Abstract
Background. The effectiveness of guided tissue regeneration (GTR) has been thoroughly documented. Since most post-GTR complications are related to the exposure of the membrane and its subsequent bacterial contamination, clinicians treat the incorporation of systemic antibiotics as an integral component of the standard surgical procedure. However, this approach remains controversial.
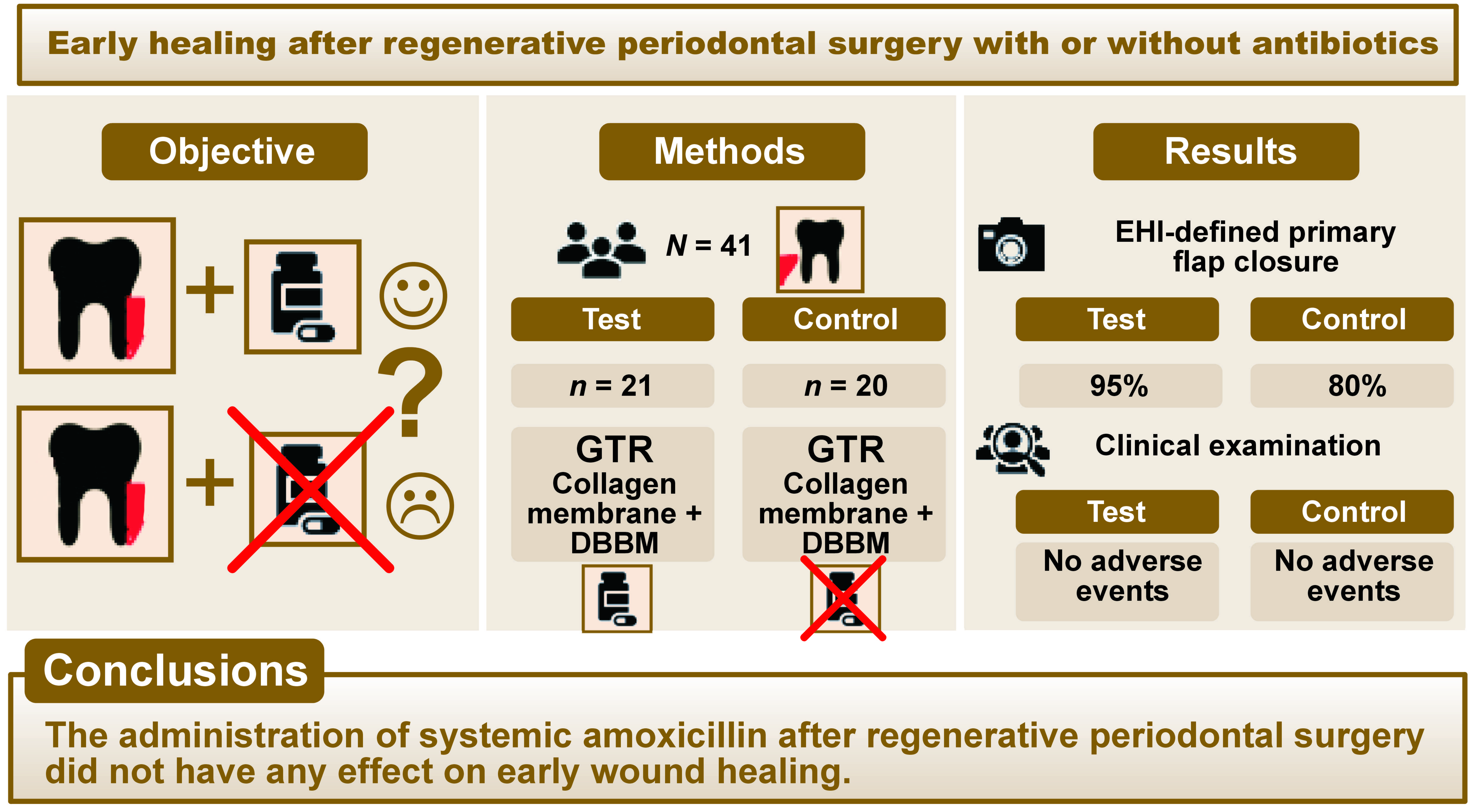
Objectives. The aim of the study was to assess the effect of postsurgical administration of antibiotics on early healing and patient morbidity after treatment of periodontal intrabony defects with deproteinized bovine bone mineral (DBBM) and a collagen membrane (GTR).
Material and methods. The study encompassed 41 patients with 41 intrabony defects. The subjects were randomly assigned to either the test group (DBBM/GTR+AB (postsurgical antibiotic)) or the control group (DBBM/GTR). In the test group, 1 g of amoxicillin was administered twice daily for 7 days. The early healing index (EHI) was assessed 1 and 2 weeks after the procedure. Patient morbidity was recorded. The clinical attachment level (CAL), probing depth (PD) and gingival recession (GR) were measured at baseline and 6 months after surgery.
Results. Early healing was uneventful in both groups. No adverse events were recorded in either group. In the second week, EHI-defined primary flap closure was evident in 95% of the test group sites and 80% of the control group sites. The CAL changed significantly in both groups: from 8.7 ±1.4 mm to 4.6 ±1.7 mm in the DBBM/GTR+AB group (p < 0.0001); and from 8.6 ±1.9 mm to 5.7 ±1.2 mm in the DBBM/GTR group (p < 0.0001). The significant outcome was in favor of the test group (p = 0.010). Probing depth significantly decreased in both groups, without any observed intergroup differences.
Conclusions. The administration of systemic amoxicillin after regenerative periodontal surgery did not have any effect on early wound healing; however, it yielded a statistically significant CAL gain after 6 months compared to the group treated without antibiotics.
Keywords: antibiotics, intrabony defects, guided tissue regeneration, regenerative dentistry
Introduction
The primary aim of periodontal therapy is to facilitate the regeneration of tissues that have been lost due to inflammation. In essence, periodontal regeneration comprises the reconstitution of the functionally arranged collagen fibers inserting into the new cementum and bone. The periodontium itself has exceptional natural, self-restoring properties that can result in regeneration after surgery, but only under optimal conditions. Wound stability, healing per primam and enhanced space provision for regeneration are the key factors that stimulate the healing process.1, 2
The establishment of knowledge on the regenerative potential of the periodontal ligament, as well as a thorough understanding of regeneration, fostered a revolutionary change in surgery.3 Papilla-preserving incisions and minimally invasive surgical techniques have largely limited the risk of developing postoperative complications.4, 5, 6, 7 Regenerative surgery has so far used barrier membranes, bone grafts, bone substitutes, bioactive agents, and their combinations. Certain surgical techniques and materials result in a reduced probing depth (PD) and greater clinical attachment level (CAL) gain. In contrast, the application of flap elevation as a standalone procedure has a lesser potential for this outcome.8, 9
The effectiveness of guided tissue regeneration (GTR) has been documented both clinically and histologically.8, 10, 11 This modality uses barrier membranes to separate the epithelium and gingival tissues from the root, thus enabling periodontal ligament cells to repopulate the isolated space.12 Histological studies have demonstrated that the primary function of the membrane is to provide space for regeneration and to stabilize the clot.13, 14
Although the routine administration of systemic antibiotics in conjunction with periodontal surgical procedures prevents postsurgical complications and optimizes expected outcomes, this approach is guided by practical experience rather than by evidence.15 Some researchers claim that perioperative antibiotics promote CAL gain,16, 17 while others argue that they offer no greater benefits than the surgery itself.18 It is also worth noting that the number of postsurgical infections is statistically low,19 and the uncontrolled use of antibiotics may favor bacterial resistance.20 In addition, hypersensitivity, allergic reactions and interactions with other drugs necessitate caution.21
Since most post-GTR complications that affect the outcome are related to the exposure of the membrane and its consequent bacterial contamination,22 some clinicians treat the inclusion of systemic antibiotics as inseparable from the standard surgical procedure.23, 24 Currently, however, the routine administration of systemic antibiotics after regenerative periodontal surgery using barrier membranes remains controversial, with no clear protocols available. It is of great importance to reduce the bacterial and viral load before surgery, a result that can be achieved through initial periodontal therapy.25, 26 Studies are also being conducted on topical agents that will be able to modulate healing in the periodontium. Plant preparations are an important focus of this research.27, 28
The process of healing is initiated by hemostasis, early clot formation and inflammatory cell infiltration. Subsequently, epithelial cells begin to proliferate, and fibroblasts migrate into the wound. Wound closure is essential for periodontal wound stability in the early phase of healing,1, 2 and the first postoperative week is deemed to be critical.29 Uneventful wound healing after GTR is a major factor for clinical success.30 Trombelli et al. have emphasized the importance of primary intention healing and have observed substantial clinical improvements in cases without membrane exposure.31 A number of indices have been developed to characterize the early wound healing process, namely the healing index (HI),32 the early healing index (EHI),7 the wound healing index (WHI),33 and the early wound healing score (EHS).34 At the same time, several surrogate parameters have been used to describe the healing process, encompassing the tissue color, bleeding, characteristics of incision margins, the presence of suppuration, the assessment of wound closure, abscess formation, fibrin and necrosis, edema, erythema, suppuration, patient discomfort, and flap dehiscence.35
Taking into consideration the complexity of periodontal healing, the present study aimed to evaluate early postoperative healing, as measured by the EHI,7 and to assess the possible complications that may arise after regenerative surgery. The assessment utilized a deproteinized bovine bone mineral (DBBM) and a collagen membrane, with or without a postoperative antibiotic regimen. The secondary objective of the study was to identify a possible relationship between early healing and the clinical outcomes measured at 6 months following regenerative surgery.
Material and methods
Study design and participants
The project was designed as a randomized, prospective, controlled clinical study. The study was approved by the Bioethics Committee of Medical University of Bialystok, Poland (approval No. R-I-002-302-2013), and was compliant with the Declaration of Helsinki. Patients diagnosed with stage III periodontitis36 and having at least 1 intrabony defect were enrolled in the study, which encompassed 41 generally healthy adults.
The patients were required to meet the following inclusion criteria: age ≥18 years; at least 1 intrabony defect with a PD ≥ 6 mm, radiographically tested using the long cone paralleling technique (radiographic depth (RxD) ≥3 mm, radiographic width (RxW) ≥2 mm); no allergic reaction to the penicillin in the family history; smoking status: non-smoker; effective plaque control (full mouth plaque score (FMPS) <20%); and signed informed consent.
Subjects who had received antibiotics within 3 months prior to the study, those diagnosed with systemic diseases that affect healing, as well as pregnant or breastfeeding women were excluded.
During the inclusion period, 99 patients with intrabony defects were screened. Twenty three subjects were excluded due to the presence of a radiological defect with a depth smaller than 3 mm. Fourteen individuals resigned without any reason. Six patients were in need of complete oral rehabilitation. Fourteen patients were excluded for the following reasons: diabetes (n = 4); insufficient oral hygiene (n = 3); age <18 years (n = 2); acute endo-perio lesions (n = 2); pregnancy (n = 1); breastfeeding (n = 1); radiotherapy (n = 1).
The random allocation software was used to allocate 41 patients into 2 groups: the study group (DBBM/GTR+AB (postsurgical antibiotic), n = 21); and the control group (DBBM/GTR, n = 20).
Clinical measurements
Prior to and 6 months after surgery, an experienced periodontist performed clinical measurements, including PD, gingival recession (GR) and mathematically calculated CAL. The probe was calibrated in 1-mm increments (PCPUNC 15; Hu-Friedy, Chicago, USA) and the readings were rounded up to the nearest millimeter. Six locations around each tooth with an intrabony defect were probed: the mesio-, mid- and distobuccal regions, as well as the mesio-, mid- and distolingual regions. The cementoenamel junction (CEJ) was used as a fixed reference point, unless the CEJ was not detectable, in which case a filling margin was used instead. The deepest value in the pre-surgery test was relevant for the statistical assessment.
The FMPS and the full mouth bleeding on probing (FMBOP) were dichotomously calculated as percentages for the 4 surfaces of each tooth.37
At 1st, 2nd and 4th week after surgery, the sites were examined for signs of suppuration (absence or presence) and dehiscence (in mm).
Photographic documentation of each site encompassed both an inner occlusal view and occlusal and non-occlusal side views. The photographs were taken with a professional camera designed for intraoral photography (Olympus OM-D E-M10; Olympus Corporation, Tokyo, Japan; lens: M. Zuiko Digital ED 60mm F:2.8 Macro; Olympus Corporation; macro flash: Metz 15 MS-1; Metz Consumer Electronics GmbH, Zirndorf, Germany).
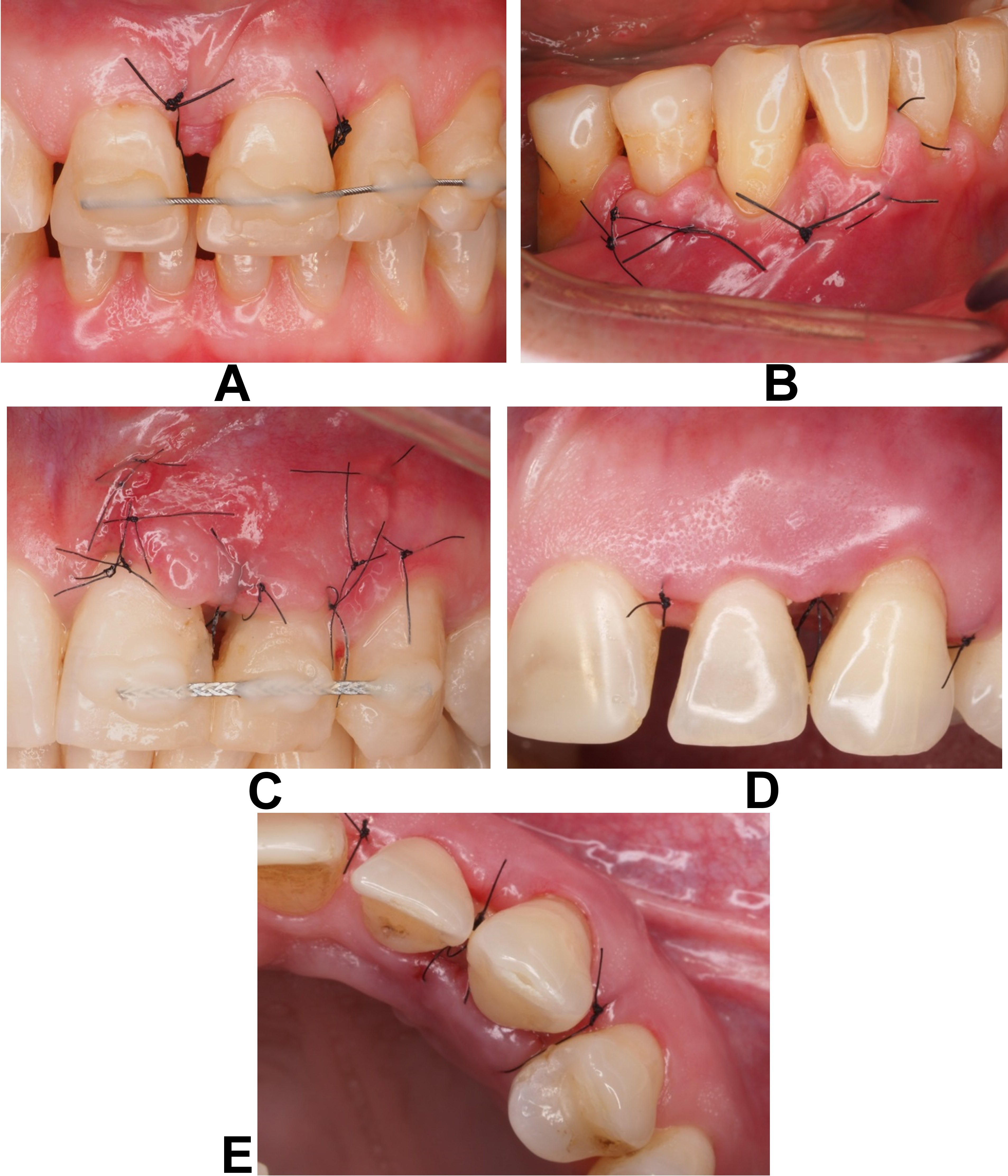
The EHI was assessed based on the photographs taken at the 1st (EHI1) and 2nd weeks (EHI2) post-surgery, as follows:
• EHI = 1: a complete flap closure with no visible fibrin line in the interproximal area (Figure 1A);
• EHI = 2: a complete flap closure with a visible fine fibrin line in the interproximal area (Figure 1B);
• EHI = 3: a complete flap closure with a visible fibrin clot in the interproximal area (Figure 1C);
• EHI = 4: an incomplete flap closure with partial necrosis of the interproximal tissue (Figure 1D,E);
• EHI = 5: an incomplete flap closure with complete necrosis of the interproximal tissue.
Surgical procedures
An experienced surgeon carried out all surgical procedures. Mucoperiosteal flaps were elevated buccally and lingually via an intrasulcular incision under local anesthesia (Ubistesin Forte; 3M ESPE, Seefeld, Germany). Vertical incisions were made exclusively in cases where easier access to the defect was necessary. The modified papilla preservation technique (MPPT) or simplified papilla preservation flap (SPPF)4, 5 was employed within interdental spaces. Subsequently, the granulation tissue was removed, and the root surfaces were thoroughly scaled and root planed using conventional Gracey currettes (Hu-Friedy) and an ultrasonic scaler (Tip PS, Piezon EMS; EMS, Nyon, Switzerland). The defect was then filled with the gently pressed DBBM (cerabone®; botiss biomaterials GmbH, Zossen, Germany) and covered with a trimmed collagen membrane (collprotect®; botiss biomaterials GmbH). Once the membrane was in place, the mucoperiosteal flap was coronally displaced and stabilized over the defect with vertical modified mattress sutures. The remaining papillae and vertical incisions were closed with loop sutures (monofilament non-resorbable Ethilon™ 5-0; Johnson & Johnson Company, New Brunswick, USA).
Intrasurgery measurements
The elevation of the flap and debridement allowed for the assessment of the extent of each intrabony defect with a periodontal probe (PCPUNC 15) and its subsequent classification as one-, two- or three-walled according to the morphological criteria. In instances of evident tooth mobility after surgery, the tooth was immobilized.
Postoperative care
On the day of the surgery, patients randomized to the test group were instructed to take 1 g of amoxicillin every 12 h for 7 days (Ospamox; Sandoz GmbH, Kundl, Austria). Additionally, they were advised to use mouthrinses containing 0.2% chlorhexidine digluconate solution for 2 weeks (Eludril; Pierre Fabre Laboratories, Paris, France). After that time, the sutures were removed, and the patients were permitted to resume brushing in the healing area with an ultra-soft brush.
The 6-month observation period was a time of scrupulous periodontal care for the patients. The 1-, 2- and 4-week postoperative appointments covered an evaluation of the patient’s general oral hygiene, polishing of the site, and detection of potential suppuration and/or wound dehiscence. In addition to supragingival scaling, the 2-, 3- and 6-month appointments involved the assessment of FMPS and FMBOP. Intraoral photographs were taken at each appointment. No subgingival instrumentation was performed in the treated areas during the first 6 months after surgery.
Statistical analysis
The data analysis was conducted using the Statistica™ 13.1 software (StatSoft, Tulsa, USA), with each patient considered a statistical unit. The primary outcome variables encompassed EHI characteristics and the parameters corresponding to suppuration, wound dehiscence and severe postsurgical pain. The alterations in CAL and PD, as determined by probing at the 6th month, and their association with EHI, were considered secondary variables. The analysis used the maximum value of CAL recorded at baseline. The variables were expressed as the mean (M) and standard deviation (SD), as well as the median (Me) and quartiles. Within each group, a statistical analysis was performed using the Wilcoxon matched-pairs signed-rank test, while between-group comparisons were conducted employing the Mann–Whitney rank sum test. The value of p < 0.05 was considered statistically significant. In addition, the Spearman’s rank correlation coefficient was calculated to assess the relationship between early healing, as described by EHI1 and EHI2, and 6-month clinical parameters.
The sample size calculation was performed a priori, under the assumption that the SD of the change in CAL was 1 mm, and that a mean difference of 1 mm could be detected with a test power of 80% in 32 subjects. However, we considered the possibility of dropouts and thus recruited and randomized 41 patients.
Results
The earliest outcome of the study, in which 41 adult patients underwent a surgical procedure (27 women, 14 men, mean age: 41.78 years), revealed predominantly undisturbed healing in both the test and control groups. The only exceptions were slight, temporary dehiscences. Otherwise, we did not observe any signs of suppuration or severe swelling, and the patients did not complain of intense pain, fever or feeling unwell. Only 2 patients in the test group experienced tooth hypersensitivity, and 1 subject in the control group reported a cold sore in the corner of the mouth. The characteristics of the defects at baseline are presented in Table 1.
One week after surgery, the mean EHI1 scores were 2.14 ±1.2 for the DBBM/GTR+AB group, and 2.4 ±1.3 for the DBBM/GTR group. Two-week postoperative EHI2 scores were 1.48 ±0.87 and 2.05 ±1.28, respectively. None of the sites exhibited an EHI score of 5, indicating complete necrosis of the interproximal tissue. An incomplete flap closure resulting in a dehiscence was observed in 4 test patients and 6 control patients at week 1, while at week 2, it was observed in 1 test patient and 4 control patients. After 4 weeks, dehiscence was observed in 3 test patients and 1 control group patient. However, the observed dehiscences did not exceed 2 mm. After 2 weeks, 95% of the DBBM/GTR+AB sites and 80% of the DBBM/GTR sites demonstrated primary flap closure. The distribution of the EHI scores is reported in Table 2.
At baseline, no statistically significant differences were observed between the groups in terms of PD, GR and CAL. At 6 months, a statistically significant decrease in PD and CAL gain was observed in both groups. Additionally, GR increased significantly in the control but not in the test group. The between-group comparison revealed statistically significant differences in terms of CAL, while PD and GR were not significantly different (Table 3).
The investigation revealed a modest correlation between EHI1 and EHI2 paired with GR and CAL in the control group after a 6-month period. There were no statistically significant correlations in the test group (Table 4).
The patients in both groups maintained high standards of oral hygiene throughout the study, as evidenced by FMPS < 20% and FMBOP < 20%.
Discussion
The primary aim of the present study was to determine the impact of a routine course of antibiotic therapy on the early healing of periodontal wounds after regenerative surgery employing collagen membranes and DBBM. Early postoperative complications, such as suppuration, wound dehiscence and severe postoperative pain were meticulously monitored to ascertain the necessity of an antibiotic regimen following regenerative surgery for intrabony defects. Irrespective of the presence or absence of an antibiotic regimen, no cases of suppuration or severe inflammatory complications were observed in any of the study groups.
The rationale for selecting the EHI was based on the fact that this index was specifically developed to monitor the healing of surgically treated intrabony defects. The EHI scores correspond to 5 characteristics indicative of complete or incomplete flap closure, taking into account the presence of fibrin and necrosis. None of the sites in our study displayed an EHI of 5, which would indicate a complete necrosis of the interproximal tissues. However, the present study revealed that the EHI scores were slightly higher in comparison to those documented by Wachtel et al.7 In that study, EHI decreased from 1.85 to 1.39 within 2 weeks in the enamel matrix derivative group and from 1.65 to 1.19 in the control group.7 The observed discrepancy in the results may be ascribed to the different surgical technique and biomaterials used for periodontal tissue regeneration. The surgical interventions performed in this study incorporated grafting materials and collagen membranes, necessitating more extensive flap preparation. Notably, unlike enamel matrix derivative, the biomaterial itself was required to preserve a space for regeneration under the flap.
The importance of primary intention healing has been demonstrated in the study on GTR procedures by Trombelli et al.31 The paper demonstrated significantly lower values of bone gain when the membrane was previously exposed.31 Wound closure for periodontal regeneration enables primary intention healing and is considered a prerequisite to stabilize the blood clot and promote a regeneration process.35 The EHI is a clinically available method to determine the early healing phase of periodontal wounds. The relevance of EHI as a predictor of the outcomes of regenerative procedures has been investigated by Farina et al.38 However, the study was not able to demonstrate the association between early healing and 6-month clinical results, such as a CAL gain and pocket depth reduction. The authors hypothesized that the observed findings could be related to a short observation period, suggesting that the EHI may not be sensitive enough to detect significant differences in early postoperative healing, which could have a substantial impact on the outcomes over a period of half a year.38 The influence of the EHI on 1-year CAL gain was examined by Liu et al.39 Lower EHI scores positively influenced the CAL gain. Sites demonstrating optimal healing were more likely to achieve a greater CAL gain during the 1-year observation period.39 Apart from the abovementioned success, it is important to acknowledge that regenerative therapy is multifactorial and contingent on numerous patient-related and site-specific factors, other than the EHI.40
The results of the current study demonstrated that GTR with DBBM led to a notable decrease in PD and an increase in CAL after 6 months, irrespective of whether the surgery was followed by a course of postoperative antibiotics. The only exception was an unchanged GR in the DBBM/GTR+AB group, which proved advantageous when the expected outcome was a CAL gain rather than a recession. Thus, the efficacy of both postoperative protocols has been demonstrated.
Six months after surgery, the attained gain in CAL was 4.6 ±1.7 mm in the test group and 2.9 ±1.4 mm in the control group. The difference between the groups was statistically significant, favoring the antibiotic regimen. However, the statistical significance in the CAL gain was not confirmed after the 1-year observation period. As previously documented, a 1-year CAL gain in the test group was 3.6 ±1.6 mm, while in the control group it was 2.7 ±1.6 mm.41 There were no statistically significant intergroup differences.41 Other authors who used collagen membranes and xenogeneic biomaterials with antibiotics reported comparable gain in CAL. Camargo et al. observed a mean gain of 3.2 mm after 6 months.42 Sculean et al. reported a mean gain of 4.0 mm after 1 year.43 Esposito et al. noted a probing attachment level (PAL) of 3.6 mm after 1 year.44 Similar clinical studies without postsurgical antibiotics revealed a mean CAL gain of 4.1 mm45 and 3.7 mm after 1 year.46
Histological studies in humans have confirmed the effectiveness of GTR. For instance, in the study by Sculean et al., concurrently with a mean attachment gain of 3.6 mm, there was 2.4 mm of new cementum and 2.1 mm of new bone.47 Thus, it may be anticipated that the clinical improvements obtained in the present study may also reflect, at least to a certain extent, periodontal regeneration. However, only a histological analysis can provide definite evidence regarding the quality of the newly formed tissues, which, due to evident reasons, could not be performed in the present study.48
Postsurgical antibiotic regimens vary in type and dosage. They range from 250 mg tetracycline administered 4 times daily for a week4 to 1.5 g of amoxicillin once a day for a week,49 200 mg of doxycycline administered daily for a week,50 or 1 g of amoxicillin plus clavulanic acid once a day for 8 days.51 What is more, clinicians are not unanimous in their views on the potential benefits of antibiotic treatment. The main postoperative hazards to prevent are membrane exposure and wound infection. A probable bacterial contamination of the exposed membrane impedes clinical effects of the procedure. The novel papilla preservation techniques (MPPT, SPPF)23 have led to a notable decrease in the incidence of such complications.23 These methods ensure the secure placement of the flap above the barrier membrane. It is, however, practically impossible to keep the membrane aseptic, as it becomes contaminated within the first 3 minutes in the oral cavity.22
Antibiotics are also frequently recommended in another modality of regenerative therapy that uses enamel matrix derivative (EMD), even though the risk of infection is lower.47, 52 However, the results of randomized clinical studies have failed to reveal any advantages of a postoperative antibiotic regimen on the clinical outcomes obtained after regenerative surgery with EMD, thus corroborating our findings.26, 53
A review of the relevant literature from 2005 to 2014 reveals that systemic antibiotics were routinely administered in 75.4% of cases involving flap elevation despite the low overall proportion of postoperative infections. These amounted to 0.073% when antibiotics were included and to 0.693% when they were not. This difference was neither statistically nor clinically significant.54 Previous studies have noted a limited occurrence of postoperative complications following periodontal surgery,19, 55 with reported rates of 2.09% and 4.2%, respectively. Some of these complications may be related to inadequate preoperative preparation of the patient. A thorough execution of the hygienic phase, comprising subgingival mechanical debridement (i.e., scaling and root planing), is imperative to reduce the bacterial load and minimize postoperative complication rates.26 Finally, a strictly executed, asepsis-oriented surgical protocol, as evidenced in the present study, is crucial to minimize the need for postsurgical antibiotic therapy.56
Conclusions
The results of the present study do not support the routine administration of antibiotics in systemically healthy patients treated with a collagen membrane and DBBM xenograft. This type of regenerative therapy, carried out under aseptic conditions, ensured undisturbed healing. Although a supplemental 1-g dose of amoxicillin did not significantly influence EHI and PD reduction, it led to a CAL gain after 6 months.
Ethics approval and consent to participate
The study was approved by the Bioethics Committee of Medical University of Bialystok, Poland (approval No. R-I-002-302-2013), and was compliant with the Declaration of Helsinki. All patients provided signed informed consent.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.