Abstract
The temporary use of contingent electrical stimulation with the GrindCare® device may offer a practical, non-invasive solution to reduce bruxism and protect restorations during vulnerable phases of phased dental rehabilitation.
Keywords: bruxism, tooth wear, electrical stimulation, resin composites
Bruxism, whether manifesting during wakefulness or sleep, is a commonly encountered phenomenon in clinical dental practice. It continues to stimulate discussion and research due to its multifactorial nature and diverse clinical implications. Notably, our current understanding suggests that bruxism may not always be a harmful behavior; instead, it can serve as either a risk and/or a protective factor, depending on the individual case and context.1 This dual nature presents a unique challenge for clinicians tasked with discerning when, and how, to intervene.
Bruxism diagnostic tools are currently categorized into 3 diagnostic levels.1, 2 They are termed subject-based when they rely solely on patient self-reporting; clinically based when accompanied by clinical signs, such as the linea alba, impressions on the tongue or cheek, or tooth wear; and device-based when confirmed via electromyographic (EMG) or polysomnographic (PSG) recordings. While these definitions aid in structuring clinical evaluation, they do not always clarify whether bruxism is active or residual, further complicating treatment decisions.
Importantly, bruxism is no longer regarded as a disorder in itself. Instead, it is understood as a behavior that warrants treatment only when it results in negative outcomes (e.g., tooth wear, pain). One promising approach in such cases is contingent electrical stimulation (CES), where muscle activity is detected and interrupted via mild electrical impulses.2 One of CES tools is Butler® GrindCare® (Sunstar Suisse SA, Etoy, Switzerland), a single-channel EMG-based device that exemplifies this approach.2 It records activity from the temporalis muscle and, when in the therapeutic mode, responds to bruxism events with mild electrical stimulation intended to disrupt the event without causing discomfort. The device has utility for both diagnosis – by establishing a baseline level of activity – and therapy, by potentially reducing excessive muscle contractions during sleep. Following a standard protocol, patients wear the device for a two-week assessment period. If more than 15 episodes per hour are recorded, treatment is recommended.2, 3
As one of the possible negative outcomes of bruxism, tooth wear is a multifactorial process in itself, and while bruxism plays a central role, other contributors must be acknowledged. Among these are opposing restorations, especially when constructed from hard materials, such as ceramics or metals. These can exacerbate wear patterns and pose additional risks during restorative treatment.
Tooth rehabilitation strategies are typically categorized into direct or indirect methods.4 Direct composite restorations offer a conservative approach. They are minimally invasive, easier to repair, and generally exhibit mechanical properties, such as elasticity and wear resistance, more closely aligned with natural dentin.5 On the other hand, they may be prone to discoloration over time, and because of placing multiple restorations over several appointments due to time constraints, patients might experience an open bite. In some cases, a Dahl plateau is created to establish a stable raised bite following the first appointment. However, this may not always be feasible due to the existing prosthodontic restorations or periodontal considerations. Between the 1st and 2nd session, there is an elevated risk of mechanical overloading with limited options for protecting the teeth during this time interval.
In this perspective, we illustrate the above notions with a case where the GrindCare was utilized temporarily during a dental rehabilitation procedure to reduce the risks posed by bruxism during a transitional, high-risk period between appointments.
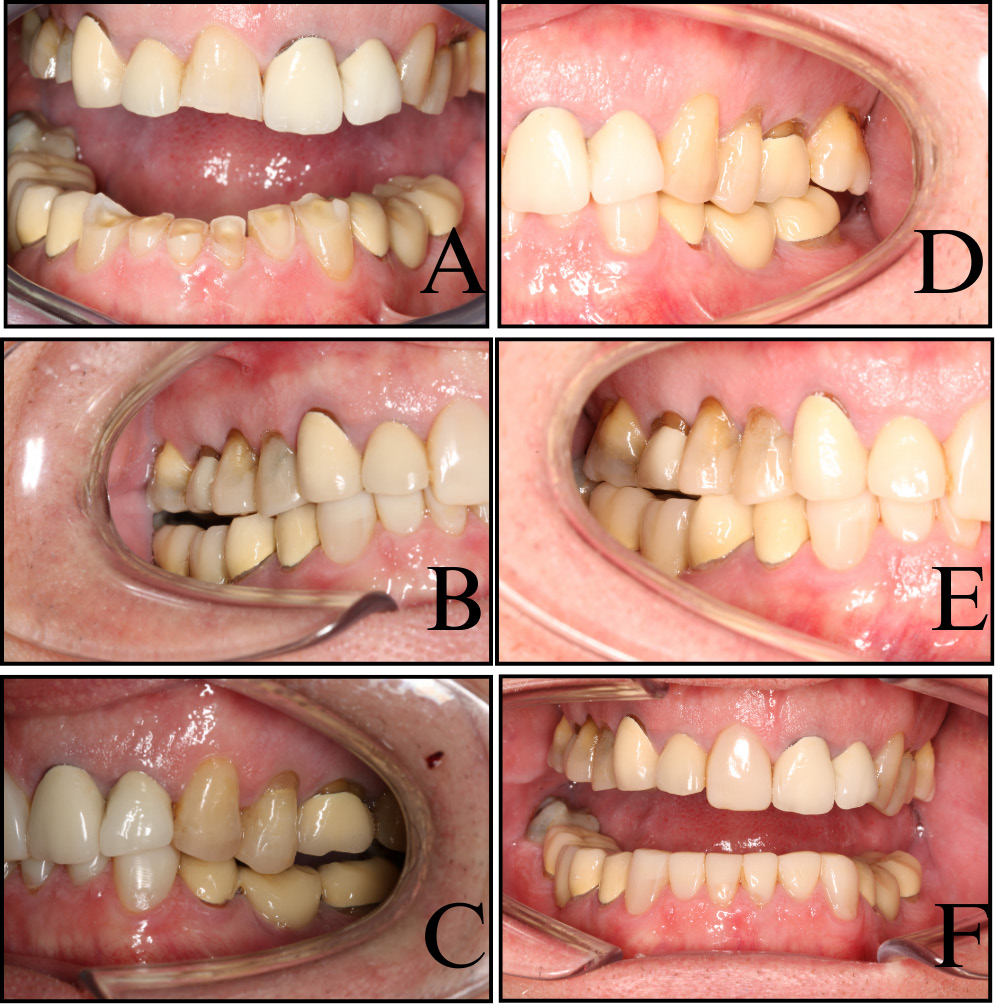
A 67-year-old male patient was referred to our tertiary university clinic for the evaluation of severely worn lower anterior teeth. His primary concerns were both functional (lip biting during eating) and esthetic (dissatisfaction with the shortened appearance of the teeth) (Figure 1A). His general medical and psychosocial histories were non-remarkable. Snoring, alcohol, caffeine and acidic beverage intake, obstructive sleep apnea (OSA), gastroesophageal reflux disease (GERD), eating disorders, medication use, depression, anxiety, stress, and dry mouth were assessed via structured interviews and validated self-report instruments, including Epworth Sleepiness Scale (ESS), GERD Questionnaire (GerdQ), eating habits questionnaires (Eating disorder Screen for Primary care (ESP) and the Sick-Control-One stone-Fat-Food (SCOFF)), Diagnostic Criteria for Temporomandibular Disorders (DC/TMD), Perceived Stress Scale 4 (PSS-4), Trauma Screening Questionnaire (TSQ), and Xerostomia Inventory (XI). All scores were zero except XI (score: 1). Sleep bruxism was assessed using the GrindCare and DC/TMD questionnaires, focusing on the past month. From a dietary standpoint, acidic intake was modest, with the patient consuming one piece of fruit daily and a small glass of fruit juice each morning.
Intraoral examination revealed healthy periodontal tissues with no probing depths exceeding 3 mm, and intact oral mucosa without signs of clenching. Overjet was 1 mm, overbite was 3 mm, and the patient had Angle class 1. Yet, there were visible bruxopositions (i.e., visible matching wear facets during laterotrusion and protrusion) on incisors and canines. There was a significant number of existing prosthetic restorations present, and their materials and occlusal design were contributing factors in the observed wear. Using the Tooth Wear Evaluation System (TWES),6 we documented localized, extreme tooth wear in the lower anterior region, of a mechanical nature, influenced by the opposing ceramic restorations.
Cold sensitivity testing revealed delayed but positive pulp responses in lower incisors, while radiographic examination showed no underlying pathology. Based on these findings, bruxism was classified as clinically based wake or sleep bruxism, although clinical signs alone were insufficient to determine whether bruxism was ongoing.
To further investigate whether this process was active, we initiated a two-week assessment period, using the GrindCare.2 The collected data revealed 246–582 bruxism episodes per night, translating to an hourly rate of 3–147 episodes – well above the clinical threshold of 18 events per hour.2, 7 This confirmed the presence of active sleep bruxism.
Our treatment strategy unfolded in 2 phases:
Phase 1 involved restoring teeth 15, 14, 11, 23, and 24, using direct composite restorations to raise the vertical dimension and create space for lower anterior reconstruction. We intentionally avoided pre-treatment mock-ups, wax-ups or guides, relying instead on direct intraoral assessment and real-time adaptation. The materials used included AP-X A3 (Kuraray Noritake Dental, Tokyo, Japan) for occlusal and palatal surfaces, and the Clearfil™ Photo Bright composite in shade UO (Kuraray Noritake Dental) for a veneer on tooth 11. Adhesion followed a standardized protocol with Clearfil Photo Bond (Kuraray Noritake Dental), 37% orthophosphoric acid etching and Clearfil SA Primer (Kuraray Noritake Dental).
Following the 1st session, the patient began using the GrindCare in the therapeutic mode, configured to deliver CES during episodes of increased temporalis activity. The device offers 10 intensity settings. Patients are guided to start at the lowest level, gradually increasing every night until the stimulation wakes them up, and then stepping down by one level. During this period, bruxism episodes dropped to 54–240 per night, indicating a substantial initial reduction.
Phase 2 addressed the lower anterior segment. The composite was applied to the lingual surfaces, using AP-X A3, and veneers were layered with Clearfil Photo Bright in multiple opacities (UO, YO, LO). The bonding protocol matched the one used in the upper arch. The posterior occlusion remained open, relying on passive eruption per the Dahl principle to close vertical gaps over time (Figure 1B,C).8 During this phase, the patient continued using the GrindCare nightly, aiming to maintain reduced muscle activity.
Over the next 12 months, follow-up assessment was performed at 3, 6 and 12 months. At 3 months, CES was discontinued, as the patient reported diminished responsiveness, and the bruxism index (BI) returned to baseline by week 8 (166–462 episodes/night). The posterior occlusion continued to re-establish, and the patient reported no functional limitations or eating restrictions.
At the 24-month review, molar contacts were restored through natural eruption, and all restorations remained intact. No fractures, debonding or chipping were noted. However, the crown on tooth 21 had begun to cause accelerated wear on the opposing restoration at tooth 32, warranting future monitoring (Figure 1D–F).
This case highlights the pragmatic utility of the GrindCare during the transitional phases of tooth rehabilitation. When conventional occlusal splints are impractical – during occlusal instability or open-bite stages – devices like the GrindCare offer a non-invasive, patient-directed alternative for short-term bruxism management. While the mechanism behind CES remains only partially understood, its ability to temporarily modulate muscle activity is noteworthy. However, the observed decrease in muscle activity during GrindCare use between sessions may be attributed not only to the biofeedback mechanism of the device, but also to the transient occlusal changes induced by the interim composite restorations, which potentially altered proprioceptive input and reduced parafunctional triggers.9
Yet, our experience also underscores the temporary nature of CES efficacy. As in this case, initial reduction may not persist beyond 2 months, raising important questions regarding long-term viability. Further research is needed to explore adaptive mechanisms, such as sensory accommodation or neuroplasticity, that may limit prolonged effectiveness.
In terms of restorative choice, our decision to pursue direct composite restorations aligns with growing evidence that such materials offer repairability, biomimetic elasticity and acceptable esthetics in bruxism patients.4 While ceramic options may provide superior esthetics, they pose a greater risk of total failure and contribute more aggressively to antagonist wear, especially in cases with the pre-existing ceramic restorations.
Ultimately, our experience affirms that the GrindCare can serve as a useful adjunct, particularly during vulnerable phases of treatment. However, randomized controlled trials are essential to validate its long-term role and clarify its mechanism of action. Until then, CES should be viewed as a complementary, rather than primary, modality in managing bruxism during complex restorative cases.
Patient consent
Written informed consent was obtained prior to the submission of this perspective. The patient authorized the use of clinical history, data and imagery for academic publication, under the condition that identifying features remain obscured.