. However, the absence of an association between the 2 measurements should not be a limitation for diagnosing nasal obstruction alone, independent of symptoms, as children are treated not only based on their self-reported symptoms. Nasal breathing is in itself a favorable outcome. As previously discussed, adequate nasal breathing promotes optimal facial growth, dental health, and reduced incidence of middle ear illnesses.24 Therefore, the objective of diagnosing and treating nasal obstruction could be complementary to diagnosing and treating its symptoms.
Another highly debated question in pediatric rhinomanometry is whether the procedure should be anterior or posterior. In the anterior rhinomanometry, the differential pressure is measured anterior to the adenoids. However, in the posterior rhinomanometry, the pressure is measured in the oropharynx, inferior to the adenoid pad. Conceptually, posterior rhinomanometry is a superior method for diagnosing nasal obstruction caused by adenoid hypertrophy. However, we decided to use anterior rhinomanometry, given that children tend to be less collaborative with the posterior method.25
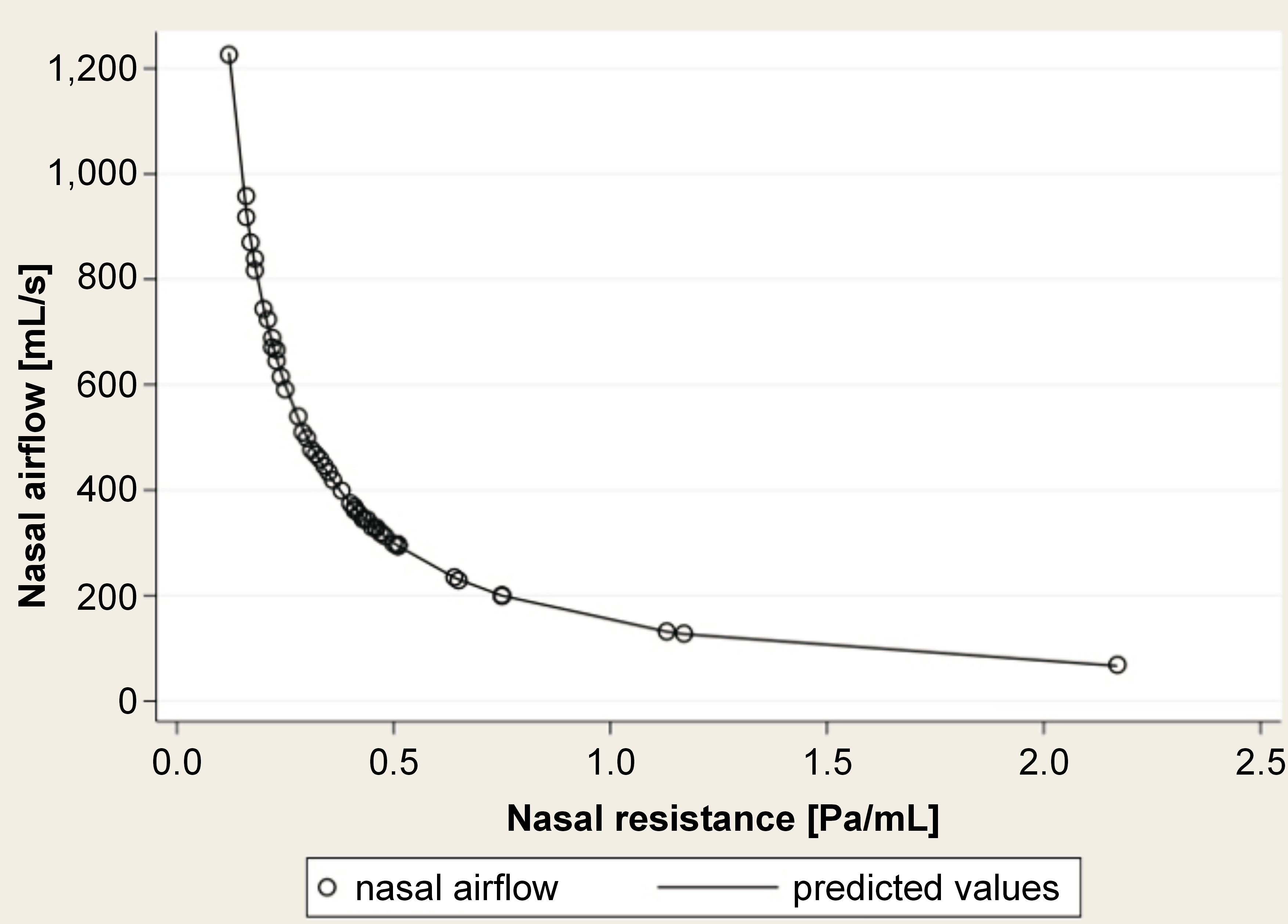
When assessing nasal obstruction, oral screen tests do not measure the severity of the obstruction but whether a certain cut-off value is surpassed or not. Once the nasal cavity is obstructed, oral breathing is forced, thereby precipitating the majority of the observed complications. This phenomenon can be attributed to the non-linear relationship between nasal airflow and nasal resistance, which exhibits an exponential relationship to the fourth power (Figure 4). Therefore, at around 0.3 Pa/mL, small changes in nasal resistance are related to substantial variations in nasal airflow. The oral screen test is a diagnostic tool that can be used to identify cases of oral breathing, irrespective of the extent of nasal obstruction.
The present study did not use nasal decongestants, which have been demonstrated to be effective in addressing turbinate hypertrophy26 and adenoid hypertrophy27 in children. Future studies will assess this topic.
Limitations
Despite the aforementioned strengths of the study, it is important to acknowledge its limitations. The study was a validation test, and a small sample size was used. However, given the characteristics of the test, it is our intention to progressively increase this sample size in order to obtain more solid results. The second limitation is that nasal obstruction can be caused by several entities, such as septal deviation, allergic rhinitis or adenoid hypertrophy, among others.28 In this study, we have included different causes of nasal obstruction in order to diminish the potential selection bias. Even though not all causes of nasal obstruction have been included, the findings of this study should be interpreted with caution.
Conclusions
Nasal obstruction in pediatric patients has garnered increasing attention from otolaryngologists, pediatricians and odontologists. This trend is evidenced by a notable increase in the number of papers addressing this subject. This study is the first to assess an odontologic tool, the oral screen, as a screening method for diagnosing pediatric nasal obstruction. It demonstrated a high level of sensitivity and a positive predictive value. Given the characteristics of the test and the high prevalence of nasal obstruction, the oral screen test can be incorporated into the daily practice of professionals who treat pediatric patients.
Ethics approval and consent to participate
The parents of the children who participated in the study provided informed consent.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.