Abstract
Background. The Montreal Cognitive Assessment (MoCA) is the most accurate cognitive screening tool for early diagnoses of mild cognitive impairment (MCI). However, the majority of research on the correlation between MCI and periodontitis has been conducted using the Mini–Mental State Examination (MMSE).
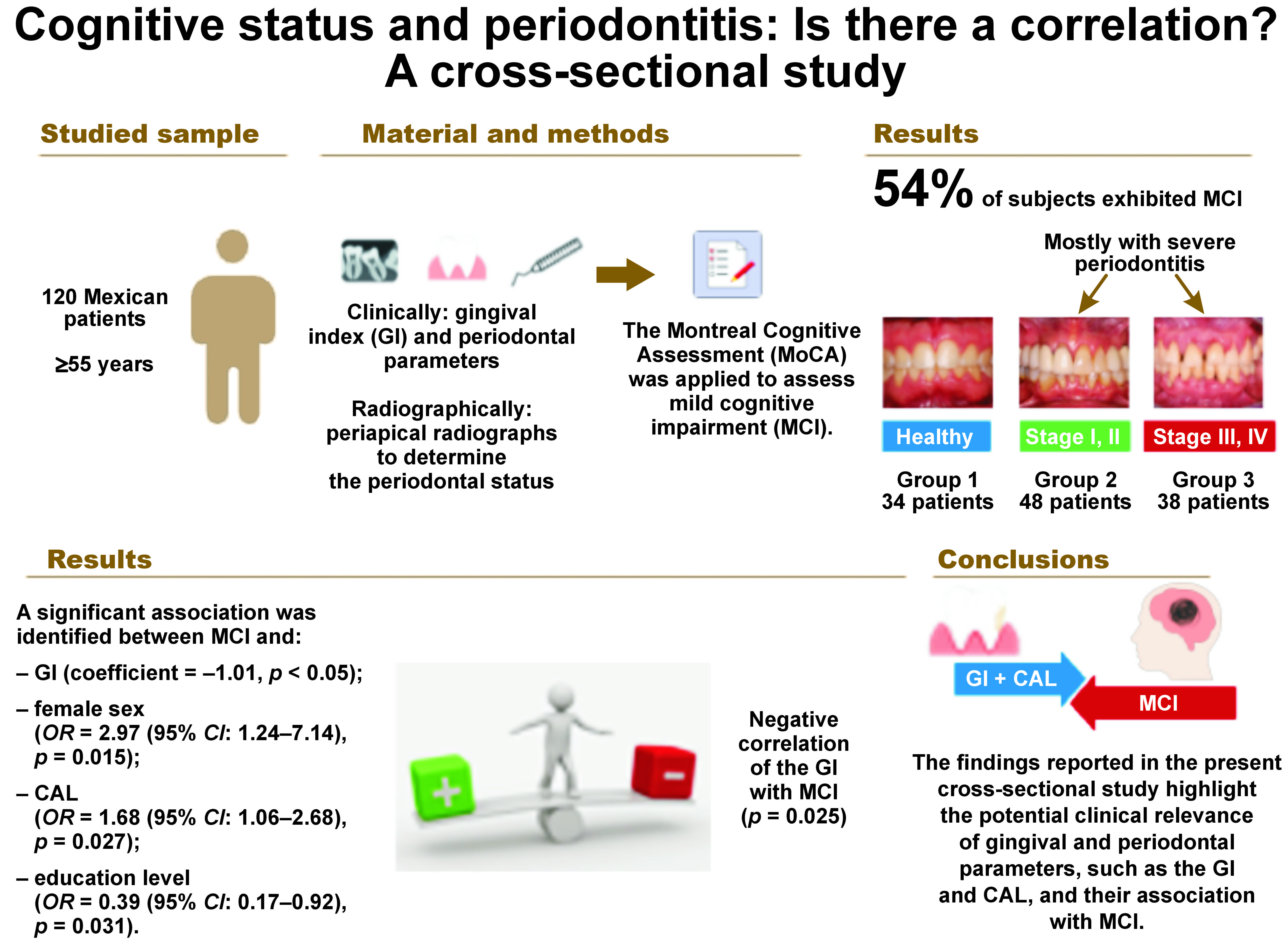
Objectives. The study aimed to determine a possible correlation between the gingival index (GI), periodontitis staging and MCI as evaluated by the MoCA in adults aged ≥55 years.
Material and methods. A total of 120 Mexican patients with or without periodontal disease who met the selection criteria were included in this cross-sectional study. A comprehensive clinical and radiographic evaluation was conducted to determine the periodontal status of all patients. The MoCA was applied to assess cognitive impairment. Descriptive statistics and logistic model-based multivariate statistical analyses were performed to identify variables associated with MCI.
Results. The cognitive status of subjects aged ≥55 years with and without periodontitis was mildly impaired in more than half of the studied sample. Thirty-four patients were diagnosed as periodontally healthy (group 1), 48 were diagnosed with mild to moderate periodontitis (group 2; stages I and II), and 38 had severe periodontitis (group 3; stages III and IV); 54% of the subjects exhibited MCI, mostly in groups 2 and 3. A negative correlation was observed between GI and MCI (p = 0.025). Other prognostic variables associated with MCI were loss of clinical attachment level (CAL) (odds ratio (OR) = 1.68 (95% confidence interval (CI): 1.06–2.68)), sex and education level.
Conclusions. These findings highlight the potential clinical relevance of gingival and periodontal parameters, such as GI and CAL, and their association with MCI in patients of advanced age.
Keywords: mental status, neurocognitive tests, Montreal Cognitive Assessment, chronic periodontitis
Introduction
The process of aging in humans is frequently associated with an increased prevalence of oral diseases, including periodontitis. There is a bidirectional link between periodontitis and ischemic heart disease,1 pregnancy,2 myocardial strain,3 and cognitive changes, including neurodegenerative diseases.4 The early detection of cognitive dysfunction can be accomplished by the utilization of several screening and evaluation instruments to identify affected individuals.5, 6, 7
The Montreal Cognitive Assessment (MoCA) is a brief, standardized, and scoring cognitive screening tool that is one of the most widely used screening tests in healthcare settings for detecting cognitive impairments that may not be as readily apparent on other cognitive tests.5, 8, 9 It is a freely available tool that includes extensive neuropsychological test batteries covering several cognitive conditions.7 The global score ranges from 0 to 30 points, and a higher score reflects better cognitive function. The test’s sensitivity and specificity for the detection of mild cognitive impairment (MCI) are 90% and 87%, respectively.7, 8, 10 Therefore, it is critical to early identify the associated risk factors for this neurological condition to implement opportune therapeutic interventions and prevent complications.6, 11
Previous reports have linked temporomandibular disorders (TMD), impaired masticatory function12 and periodontitis to an increased risk of MCI. Additionally, reduced masticatory function,12 TMD and periodontitis have been reported as risk factors associated with MCI. Temporomandibular disorders are characterized by pain in the temporomandibular joint and masticatory muscles, which decreases chewing efficiency. Chewing is a constant sensory stimulus for the brain. Impaired memory functions in the hippocampus, masticatory muscle loss and weakness are associated with systemic and central inflammation and dementia.12 On the other hand, mastication activates various cortical regions of the brain, and increased cerebral blood oxygenation levels in the hippocampus and prefrontal cortex facilitate learning and memory processes.13 Periodontitis causes structural damage to the tissues surrounding the alveolar bone and periodontal ligament collagen fiber-affected teeth, leading to tooth loss.14 The host response is a complex interplay between numerous cells and inflammatory mediators.14, 15 The host response to periodontal bacteria may induce chronic systemic inflammation, which, in turn, stimulates the production of high levels of inflammatory mediators (P-selectin, receptor activator of nuclear factor kappa-Β ligand (RANKL), intercellular adhesion molecule 1 (ICAM-1)), cytokines (tumor necrosis factor alpha (TNF-α), interferon gamma (INF-γ), interleukin (IL)-1, IL-6, prostaglandin E2 (PGE2)), prostanoids, and matrix metalloproteinases (MMPs).15 The evidence suggests that these inflammatory mediators reach all systems through blood circulation.15 Studies have proposed that peripheral inflammation and dysbiotic conditions contribute to the pathogenesis of MCI.14, 15 Epidemiological data has demonstrated an association between the systemic inflammatory influence of periodontal disease and an increase in the neuroinflammatory response, which can result in cognitive impairment in elderly individuals.15 These studies have reported odds ratios (ORs) and hazard risk ratios within the mild to moderate range.11, 16 Other studies,14, 16, 17, 18, 19, 20, 21, 22, 23 including 3 systematic reviews,14, 16, 17 have also suggested a relationship between periodontitis and cognitive impairment.
The evidence presented thus far suggests that periodontal disease modulates neuroinflammation and neurodegeneration, which may result in cognitive impairment. Therefore, clinicians must objectively assess the medical comorbidities of their patients, including possible cognitive dysfunctions.24 The present analytical cross-sectional study aimed to find a correlation between the gingival index (GI) staging, grading of periodontitis and MCI, as evaluated through the MoCA in Mexican adults aged ≥55 years.
Material and methods
Study population
This cross-sectional study was approved by the Institutional Ethics Committee of the Faculty of Dentistry at the Autonomous University of San Luis Potosí in Mexico (approval No. CEI-FE-036-021). Individuals attending the outpatient Periodontics Clinic (Periodontics Postgraduate Program, University of San Luis Potosí) from October 2022 to June 2023, who met the inclusion criteria, were consecutively selected for the study (non-probability sampling). Prior to enrollment, written informed consent forms were obtained from all participants.
The inclusion criteria were as follows: participants aged 55 years or older with at least 10 natural teeth present; individuals with or without periodontal disease, at different stages of severity, diagnosed through a full-mouth clinical and radiographic examination at baseline. Patients were excluded from the study if they had a history of severe cranial trauma, cerebrovascular disease, chronic headache or migraine, neurodegenerative conditions, brain tumors, chronic subdural hematoma, cryptococcosis, pellagra, hypothyroidism, uncontrolled hypertension, current medication likely to affect the cognition level, or oral acute infectious process.
The demographic information collected encompassed various parameters, such as age, sex, education level, diet, smoking or alcohol consumption, and medical history.
Clinical and radiographic intraoral examination
The number of present teeth and the degree of tooth mobility were recorded. Additionally, the GI was determined according to the Löe–Silness criteria.25 Then, an experienced and precalibrated periodontist documented the probing pocket depth (PPD) and clinical attachment level (CAL) with the use of a North Carolina periodontal probe. These procedures were performed at 6 sites around each tooth (mesiobuccal, buccal, distobuccal, mesiolingual/palatine, lingual/palatine, and distolingual/palatine). Probing pocket depth was measured as the distance from the gingival margin to the base of the pocket. The CAL was determined by measuring the distance from the cementoenamel junction to the base of the pocket. The pocket’s base was established at the level where the periodontal probe encountered maximum resistance.
The presence of bleeding was evaluated through careful probing around each present tooth, and the total percentage of bleeding sites was recorded.26 Additionally, periapical radiographs were taken in all subjects to assess bone height loss and the pattern of loss, as well as other periodontally relevant features, such as loss of cortical and crest bone continuity, widening of the periodontal ligament space, calculus deposits, and periapical/furcation lesions.26
Each subject was diagnosed according to the periodontal parameters described in the current classification of periodontal diseases26: CAL; PPD; the percentage of bone loss; the presence and extent of angular bony defects and furcation involvement; tooth mobility; and tooth loss due to periodontitis. In addition, periodontal disease was categorized into 3 grades according to the risk of disease progression, the anticipated treatment response, and the impact on systemic health,26 as follows: grade A – low risk; grade B – moderate risk; and grade C – high risk. This evaluation includes the assessment of the patient’s general health status and the presence of additional risk factors, such as smoking or diabetes mellitus. The staging of periodontitis was determined as follows: stage I – initial periodontitis (no tooth loss), CAL: 1–2 mm; stage II – moderate periodontitis (no tooth loss), CAL: 3–4 mm; stage III – severe periodontitis with potential for additional tooth loss (≤4 lost teeth), CAL ≥ 5 mm; and stage IV – advanced periodontitis with extensive tooth loss and potential for loss of dentition (≥5 lost teeth), CAL ≥ 5 mm. The CAL was measured interdentally at the site of the greatest loss. The control group consisted of patients with a confirmed absence of periodontal disease, as determined by clinical and radiographic examination.
Cognitive assessment
A complete cognitive evaluation was conducted for each participant. The validated Spanish version of the MoCA tool7, 8, 10 was used to assess the presence of cognitive impairment among the study participants. The test took approx. 10 min to complete and evaluated multiple cognitive skills. Following the intraoral examination, the MoCA test was administered to each patient. The participant was provided with detailed instructions as they proceeded with the test. Ultimately, a single trained evaluator (AMG) interpreted the individual results. The MoCA score ranges from 0 to 30 points. The severity of cognitive impairment was classified according to the final score, as follows: 10–17 points – moderate; 18–26 points – mild; and >26 points – absent.
Evaluator calibration and statistical analysis
To ensure the quality of the measurement of clinical and radiographic periodontal variables, the evaluator (AMG) was calibrated with an expert (interobserver agreement) through kappa statistic (0.82). With regard to the performance of the MoCA tool, the same evaluator was previously trained and duly certified (MoCA Certificate of Completion, 2021/10/07).
A preliminary descriptive analysis was conducted on the collected data. It incorporated the calculation of various statistical measures, including frequencies, means and standard deviations, medians, interquartile ranges, ranges, and percentages. The distribution of the data was evaluated using the Shapiro‒Wilk test. Based on the results, diverse statistical hypothesis tests, both parametric and non-parametric, were carried out for quantitative and categorical data. Initially, exploratory correlation analyses were conducted between quantitative variables, reporting Pearson’s correlation coefficients (r and corresponding p-values), but only of those with significant correlation. Then, multiple binary logistic regression analyses were performed, incorporating diverse prognostic variables in the models. The approach yielded respective ORs (95% confidence intervals (CIs)), which were subsequently used to predict MCI. The statistical analysis was conducted using the GraphPad Prism v. 8 (GraphPad Software, Boston, USA) and STATA v. 14 (StataCorp LLC, College Station, USA) software. More than 80% of the power was achieved, indicating an adequate sample size to contrast the hypothesis of the association between the periodontal status and MCI.24 A statistically significant value of 0.05 was set.
Results
Demographic characteristics of the participants
A total of 120 participants were included in the study; 50% were female, and 50% were male, with a mean age of 63.34 ±4.86 years. Their median age was 59.5 years (interquartile range: 55–95 years). Regarding education level, 46% of the participants had finished undergraduate studies, while only 6% obtained a postgraduate degree. Patients were divided into 3 groups according to their periodontal characteristics. The control group (group 1, n = 34) comprised periodontally healthy individuals (<10% bleeding on probing and PPD < 4 mm), with or without minimal insertion loss. The second group (group 2, n = 48) consisted of patients diagnosed with stage I and II (moderate) periodontitis, grades A–C. The third group (group 3, n = 38) consisted of patients diagnosed with stage III and IV (severe) periodontitis, grades A–C (Table 1).
There were 15% smokers in the sample, with the majority (20%) belonging to group 2. Regarding the body mass index (BMI), it was observed that all 3 groups were overweight. Additionally, the severity of periodontal disease increased along with the BMI. A comparison of the BMI among the 3 study groups revealed no statistically significant differences (p = 0.268). Regarding comorbidities, 68% of the participants exhibited one or more systemic diseases, with the highest prevalence observed among individuals with stage III and IV periodontitis (84%). Thirty different types of diseases were identified. The most prevalent conditions were arterial hypertension (33%; predominantly observed in group 3) and type 2 diabetes mellitus (20%). The occurrence of benign prostatic hyperplasia, hypothyroidism and depression was also documented. Furthermore, 74 different types of medications were reported, with metformin, losartan, enalapril, and omeprazole being the most prevalent.
Periodontal characteristics
The clinical parameters (number of teeth, bleeding sites, bleeding on probing, PPD, GI, and CAL) were described and compared between the study groups (Table 2). Significant differences were identified between the groups. The severity of these parameters increased concomitantly with the severity of periodontitis, thereby validating the periodontal diagnosis and classification.
Cognitive assessment
Fifty-four percent of the participants exhibited MCI, with a cut-off point of 23, as established by Thomann et al.7 When the sample was divided according to the periodontal status, the percentage of MCI was obsrved to increase with the severity of periodontitis. Patients in groups 2 and 3 showed a mild degree of cognition, while the overall score of healthy patients corresponded to a normal cognitive level. However, no statistically significant differences were identified between the groups (p > 0.05) (Table 3).
Cognitive status and gingival index
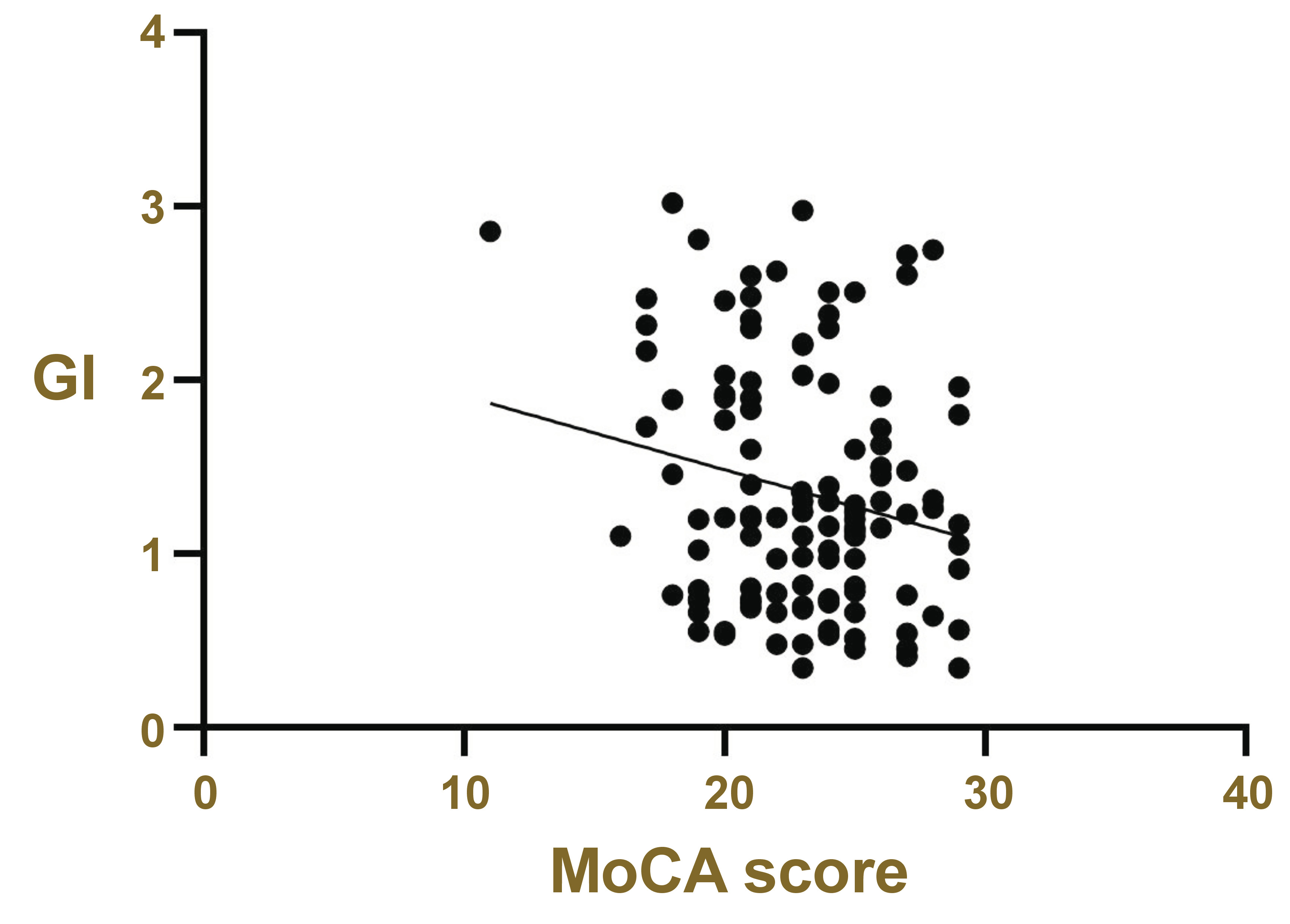
Possible relationships between different gingival and periodontal variables and MCI, as evaluated by the MoCA, were determined. Only the GI showed a significant negative correlation with MCI (p = 0.025) (Figure 1). In light of these findings, MoCA scores were obtained for all participants, who were then classified into 3 groups according to the extent of inflammation: mild (23.28 ±3.03); moderate (23.29 ±3.22); and severe (21.50 ±2.86).
A correlation was identified between the GI and the MoCA score (coefficient = –1.01, p = 0.032) with adjustments made for age, sex, BMI, education level, and comorbidities (arterial hypertension, diabetes mellitus and depression) (Table 4). Furthermore, the likelihood of presenting MCI in cases of severe gingival inflammation was 3.03 (p = 0.062), and the individuals with a high level of education showed a 63% decrease in the likelihood of developing MCI compared to those with a low level of education (OR = 0.37, 95% CI: 0.15–0.86, p = 0.021) (Table 5).
Cognitive status according to sex and age
Based on the MoCA results, female patients with periodontal disease exhibited a greater tendency for developing MCI than males. However, this difference was not statistically significant. The participants were divided into 3 age groups (55–59, 60–74, and ≥75 years). Lower MoCA scores were observed among the oldest participants. The presence of MCI was detected only in patients over the age of 60.
Association of mild cognitive impairment with periodontal parameters and other variables
Another logistic regression model for the occurrence of MCI was conducted, with adjustments made for the number of teeth, age, BMI, sex, comorbidities (arterial hypertension, diabetes mellitus, depression), and education level. Participants with loss of CAL exhibited a 68% higher possibility of developing MCI than participants without loss of CAL (OR = 1.68, 95% CI: 1.06–2.68, p = 0.027). Female participants were 2.97 times more likely to have MCI than male subjects (OR = 2.97, 95% CI: 1.24–7.14, p = 0.015) (Table 6).
Discussion
This cross-sectional study aimed to determine the cognitive state evaluated by the MoCA in Mexican adults aged ≥55 years, with or without periodontal disease. A contemporary classification system for periodontal diseases was implemented, encompassing the general status of each patient and the presence of comorbidities or risk factors.25, 26, 27, 28, 29 Consequently, it was determined that more than 50% of the subjects exhibited MCI. This finding can be explained by the presence of chronic inflammation caused by periodontal disease and the production of inflammatory mediators and bacterial products. These factors may exacerbate neurodegeneration processes systemically or trigeminally, favoring the presence of MCI.20, 21 However, in periodontally healthy subjects, MCI was present in more than half of the participants, which may be the result of other risk factors associated with systemic inflammation comorbidities, such as obesity, smoking and genetic alterations.15, 30, 31
The evidence demonstrates that severe gingival inflammation and/or chronic periodontitis are peripheral sources of inflammatory cytokines. The cytokines increase the levels of C-reactive protein, which favors the activation of microglial cells and the production of misfolded proteins. These proteins are biomarkers that contribute to the occurrence of dementia and accelerate neurodegeneration.11, 31, 32, 33 Consequently, subjects with severe gingival inflammation show a greater tendency to MCI compared to those with a mild or moderate GI. Likewise, a significant negative correlation was observed between the MoCA score and the level of gingival inflammation, suggesting that reduced gingival inflammation is associated with enhanced cognitive function. This finding indicates that gingival inflammation may contribute to the development of MCI, which is consistent with the results of studies by Hategan et al.11 and Stewart et al.,34 who found a positive correlation between inflammation, IL-1β levels and MCI. Moreover, a logistic regression model implemented in our study demonstrated a tendency toward MCI in cases of severe gingival inflammation. In this regard, Shin et al. stated in their cohort study that the presence of chronic periodontal inflammation (with severe gingivitis and CAL) is not sufficient to observe its positive association with cognitive decline.16 A period of persistent inflammation is also necessary for the cognitive status to be affected.
In the present study, a negative correlation was observed between MCI and the GI. A case–control study conducted on patients with active periodontitis reported significant cognitive impairment over a period of 6 months compared to patients with inactive periodontitis.35 This evidence suggests that the level of disease activity, the amount of bone destruction, inflammation, and exposure time are potential factors that activate neurodegeneration and trigger MCI. Yoneyama et al. found that there was an increase in the Mini–Mental State Examination (MMSE) test scores in a 24-month study of oral care in older adults.36 The MMSE is a brief screening tool commonly used to assess dementia, orientation, memory, attention, language, and visuospatial skills. These findings demonstrated that reducing periodontal inflammation can reverse the progression of MCI.36 In addition, clinical and animal studies have reported that the administration of paracetamol, ibuprofen, and non-steroidal anti-inflammatory drugs (NSAIDs) is associated with a significant reduction in IL-1β, IL-6, TNF-α, and PGE2, improved cognitive function, and reduced systemic inflammation.37, 38, 39, 40 It can thus be inferred that neuroinflammation is related to inflammatory systemic states, and that the modification of the related processes could have a positive effect on cognitive function.
Concerning clinical attachment loss, which is equally essential to the diagnosis of periodontitis, the present study identified a positive association between clinical attachment loss and MCI, with an OR of 1.68 (95% CI: 1.06–2.68). Subjects with evident loss of CAL were 68% more likely to develop MCI than those without CAL loss. The progression of clinical attachment loss is contingent upon the stage of periodontal disease. Therefore, the longer the periodontal disease, the greater the loss and the subsequent development of chronic neuroinflammation and MCI. The phenomenon of cognitive deterioration is preceded by a loss of insertion. Furthermore, Gil Montoya et al., in their case–control study, used cognitive tests (“Phototest”) in conjunction with 29 inflammatory biomarkers to determine the level of peripheral inflammation and to establish a potential association between periodontal disease and MCI or dementia.18 The authors demonstrated that clinical attachment loss was significantly associated with MCI, reporting an OR of 2.97 (95% CI: 1.61–5.48).18
Other periodontal variables have been linked to MCI. In the current study, as the severity of periodontitis increased, a greater tendency toward the condition was observed. Periodontally healthy subjects showed no impairment (with an average MoCA score of 23.15 points) compared to groups with periodontitis. However, this difference was not statistically significant, possibly due to the cross-sectional design of the study. The study design is subject to limitations, particularly due to constraints in sample size and the measurement of all variables at a single time point. Nevertheless, robust evidence supports a link between periodontal indicators, specifically the GI and CAL, and cognitive deterioration.4, 11, 14, 15, 16
This outcome is consistent with the results of the study by Hategan et al., which revealed that subjects diagnosed with aggressive periodontitis had lower MoCA scores compared to those with mild periodontitis or periodontally healthy subjects.11 A lower test score was also observed in subjects with moderate periodontitis compared to those without periodontal disease. Two other studies have reached similar conclusions. These studies were conducted in 2021 by Hu et al.14 (using a cohort design) and Guo et al.41 through a systematic review/meta-analysis. On the other hand, a cross-sectional study conducted by Naorungroj et al. revealed no significant association between severe periodontitis and MCI.42
In their meta-analysis, Guo et al. included 20 observational studies.41 When periodontitis and cognitive impairment were associated, the OR was 1.77 (95% CI: 1.31–2.38), indicating a strong relationship between the 2 conditions. However, the statistical analysis revealed no significant effect of periodontitis on dementia, with an OR of 1.59 (95% CI: 0.92–2.76). Additionally, Guo et al. stated that the MMSE is less sensitive and specific than the MoCA, and that it could generate false positive results. In addition, they considered the MMSE as a test designed specifically for dementia and not for MCI, as the MoCA is.41 In this regard, and according to Nasreddine et al.,8 the MoCA is specifically designed to detect subtler cognitive deficits, which characterize MCI, earlier and more accurately.
Multiple chronic comorbidities, such as high blood pressure, hyperlipidemia, coronary heart disease, or heart attacks, are considered high-risk prognostic factors for the development of MCI. Depression, diabetes mellitus and chronic obstructive pulmonary disease have been identified as moderate risk factors.15, 18, 32, 43 Several chronic conditions were present in the studied sample, which were subsequently incorporated into multivariate regression models. However, a significant association could not be demonstrated in all 3 study groups between hypertension and type 2 diabetes mellitus with MCI. Both diseases are prevalent among the Mexican population and are considered major contributors to mortality after the age of 50.44 No association was identified between depression and MCI.
Regarding the variables of age, sex and education level, a positive association was identified between MCI and female sex (OR = 2.97 (95% CI: 1.24–7.14)). In postmenopausal women, the amount of estrogen, a vital component of cognitive function, decreases. Estrogens increase synaptic properties in neurons, act as antioxidants to protect neurons from free radicals, improve mitochondrial function in the brain, and induce DNA repair. Our results are consistent with several previous reports.40 The logistic regression model did not demonstrate a correlation between the age of the participants and MCI. However, as expected, a decline in performance was evident with increasing age, particularly in the 75–90 age group. This decline was not statistically significant. Cecato et al. reported analogous results in their cross-sectional study of 136 elderly participants with more than 4 years of education.45 The current study found a positive association between the education level and MCI (OR = 0.39 (95% CI: 0.17–0.91)), in concordance with the study of Langa and Levine.46
It is important to note that a rapid growth of dementia usually occurs in subjects of advanced age. Currently, there is no known cure for this disease. Therefore, it is necessary to apply diagnostic tests during the early stages of the condition, encompassing electrophysiological studies, magnetoencephalography, and magnetic resonance spectroscopy.47, 48, 49 However, these technologies are not available in a traditional dentist’s office. The present study claims that the application of the MoCA in periodontitis-affected patients may facilitate the identification of an association with their cognitive status.
According to recent research, the management of periodontal disease may play a pivotal role in the prevention of cognitive impairment. In a recent retrospective cohort study, Lee et al. examined the extent and temporal aspect of the relationship between periodontal disease, dental health and dementia.50 The participants were assigned to one of the 4 study groups, namely dental prophylaxis, intensive periodontal therapy, tooth extraction, and no treatment at all. Next, a comparison was made between the groups’ dementia incidence rates. The study found that the incidence of dementia was notably higher in the groups that did not receive treatment (0.76% annually) and those that underwent tooth extraction (0.57% annually) compared to the groups that received dental prophylaxis (0.39% annually) and underwent intensive periodontitis treatment (0.35% annually). The risk of dementia was higher in subjects with more severe periodontal disease or in those who did not receive any periodontal therapy.
Strengths and limitations
This study, conducted in Mexico, is among the first to demonstrate a connection between periodontitis and MCI using the MoCA. The Montreal Cognitive Assessment is more precise and accurate in detecting MCI compared to the widely used the MMSE test. In addition, the test was administered by a researcher who had received certification for its application and interpretation of subsequent results. Another strength of the study is the implementation of a new and improved classification of periodontal diseases, as proposed by Tonetti et al.26 and Caton et al.30
The study’s main limitations were the limited sample size and the narrow range of selection criteria chosen. The following risk factors associated with MCI were not considered: lifestyle; intake of NSAIDs; family history of cognitive dysfunction; socioeconomic status; and sedentary lifestyle. There was no strict control of potential confounding variables. The authors propose the execution of future longitudinal clinical studies of higher methodological quality, with the objective of establishing whether periodontal treatment modifies the MoCA score.
Conclusions
The findings reported in the present cross-sectional study highlight the potential clinical relevance of gingival and periodontal parameters, such as GI and clinical attachment loss, and their association with MCI. A comprehensive periodontal evaluation is imperative for patients aged ≥55 years old, as it can serve as an objective indicator of their cognitive status within the context of periodontal care.
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of the Faculty of Dentistry at the Autonomous University of San Luis Potosí in Mexico (approval No. CEI-FE-036-021). Prior to enrollment, written informed consent forms were obtained from all participants.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.