Abstract
Background. The post-coronavirus disease (post-COVID) syndrome (PCS), which occurs after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, can manifest a variety of symptoms in the oral cavity. Changes to the tongue tend to persist longer than other symptoms in this area.
Objectives. The aim of the study was to present the changes and lesions that occur on the tongue after SARS-CoV-2 infection, as well as their healing as a consequence of the therapy used or lack thereof.
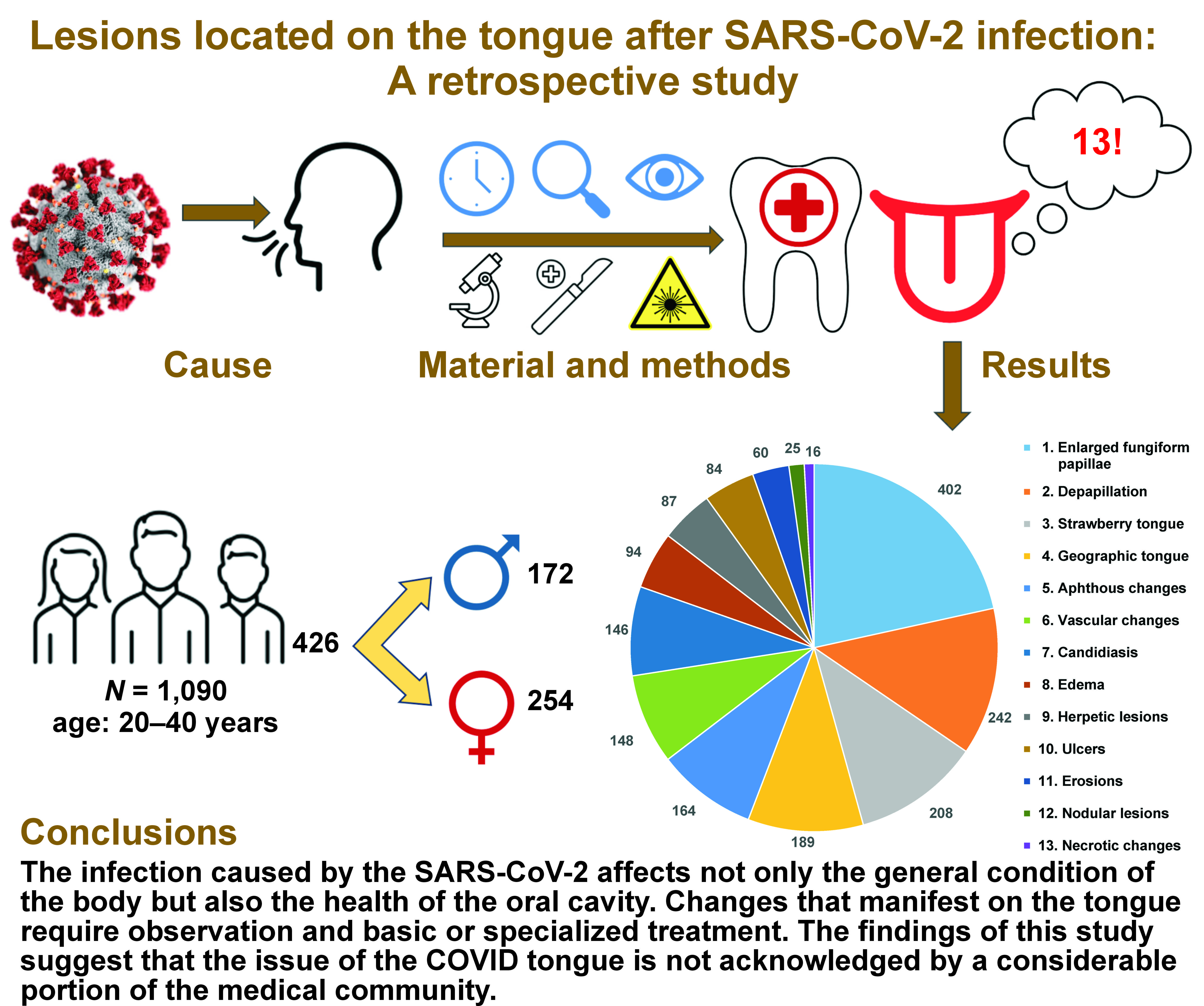
Material and methods. The study sample included 426 individuals who had contracted SARS-CoV-2 and presented with changes on the tongue. Periodic checkups enabled to determine their variability and duration in response to treatment or lack thereof.
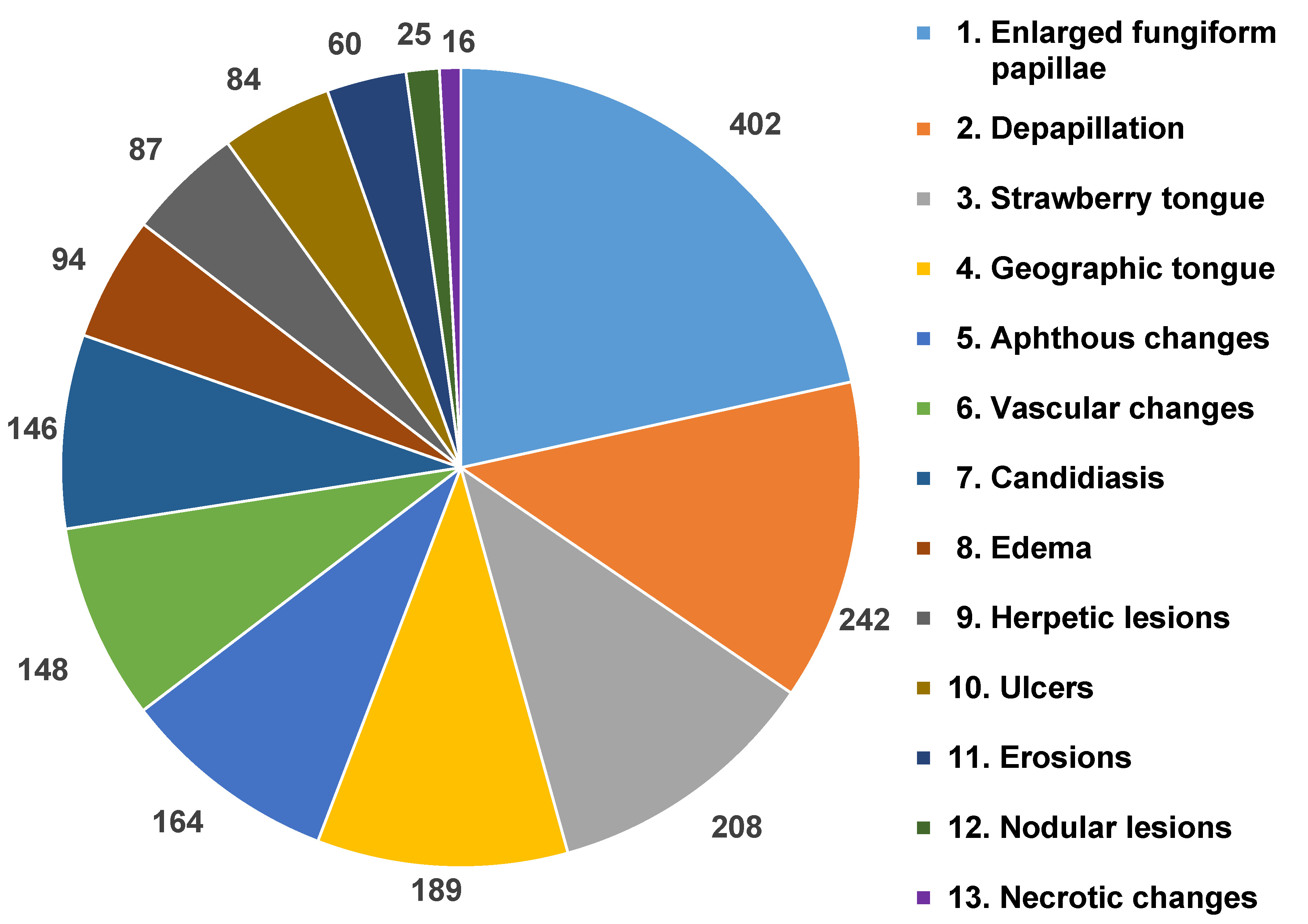
Results. The presence of various oral manifestations was reported, including strawberry tongue (women (F): 143; men (M): 65), depapillation (F: 86, M: 156), geographic tongue (F: 65, M: 124), vascular changes (F: 102, M: 46), aphthous changes (F: 106, M: 58), candidiasis (F: 89, M: 57), edema (F: 42, M: 52), herpetic lesions (F: 38, M: 49), ulcers (F: 38, M: 46), erosions (F: 32, M: 28), nodular lesions (F: 6, M: 19), and necrotic changes (F: 9, M: 7). Fungiform papillae were found to be enlarged in 189 women and 213 men. On average, from 3 to 5 changes were identified concurrently. In the majority of cases, the changes disappeared on their own and persisted from 4 weeks to 36 months. In 20% of cases, they recurred. Local therapy resulted in a 50% reduction in the duration of PCS.
Conclusions. Changes that manifest on the tongue require observation and basic or specialized treatment. In the absence of pain, monitoring is recommended for a period of 4 weeks, after which a spontaneous disappearance should be expected. In the event that various changes occur in the oral cavity, the patient should be referred for specialized treatment.
Keywords: treatment, duration, SARS-CoV-2, long COVID, tongue changes
Introduction
Coronavirus disease 2019 (COVID-19) manifests a variety of symptoms in the oral cavity1, 2, 3, 4 and promotes the appearance of the post-COVID syndrome (PCS). The syndrome occurs in individuals who have been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), persists for a minimum of 2 months, and cannot be explained by an alternative diagnosis. A variety of symptoms may occur in the oral cavity during the acute phase of COVID-19 or after infection, and they are recurrent.5 Single cases of autoimmune diseases have also been observed in the aftermath of SARS-CoV-2 infection.6 Oral symptoms may also emerge following COVID-19 vaccination.7 The mechanism of action of the SARS-CoV-2 is not known. Despite the passage of time, it is not possible to confirm whether the changes located in the oral cavity are the result of the systemic reaction to the viral infection, the congenital immune response to those infections, the action of cytokines,8, 9 or are secondary.10, 11 However, it has been validated that the oral cavity is the gateway and reservoir of the virus because the high concentration of angiotensin-converting enzyme 2 (ACE2) receptors in this area predisposes to the appearance of pathological changes.12, 13 The virus causes the activation of cytokines, leading to cell apoptosis and subsequent loss of taste buds, resulting in dysfunction of taste sensation. It also affects the trajectory of the gustatory tract by damaging cells in peripheral taste neurosensory chemoreceptors and/or by directly damaging cranial nerves (VII, IX and X) that are responsible for taste.14
The progression and severity of the infection are also influenced by the patient’s oral hygiene, which is inseparably linked to their mental health.15 A retrospective analysis of patients after the SARS-CoV-2 infection has revealed that tongue lesions tend to persist longer than other changes in this area.
Initially, tongue lesions were observed in 38% of patients with confirmed SARS-CoV-2 infection.3 The first cases of tongue swelling were reported at the beginning of the pandemic. It affected hospitalized patients with severe COVID-19, intubated patients and Black individuals.16, 17 An image of a strawberry tongue was featured on social media in January 2021.18 Fungal lesions were also prevalent, resulting from poor hygiene practices. Subsequent examinations revealed depapillation of the tongue (red papilla-free areas surrounded by an irregular white border), swelling, inflammation, ulceration, nodules, and geographic tongue. The manifestation of these symptoms in conjunction with the SARS-CoV-2 infection is referred to as “the COVID tongue.”19, 20 However, it remains unclear whether the COVID tongue is an early symptom of the disease or develops with its progression. Geographic tongue resulting from the SARS-CoV-2 infection persists for years without causing pain, only discomfort when consuming spicy foods. Vascular,21 drug-induced22 and reinfection-related23 lesions have also been documented.
A review of the available literature revealed no publications describing the occurrence, treatment and disappearance of all 13 tongue pathologies that appeared throughout the pandemic and were the result of infection with all known variants of the SARS-CoV-2 virus.
The objective of this study is to present the changes that occur on the tongue after SARS-CoV-2 infection, as well as their healing as a consequence of the therapy used or lack thereof.
Material and methods
The study was conducted according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. The Bioethical Commission of Medical University of Warsaw, Poland, has approved the scope of this retrospective study (commission statement No. AKBE/318/2023). The medical records of 1,090 patients aged 20–40 who had contracted the virus between 2020 and 2023 were transferred from the IT system of the dentistry clinic to an external hard drive.
The data was password-protected using the AES 256 encryption in the VeraCrypt software (https://veracrypt.io/en/Home.html). A random ID number was assigned to each patient, distinguishing between male and female subjects, and was not associated with their existing medical data.
The documentation was searched using the following terms: “SARS-CoV-2”; “RT-PCR (reverse-transcription polymerase chain reaction)”; “post-COVID-19 syndrome”; different tongue changes and treatment methods (“enlarged fungiform papillae”, “depapillation”, “strawberry tongue”, “geographic tongue”, “vascular changes”, “aphthous changes”, “candidiasis”, “edema”, “herpetic lesions”, “ulcers”, “erosions”, “nodular lesions”, and “necrotic changes”). After analyzing the data, the documentation of 426 individuals (254 women and 172 men) who had mild forms of SARS-CoV-2 infection that persisted for up to 7 days and manifested with lesions on the tongue was included. The patients were previously healthy and did not exhibit any oral lesions. The exclusion criteria were comorbidities, pregnancy and addictions.
During the initial visit, all patients underwent a comprehensive examination, in accordance with the checklist presented in Table 1. Particular attention was paid to the presence of lesions on the tongue. As outlined in the documentation, periodic checkups allow to determine variability and duration of lesions, depending on the therapy or lack thereof.
In the case of ulcers, erosions and aphthous changes, laser therapy was recommended (5 treatments every 3 days) using a semiconductor laser (SMART; Lasotronix, Piaseczno, Poland). The treatment area was rinsed 3 times a day for 14 days with a 0.2% chlorhexidine solution or Alfa Implant fluid (ATOS, Warsaw, Poland), which contains sage, chamomile, arnica, oak bark extracts, linseed, xylitol, and chlorhexidine. For the treatment of fungal lesions, Nystatin (Teva Pharmaceuticals Polska Sp. z o.o., Warsaw, Poland) at a concentration of 100,000 IU/mL was applied topically to the tongue (2–3 times a day) until the symptoms disappeared and for 48 h after their disappearance.
All patients with lesions located on the tongue were advised, in addition to standard hygiene practices, to brush their tongue twice a day and to use undiluted antiseptic rinses 3 times a day for at least 30 s.
Statistical analysis
The following variables were used for the statistical analysis of quantitative data (MS Excel 365 (Microsoft Corporation, Redmond, USA)): the total number of patients (N); the number of valid observations in the occurrence of different tongue pathologies divided by gender (n); minimum value; maximum value; arithmetic mean (μ); median (Me); and standard deviation (SD). Additionally, the percentage of patients affected by these changes and the duration of symptoms relative to the therapy or lack thereof were calculated. The study adopted a significance threshold of α = 0.05.
Results
The distribution of different types of changes observed on the tongue is shown in Figure 1. The most prevalent symptom was the enlargement of fungiform papillae, which was found in 44.4% of women and 50% of men. According to the findings, 36.6% of the male population presented with depapillation, 33.6% had strawberry tongue and 29.1% demonstrated geographic tongue. However, in the case of women, 23.9% had vascular changes, 20.9% – candidiasis, 20.2% – depapillation, and 19.7% – aphthous changes. The least common symptoms in men were necrotic changes (1.2%), erosions (3.8%) and nodular lesions (4.5%), while in women, nodular lesions (0.7%), necrotic changes (2.1%) and herpetic lesions (4.7%) were the least prevalent.
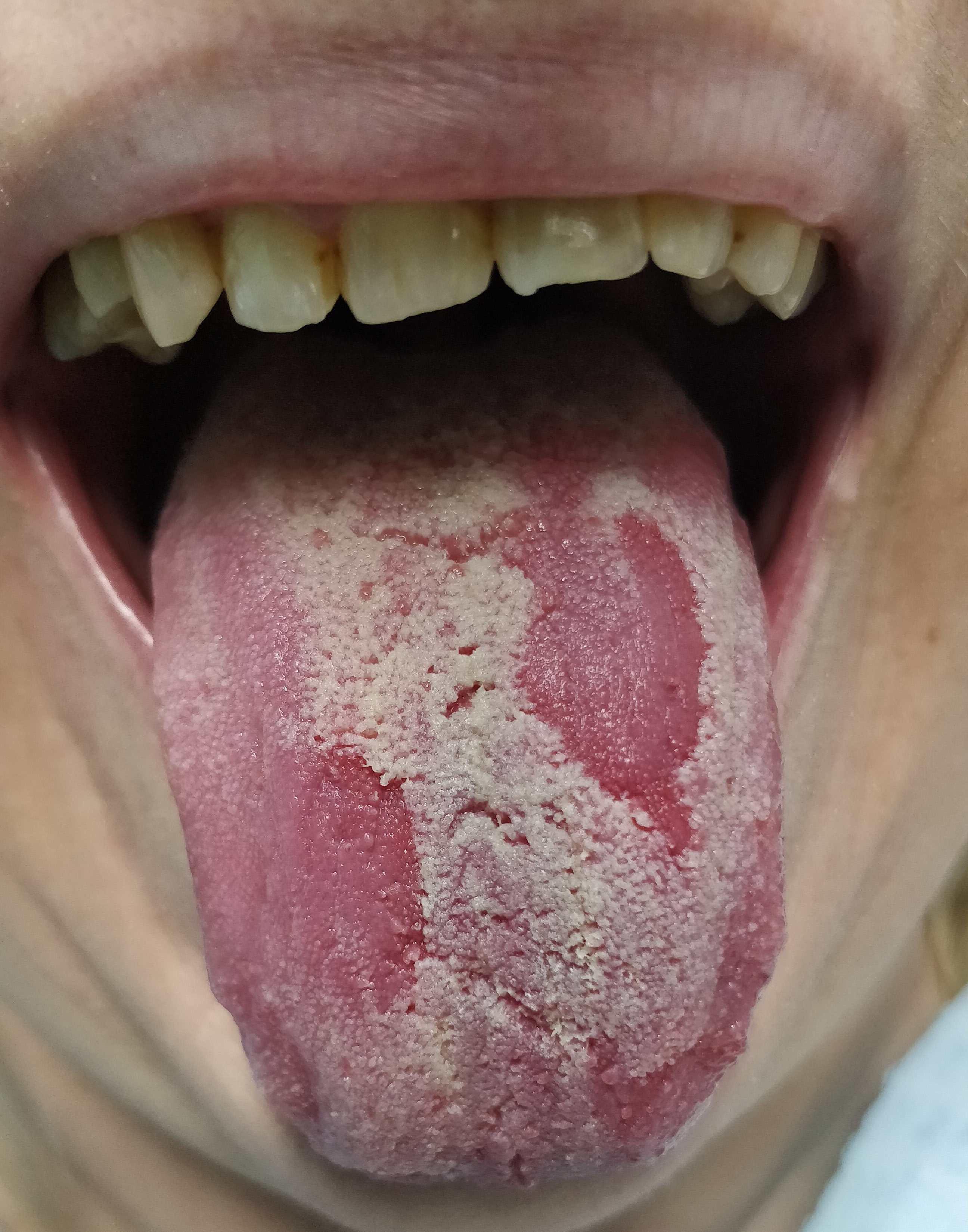
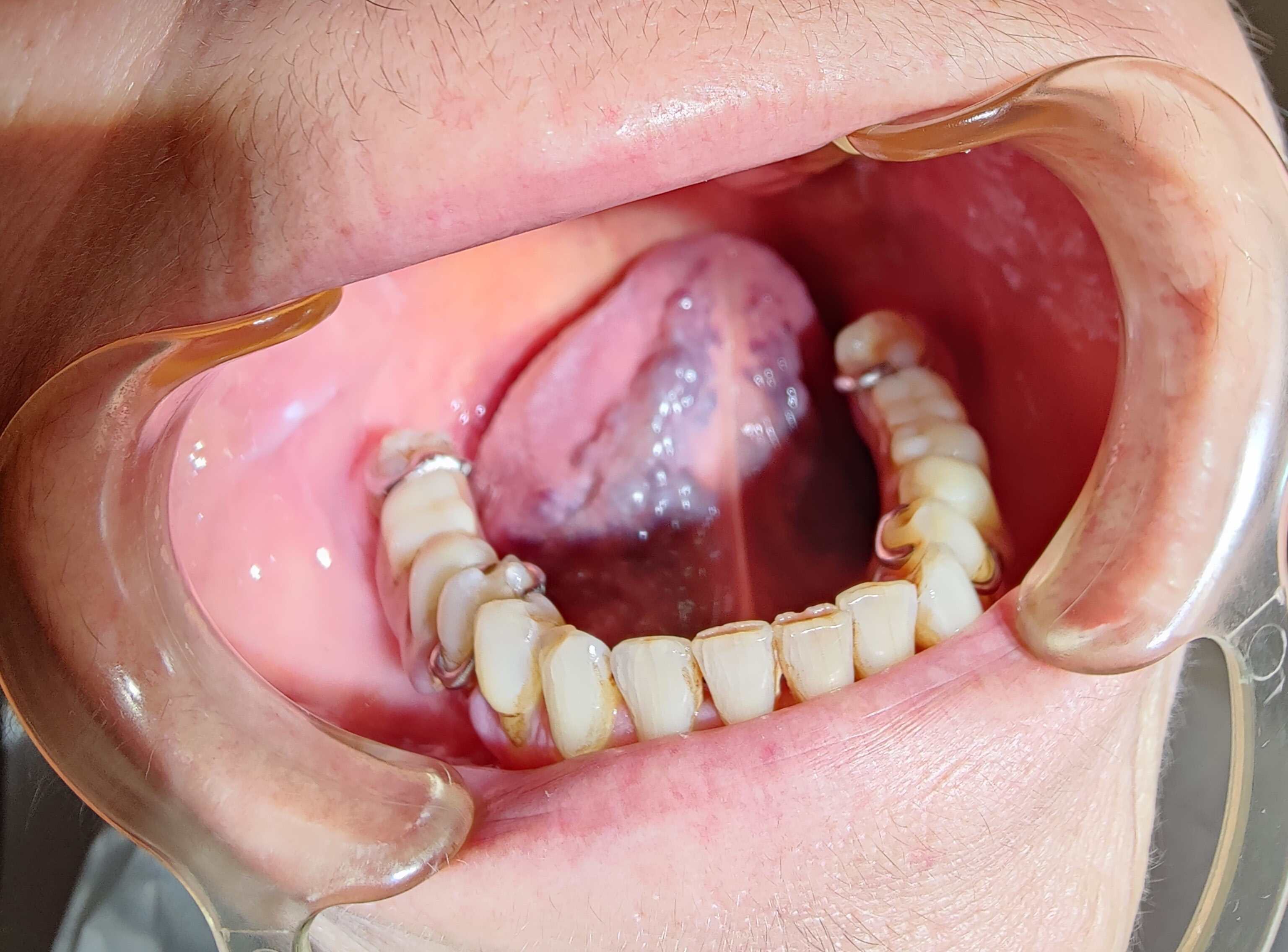
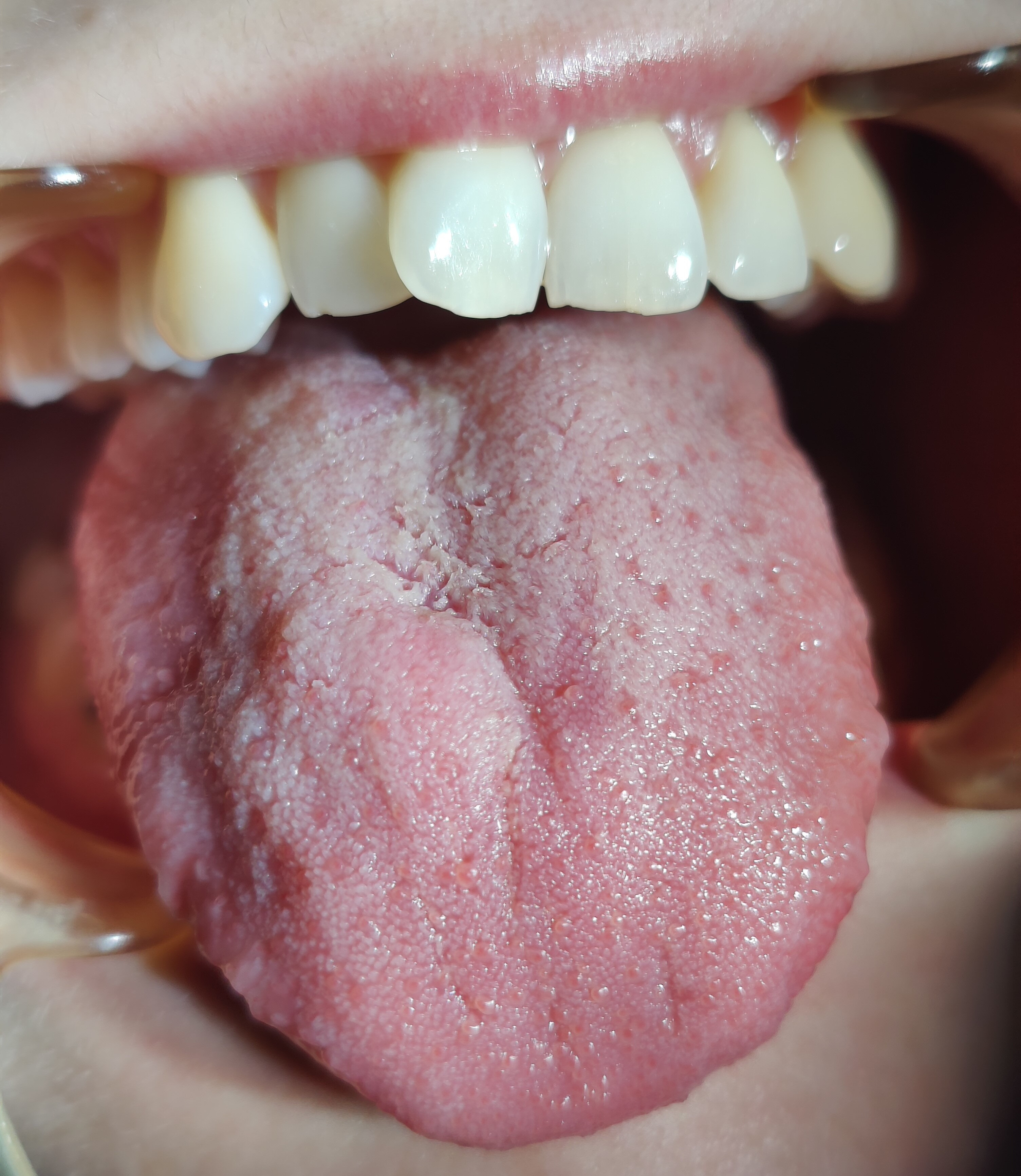
The first variants of the virus gave a characteristic picture of strawberry tongue, which occurred in 208 analyzed patients. Subsequently, depapillation (Figure 2) was identified in 86 women and 156 men. The Omicron variant caused the intensification of clinical changes on the tongue, which included geographic tongue in 65 women and 124 men with strongly marked furrows on the entire dorsal and lateral surfaces. Vascular changes on the ventral surface (Figure 3) were identified in 102 women and 46 men. Large aphthous changes were found in 106 women and 58 men, candidiasis – in 89 women and 57 men, edema – in 42 women and 52 men, herpetic lesions – in 38 women and 49 men, ulcers – in 38 women and 46 men, erosions – in 32 women and 28 men, nodular lesions (Figure 4) – in 6 women and 19 men, and necrotic changes were found in 9 women and 7 men. Enlarged fungiform papillae were reported in 189 women and 213 men.
The number of tongue changes and their duration after and without treatment are presented in Table 2. On average, from 3 to 5 changes were identified concurrently. In most cases, the changes disappeared spontaneously and persisted from 4 weeks to 36 months (4.69% of cases). In 20% of cases, they recurred.
The local therapy used resulted in a 50% reduction in the duration of PCS.
In 2 male patients, unilateral swelling of the tongue accompanied by depapillatory changes and a fungal coating was found to be cancerous. As indicated in the interview, the patients exhibited a history of good health, refrained from smoking, and did not consume alcohol. The first patient (KP), a 38-year-old male, experienced a short-term loss of smell and taste, mild fever, and muscle and bone soreness for a duration of 4 days. The second patient (WA), a 36-year-old male, exhibited a loss of taste for 7 days during the infection. The reverse transcription polymerase chain reaction (RT-PCR) test was positive in both patients. At present, patients are being treated in the oncology department.
Discussion
At the onset of the COVID-19 pandemic, the predominant focus was on respiratory and systemic manifestations. In the case of pathologies located within the oral cavity,1, 2, 3, 4 only cases of single lesions on the tongue have been documented.24, 25, 26
A survey of 665 Egyptian patients after the SARS-CoV-2 infection revealed that xerostomia occurred in 47.6% of cases, oral pain was experienced by 23% of patients, ulcerations were present in 20.4% of cases, 12% of patients reported pain in bones or joints, and 10.5% of individuals experienced halitosis. In 28.3% of cases, 2–3 symptoms manifested concurrently.27
Based on our retrospective study, it was found that lesions located on the tongue are characterized by clinical diversity and that they undergo transformation with the emergence of new coronavirus variants. All patients with tongue lesions reported no pain symptoms, only discomfort when swallowing. Patients requiring consultation with a specialist were referred to reference centers.
The documentation of the group under study revealed that 94.36% of patients demonstrated enlarged fungiform papillae, 56.81% had depapillation, 48.82% had strawberry tongue, and 44.36% presented with geographic tongue.
The tongue pathologies persisted from 5 to 1,080 days. The shortest time after treatment concerned aphthous changes, herpetic lesions, candidiasis, and erosions. The average duration of ulceration, necrotic changes, and swelling of the tongue did not exceed 12 days, and the average duration of swelling of the fungiform papillae, strawberry tongue, vascular changes, depapillation, and geographic tongue was between 5 and 22 weeks.
Based on other research, aphthous-like lesions persist from 5 to 10 days, ulcerations and erosions from 5 to 21 days, and erosions from 14 to 28 days after treatment.3 Other authors have observed that lesions in the oral cavity may disappear from 10 to 42 days after the disappearance of systemic symptoms and disappear spontaneously or after basic treatment.14, 24 The longest persisting lesion is geographic tongue (120–180 days).28
A review of patient records indicated that untreated lesions, erosions, ulcers, aphthous changes, and herpetic changes exhibited the shortest duration, ranging from 10 to 12 days. Candidiasis and edema demonstrated a duration of approx. 20 days, necrotic changes – about 11 weeks, and the remaining conditions – from 9 to 36 months.
Many authors have observed tongue pathologies related to acute SARS-CoV-2 infection, as well as the impact of commonly prescribed pharmaceutical agents.1, 3, 22 Some patients present with a white infiltrative plaque on the dorsum of the tongue, located centrally, that resembles the late stage of recurrent herpetic lesions and candidiasis, manifesting as multiple, small, yellowish, circular ulcers.3, 29 In other cases, irregular tongue ulceration has been observed,2, 3, 29, 30 accompanied by the presence of many small extravasations31 or depapillation of the tongue.26, 32
The medical records of the patients included in the study indicated that candidiasis occurred in 29.96% of patients, herpetic lesions – in 16.19%, ulcers – in 15.96%, erosions – in 11.26%, and tongue depapillation affected 56.8% of individuals.
In a study conducted in Spain in 2020, 304 out of 666 patients presented with mucocutaneous manifestations, including transient lingual papillitis (11.5%), aphthous inflammation (6.9%), glossitis with pitting on its lateral surface (6.6%), and glossitis with uneven depapillation (3.9%).31 In the current study, aphthous changes were found in 38.5% of the subjects.
Analyzing the data from the available literature, it should be stated that no clear relationship has been established between COVID-19 and changes on the tongue.33 Some researchers believe that these changes are a consequence of stress,1, 3, 32, 33 hygiene neglect,1, 3 opportunistic infections,1, 3, 8, 22, 23 immunosuppression,1, 3, 28 vascular changes,1, 20, 21 or excessive inflammatory response.34, 35, 36, 37, 38 It is also suggested that systemic health deterioration, an acute onset of infection, and multidrug therapy may induce pathological changes in the oral cavity.33 The occurrence of secondary ulcers and the immune response are associated with the presence of viral infections.29, 36, 37 Coronavirus disease may, therefore, cause the overactivation of the humoral response to inflammatory factors, resulting in a cytokine storm and immune exhaustion, which may lead to early changes in the oral cavity.35
A lack of oral hygiene among hospitalized patients connected to a respirator is a probable cause of opportunistic fungal infections.35An impaired immune system can cause recurrent herpes simplex virus (HSV-1) infections, non-specific ulcers and drug eruptions.37, 38
In the present study, the hypothesis of hygiene neglect was rejected, as evidenced by good oral hygiene and overall health of the patients. The adequate selection of oral hygiene products, especially those based on natural ingredients, ensures adequate protection of the oral cavity.39 Initially, it was thought that antibacterial mouthwashes could reduce viral load. The study on hypochlorous acid (HClO) and povidone-iodine (PVP-I) revealed no evidence to support the hypothesis that these preparations reduce the viral load.40 At the beginning of the pandemic, 73% of respondents reported feelings of fear and anxiety when considering a dental visit due to the possibility of being infected with the SARS-CoV-2.41 Isolation and anxiety caused deterioration of oral hygiene in some patients, increased caries and inflammation of the oral cavity, and temporomandibular joint (47.8%) and muscle disorders, leading to parafunctions, bruxism (31%), headaches, and mental disorders.42, 43 The resumption of medical activities was permitted after the implementation of increased hygiene and antiseptic procedures in dental offices, based on the guidelines from the World Health Organization (WHO) and the Polish Dental Association (PDA). The use of the ultraviolet C (UVC) radiation, ozone, disinfectants, and protective equipment ensured the safety of staff and patients.44, 45
Some studies suggest that changes in the oral cavity may be the direct effect of the virus.4, 21, 38
Published papers on tongue lesions during the SARS-CoV-2 infection confirm the relationship with organic damage or complications of thrombocytopenia, anticoagulant treatment, disseminated intravascular coagulation, and systemic inflammation. It is suggested that the presence of long COVID lesions results from primary or secondary vascular/hematological changes and lymphocytic thrombophilic arteritis.33, 38
Some authors hypothesize that prolonged manifestations of the disease may result from co-infections and/or secondary changes.1, 8, 32, 35
The results of the conducted cross-sectional study indicated the occurrence of erythematous spots, single ulcers (3%), atrophic glossitis (4.6%), and candidiasis (1%).38
The differential diagnosis of the COVID tongue includes herpetic glossitis, Melkersson–Rosenthal syndrome, lichen planus, and fungal infections.1 When diagnosing a patient with PCS symptoms localized on the tongue, reinfection should be considered, which may occur many months after the initial infection with the SARS-CoV-2.1, 3, 35
In addition, there have been reports of post-vaccination lesions on the tongue.46 A survey conducted among vaccinated individuals in Poland, Italy and other EU countries revealed post-vaccination symptoms in the oral cavity after the administration of the first dose of the vaccine (3.1%) and after the second dose (5.4%). The undesirable post-vaccination reactions include changes in sensitivity and facial paresis, a burning sensation, aphthous changes, taste changes, xerostomia, depapillation of the tongue, pain, stomatitis, cheilitis, and candidiasis.47 Many published studies demonstrate that changes located on the tongue are the result of acute infection, reduced immunity, polypragmasia, hygiene neglect, or stress.1, 3 This hypothesis was not confirmed in the current study.
However, the medical documentation of young patients, those without comorbidities and previous changes in the tongue, as well as the emergence of subsequent variants of the virus, indicate that they cannot be underestimated. The presence of 2 cases of cancer lesions suggest the necessity for thorough diagnostics.
Research on lesions located on the tongue should also be continued based on other age groups, as well as those affected by systemic diseases or addictions.
Conclusions
The infection caused by the SARS-CoV-2 affects not only the general condition of the body but also the health of the oral cavity. Changes located on the tongue require observation and basic or specialized treatment. In the absence of pain symptoms, the patient should be monitored for a period of 4 weeks, with the expectation that the symptoms will spontaneously resolve. In case of pain, a good solution is the application of laser bio-stimulation. If various changes co-occur in the oral cavity, it is advised that the patient be referred for specialized treatment. The findings of this study suggest that the issue of the COVID tongue is not acknowledged by a considerable portion of the medical community.
Ethics approval and consent to participate
The Bioethical Commission of Medical University of Warsaw, Poland, has approved the scope of this retrospective study (commission statement No. AKBE/318/2023). In addition to the standard consent to treatment, as required by national regulations, all patients provided written consent to participate in this study.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.