Abstract
Background. Obstructive sleep apnea (OSA) is associated with an increased likelihood of health issues, such as hypertension, cardiovascular disease and stroke. Screening is typically performed through self-report questionnaires related to OSA symptoms.
Objectives. The present study aimed to evaluate sex differences in the commonly used questionnaires for the evaluation of OSA symptoms in order to determine whether different OSA screening tools should be considered in males and females.
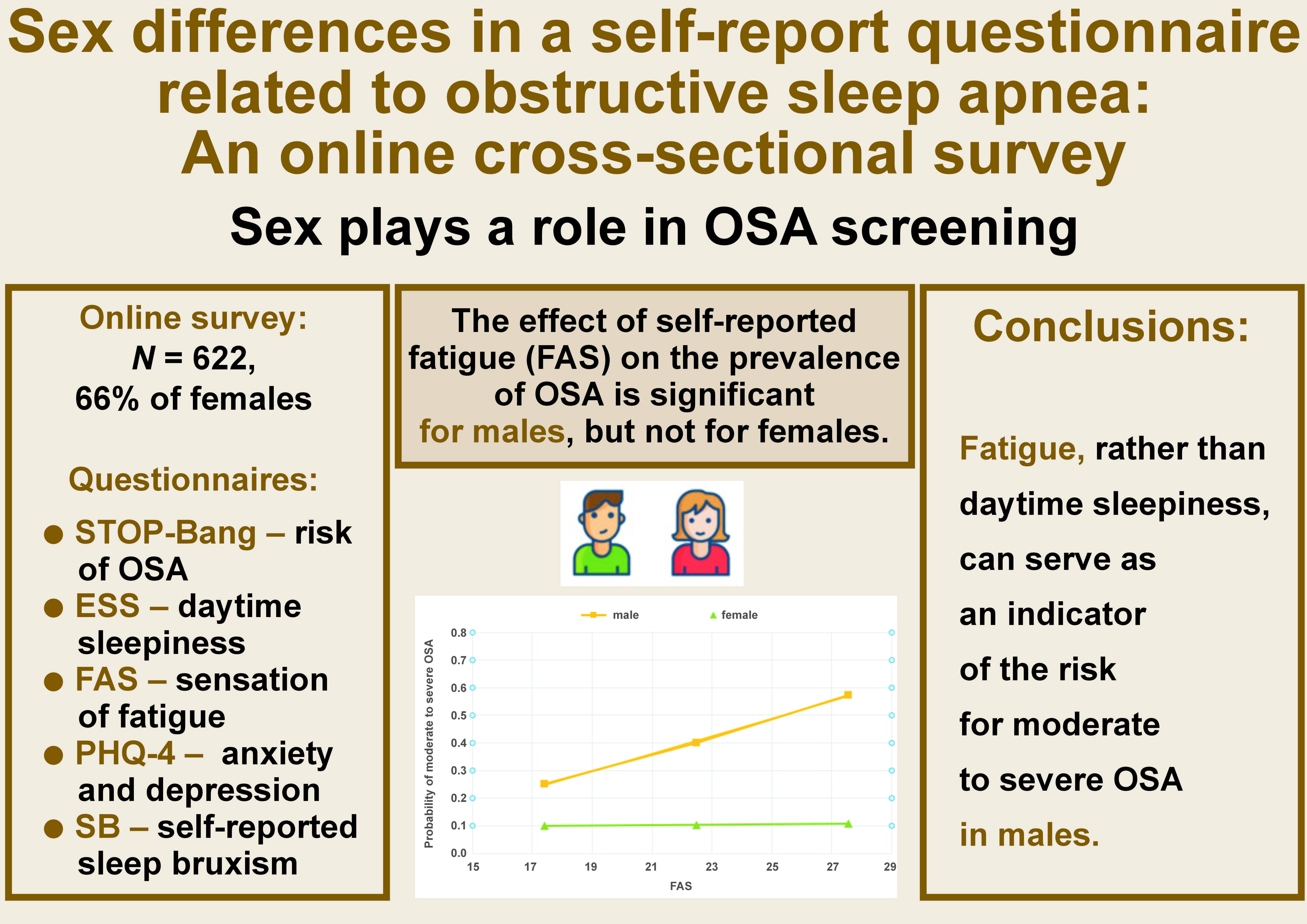
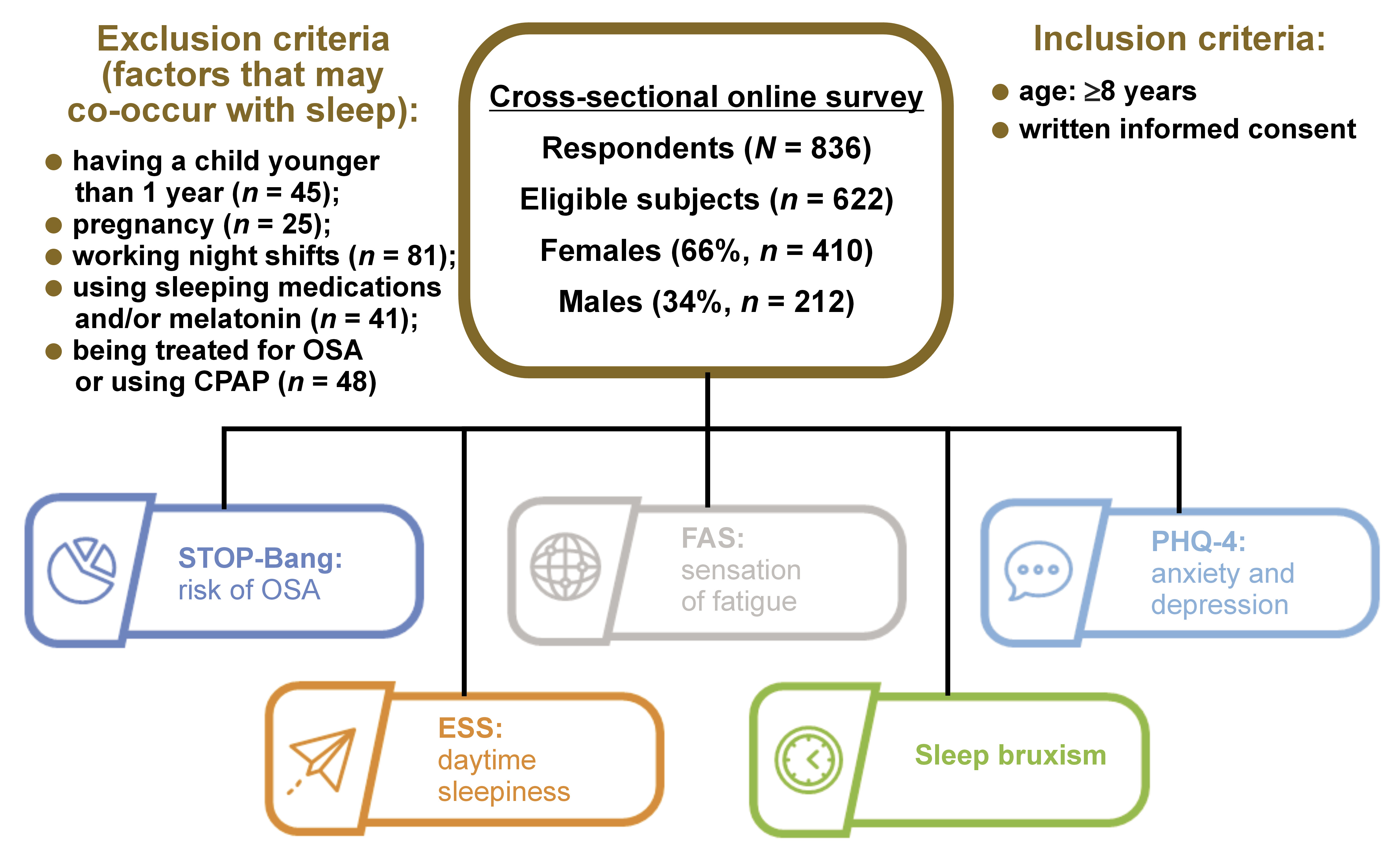
Material and methods. The data was collected from the general population (N = 622, 66% female) through an online cross-sectional survey. The survey incorporated the STOP-Bang Questionnaire, the Epworth Sleepiness Scale (ESS), the Patient Health Questionnaire-4 (PHQ-4), the Fatigue Assessment Scale (FAS), and sleep bruxism (SB) questionnaires.
Results. Female subjects exhibited elevated levels of anxiety and fatigue (p < 0.001 for both) and the potential presence of SB (p < 0.005). The logistic regression analysis revealed that the odds of moderate to severe OSA increased by 5–8% for age and sleepiness, were higher for subjects exhibiting SB (an increase of 82%), and were particularly high for males (male sex increased the odds of moderate to severe OSA by over 5 times). Despite higher fatigue scores among females, the effect of fatigue on the probability of moderate to severe OSA in females was non-significant. While male subjects demonstrated lower fatigue scores, these levels were significantly associated with the risk of moderate to severe OSA. Daytime sleepiness did not influence the OSA risk for either sex.
Conclusions. The impact of reported fatigue on the prevalence of OSA is substantial among males but non-significant among females. The efficacy of daytime sleepiness scales in evaluating OSA is poor. The fatigue scale may be more effective in the screening of OSA, at least in males. Limitations of the study include potential response bias due to participant anonymity and the use of the STOP-Bang Questionnaire instead of polysomnography, the gold standard for OSA diagnosis.
Keywords: sex, STOP-Bang, obstructive sleep apnea (OSA), Epworth Sleepiness Scale (ESS), Fatigue Assessment Scale (FAS)
Introduction
Obstructive sleep apnea (OSA) belongs to a broad list of conditions referred to as sleep-disordered breathing, which range from snoring to OSA.1 Obstructive sleep apnea is characterized by a repetitive collapse of the upper airway during sleep, frequently associated with oxygen desaturation and/or arousal from sleep.2 Although patients with nocturnal snoring tend to have smaller airway dimensions, their pharyngeal measurements do not differ from those of non-snoring individuals.3 The only parameter found to relate to upper airway volume is vertical skeletal dimension.4
Obstructive sleep apnea is closely linked to arterial hypertension. A study on patients with comorbid arterial hypertension and OSA revealed notable associations between sleep fragmentation and the levels of calcium, uric acid and magnesium in their blood samples.5 Additionally, OSA has been associated with the disruption of the circadian rhythm and metabolic dysregulation of circadian clock proteins.6
The prevalence of OSA is higher in males than females, even after correcting for age and body mass index (BMI).1, 7 The clinical presentation of OSA differs between the sexes.8, 9 These differences may originate from hormonal influences and/or anatomic differences, such as the shape of the upper airways, distribution of body fat, and craniofacial morphology. Generally, females are more symptomatic, experience prolonged partial upper airway obstruction, and report insomnia as a symptom of OSA more frequently than men.10 Males tend to report sleepiness, snoring and apnea, while females report fatigue, initial insomnia, depression, and morning headaches.8, 11 As a result, women are less likely to be diagnosed and treated for OSA than men.10, 12 In some cases, women with OSA may be misdiagnosed and treated for other conditions, such as depression, insomnia and hypothyroidism.13 A comparison of males and females with similar OSA severity reveals that women utilize healthcare resources to a greater extent than males due to atypical symptoms, a poor perception of health and the overuse of psychoactive medication.14
The gold standard for OSA diagnosis is polysomnography,10, 15 because it monitors multiple physiological parameters during sleep, ensuring an accurate and objective diagnosis. However, initial screening is usually performed using self-report questionnaires related to OSA symptoms, whose predictive performance has been widely evaluated.16, 17 Several such screening tools include the Epworth Sleepiness Scale (ESS),18 the STOP and STOP-Bang Questionnaires,19 the Gait Outcomes Assessment List (GOAL) Questionnaire,20 the Berlin Questionnaire,20 the Athens Insomnia Scale (AIS),21 and the Fatigue Assessment Scale (FAS).22 However, it is not yet clear whether there is a sex-specific influence on the predictive performance of the different questionnaires. Pataka et al. evaluated possible sex differences in various questionnaires used for OSA prediction and concluded that sex-specific evaluation of questionnaires is necessary to prevent OSA underdiagnosis.23
This study utilized an online questionnaire to effectively identify issues related to OSA. This method enables access to a broad spectrum of the population, including men and women who might be unaware of their condition or have been underdiagnosed with OSA.
Few questionnaires were assessed to determine the most effective tool for each subgroup (based on sex and age).
The present study aimed to examine sex differences in the commonly used questionnaires for the evaluation of OSA symptoms (ESS,18 STOP-Bang Questionnaire,24 FAS,22 and Patient Health Questionnaire-4 (PHQ-4))25 in order to determine whether different OSA screening tools should be considered for males and females.
Material and methods
The study was conducted as a cross-sectional online survey through the administration of anonymous questionnaires. Participation in the survey was voluntary. The survey was posted on WhatsApp groups and billboards from March 2023 to October 2023. The data for the present study was collected through Google Forms (https://docs.google.com/forms/d/1ZDd74tBezP5D-xsj0VxBj23yKZ_YnI8SaNOLkF8CDyk/edit).
The minimum required sample size was determined using a sample size calculator (https://www.calculator.net/sample-size-calculator.html). The following parameters were used: a population size of 9,000,000 (representing the population size in Israel); a confidence level of 95%; a maximum error of 5%; and a population proportion of 20%.26 Based on these calculations, a minimum sample size of 246 individuals was determined.
The study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee at Tel Aviv University, Israel, approved all study procedures (approval No. 0005883-2 15/1/2023).
Instruments and study variables
Demographic information
The following information was collected: age; sex; personal status (single, married, divorced, widowed); number of children and the age of the youngest child; pregnancy (yes/no); use of pharmacological drugs to improve sleep (including melatonin) (yes/no); treatment for disruptive sleep (e.g., continuous positive airway pressure (CPAP) machine, oral appliances) (yes/no); having a job (yes/no) and the type of work (daytime, night-time, shifts).
Risk of OSA
The Hebrew version of the STOP-Bang Questionnaire, developed and validated by Chung et al. as a screening tool for OSA,24 was used in the present study. The questionnaire showed high sensitivity, especially among patients with moderate to severe OSA.
The first part of the questionnaire consists of 4 self-report yes/no questions concerning Snoring, Tiredness during daytime, Observed apneas, and high blood Pressure (STOP). The total score on the STOP questionnaire ranges from 0 to 4.
The second part of the questionnaire (Bang) refers to BMI, Age, Neck circumference, and Gender (BANG). In the present study, data concerning neck circumference and height (necessary for the calculation of BMI) was not collected.
As the aim of the present study was to identify subjects with moderate to severe risk of OSA who should be referred for further evaluation, 2 categories of OSA were defined, as suggested by Chung et al.24:
– low risk: score of 0–2 on the self-report questions of the STOP part;
– moderate to severe risk: a combination of 2 items from the STOP part and 1 from the BANG part, as follows: STOP score of 3–4; or STOP ≥ 2 + BANG ≥ 1; or STOP ≥ 2 + male sex; or STOP ≥ 2 + age >50 years.
Daytime sleepiness (ESS)
Daytime sleepiness was assessed using the ESS.18 The questionnaire measures the subject’s general level of daytime sleepiness. The questionnaire consists of 8 items, which are scored on a scale from 1 to 4. The scale assesses daytime sleepiness through asking the subjects about their propensity to fall asleep or doze off during common daily activities (e.g., reading, watching TV, talking). The final scores for daytime sleepiness are as follows:
– 0–5: low normal;
– 6–10: high normal;
– 11–12: mildly excessive;
– 13–15: moderately excessive;
– 16–24: severely excessive.
Patient Health Questionnaire-4 (PHQ-4)
The Patient Health Questionnaire-4 is used for screening anxiety and depression.25 It is a validated ultra brief tool consisting of 2 questions about anxiety and 2 questions about depression.
Respondents rate the frequency with which they had been bothered by specific problems over the past 2 weeks on a scale from 0 (not at all) to 3 (nearly every day). The total score ranges from 0 to 12, and the conditions are usually evaluated using the following cut-off scores27:
– 0–2: normal;
– 3–5: mild;
– 6–8: moderate;
– 9–12: severe.
The questionnaire also enables to perform a separate evaluation for anxiety and depression. A total score of at least 3 for the first 2 questions indicates the presence of anxiety. A total score of at least 3 for the last 2 questions is indicative of depression.
Fatigue Assessment Scale (FAS)
The Fatigue Assessment Scale is a self-report questionnaire22, 27 designed to evaluate symptoms of chronic fatigue, both physical and mental. It is comprised of 10 questions, each rated on a 1–5 scale. The total score ranges from 10 to 50 points, as follows:
– 10–21: normal;
– 22–34: fatigue;
– ≥35: extreme fatigue.
Possible sleep bruxism
Possible sleep bruxism (SB) was evaluated according to the Oral Behaviors Checklist (component of the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD), official Hebrew version).28 The question posed to participants was as follows: “Based on the past month, how often have you clenched or ground your teeth while asleep?”. Sleep bruxism was diagnosed when subjects reported clenching or grinding their teeth at a frequency of at least 1–3 nights per week. This methodology is considered valid for the screening of bruxism among large populations.29, 30 It adheres to the definition of “possible” bruxism, as defined by the consensus papers on bruxism.31
Statistical analysis
The data was analyzed using the IBM SPSS Statistics for Windows software, v. 25.0 (IBM Corp., Armonk, USA). The χ2 test was used to compare categorical variables. The logistic regression analysis evaluated the multivariate effect of the study variables on OSA.
Finally, the Hayes PROCESS model for SPSS v. 4.132 was used to analyze the relationship between the risk of OSA and FAS and between the risk of OSA and ESS, while taking into account the effect of sex.
Results
A total of 836 subjects provided responses to the questionnaire. Given the potential impact of certain factors on nighttime sleep, the following subjects were excluded from the analyses: participants who have a child younger than 1 year (n = 45); pregnant individuals (n = 25); those working night shifts (n = 81); individuals who use sleeping medications and/or melatonin (n = 41); and subjects treated for OSA or using CPAP (n = 48). Some participants responded positively to more than one of the above categories.
The final number of subjects included in the study was 622 (74.4% of the original sample, with 66% being female). Female subjects were significantly older than males (mean age: 46.3 ±15.4 years vs. 37.8 ±7.4 years, respectively; p < 0.001) (Figure 1).
OSA
About 20% of the female respondents were classified as having a moderate to severe OSA risk, while approx. 46% of the male respondents were identified as having a moderate to severe OSA risk (significantly higher percentage than that in females; χ2 = 113.869, degrees of freedom (df) = 2, p < 0.001) (Table 1).
PHQ-4
A greater proportion of females than males were categorized as mild, moderate and severe based on their total PHQ-4 score (χ2 = 19.215, df = 3, p < 0.001). Similarly, more females than males were classified as suffering from anxiety (χ2 = 10.903, df = 10, p < 0.001) (Table 1). The differences between males and females in the depression scale were borderline significant (13.4% vs. 18.5%, respectively; χ2 = 2.523, df = 1, p = 0.069).
FAS
Significant differences were observed between males and females in terms of their level of fatigue, with more females reporting fatigue and extreme fatigue compared to males (χ2 = 15.173, df = 1, p < 0.001) (Table 1).
ESS
No differences between sexes could be detected with regard to ESS categories (χ2 = 2.070, df = 2, p = 0.558).
Possible SB
Significant differences were identified between males and females with respect to possible SB, with females exhibiting a significantly higher prevalence of possible SB compared to males (χ2 = 10.450, df = 1, p < 0.005) (Table 1).
Multivariate analyses
The logistic regression analysis was used to evaluate the relationship between age, sex, ESS, FAS, SB, anxiety, FAS and sex interaction, and OSA. As delineated in the Material and methods section, OSA was categorized as low risk vs. moderate/severe risk (Nagelkerke R2 = 0.202) (Table 2).
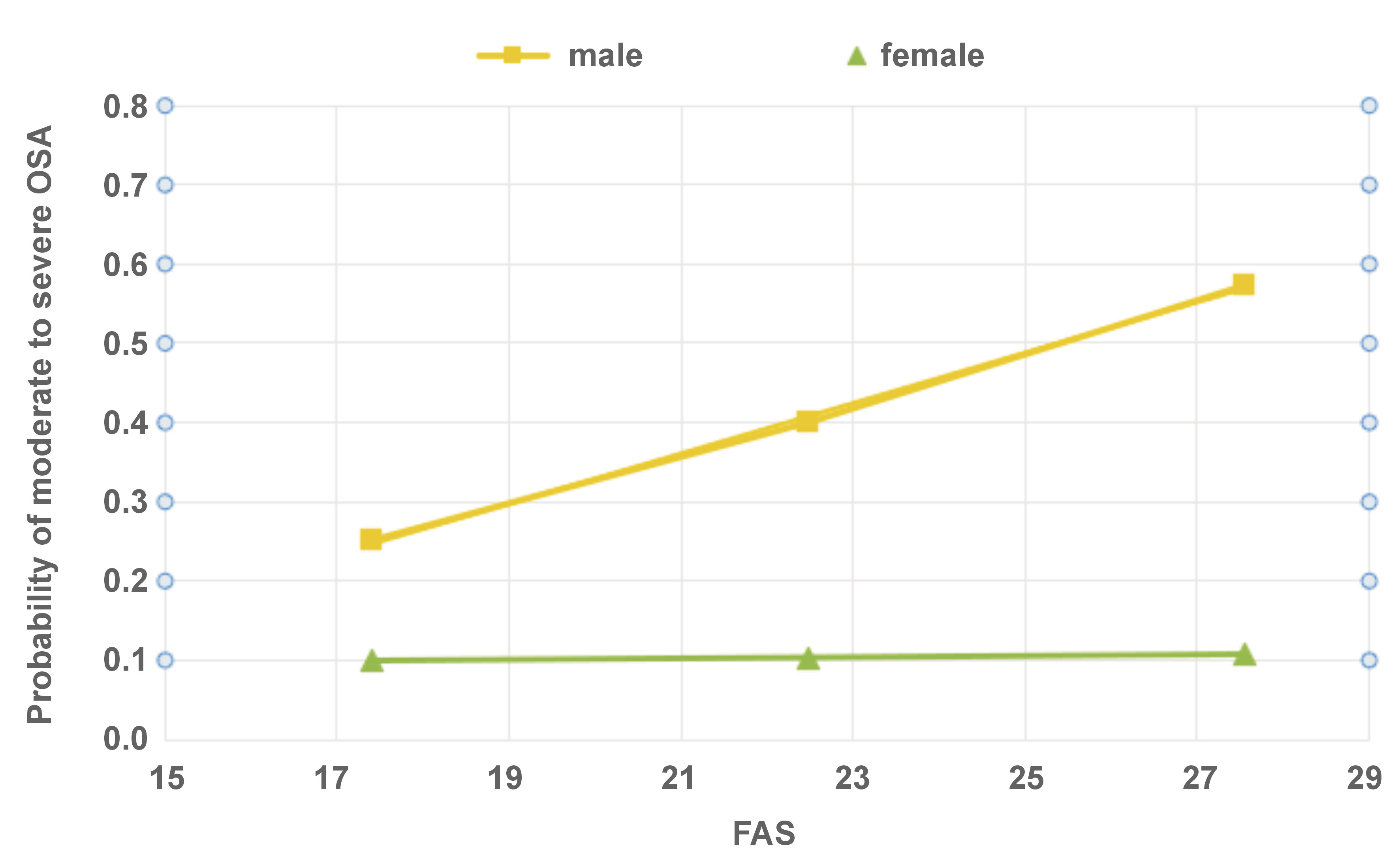
The Hayes PROCESS model was used to analyze the relationship between OSA and FAS, while taking into account the effect of sex (Figure 2). The conditional effect of FAS on the values of the male sex was as follows: effect size = 0.1539, p = 0.003 (95% confidence interval (CI): 0.0709–0.2368). The conditional effect of FAS on the values of female sex was not observed.
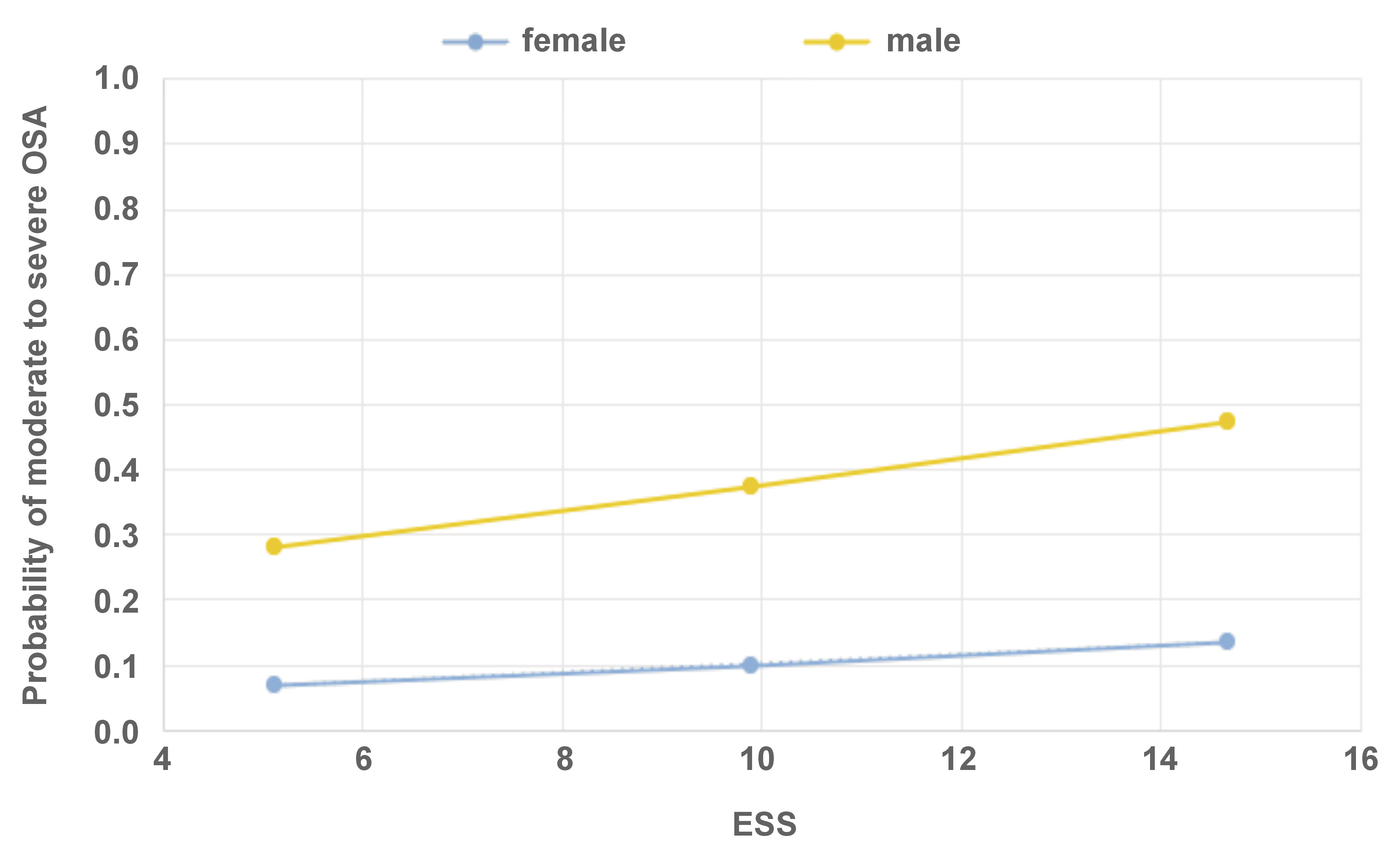
The Hayes PROCESS model was used to analyze the relationship between OSA and ESS, while taking into account the effect of sex (Figure 3). The investigation revealed no conditional effect of ESS on values of either sex.
Discussion
Obstructive sleep apnea is associated with an increased likelihood of developing hypertension, cardiovascular disease, stroke, daytime sleepiness, motor vehicle accidents, and diminished quality of life.33
The initial screening for OSA is usually conducted through the administration of self-report questionnaires. One of the most common tools for the initial screening of OSA is the STOP-Bang Questionnaire.24 Numerous studies have demonstrated its efficacy in the screening of moderate-to-severe OSA, exhibiting excellent diagnostic performance.34, 35, 36 Additionally, the tool demonstrates a high sensitivity in predicting moderate-to-severe OSA and severe OSA, when compared to the ESS.
In 2022, the United States Preventive Services Task Force (USPSTF) published a systematic review addressing the topic of screening for OSA in adults.36 The authors noted the absence of sufficient evidence to support the accuracy of screening questionnaires in identifying adults within the general population who are at an increased risk for OSA.36
Sex plays a significant role in OSA, with more males being affected by OSA compared to females, even after correcting for age and BMI.7, 23 Today, the medical community is increasingly aware that males and females may react differently to medical conditions, and that sex-specific approaches should be applied to many of the commonly used diagnostic and treatment protocols.37
In the present study, the STOP tool was used to screen for subjects who should be referred for further evaluation (namely, subjects who present moderate to severe risk of OSA). Due to the mode of the study (online survey), not all of the Bang data was collected, specifically the neck circumference and BMI. Nevertheless, the collected information enabled the accurate differentiation between subjects who do not require further intervention (low OSA risk) and those requiring referral for professional evaluation (moderate to severe risk of OSA).
As expected, there were significant differences between males and females in the OSA, PHQ-4, SB, FAS, and anxiety scores. While males showed higher rates of OSA, females exhibited heightened levels of PHQ-4, anxiety and SB. These results align with the findings reported in recent literature on the subject.7, 38, 39, 40, 41 While there were no significant differences in daytime sleepiness (ESS) scores between males and females, notable differences emerged in the levels of fatigue (FAS) and their correlation with OSA.
The logistic regression analysis demonstrated that the odds of OSA increased for age, male sex, FAS, SB, and the FAS and sex (male) interaction. The likelihood of moderate to severe OSA increased by 5–8% for age and ESS. This increase was particularly pronounced in subjects who exhibited SB behavior (an increase of 82%), and was especially high among males, as being male was associated with an over fivefold increase in the risk of moderate to severe OSA compared to females.
Sleep bruxism and OSA are closely associated. The connections between OSA and SB and OSA and sex are widely reported.42 For example, a higher prevalence of OSA has been reported in males compared to females,43 and severe OSA has been identified as a risk factor for SB.44 Interestingly, females tend to be symptomatic at lower apnea–hypopnea index (AHI) scores due to the long-term consequences of disrupted rapid eye movement (REM) sleep or more episodes of upper airway resistance during sleep, which can lead to symptoms such as daytime fatigue.43
In the present survey, female subjects exhibited higher levels of fatigue than males. However, no significant differences were observed between the sexes in terms of daytime sleepiness scores. This finding aligns with the conclusions of previous studies.10, 23 Despite the higher fatigue scores observed among females, the effect of fatigue on the probability of moderate to severe OSA in this population was not statistically significant. On the other hand, males exhibited lower fatigue scores; yet, their fatigue level had a significant effect on the risk of moderate to severe OSA. The impact of reported fatigue on the risk of OSA is significant for males and non-significant for females, despite the relatively high levels of fatigue reported by females.
There were no statistically significant differences in ESS scores between the sexes. This instrument is often used to assess subjective sleepiness.18 Although the ESS is widely used as a screening tool for OSA, its diagnostic reliability has been questioned. Kum et al. found that ESS scores increased as AHI thresholds increased, but some studies have found a weak or no correlation between ESS scores and OSA severity, suggesting that ESS may not aid in OSA diagnosis.45, 46 The results of the present study are in agreement with those by Miller et al.47 and Ghandeharioun et al.,48 suggesting that ESS is not efficient in predicting moderate-to-severe OSA. Similarly, Hamilton and Chai-Coetzer demonstrated that ESS is a poor marker of OSA.49, 50 Apparently, for AHI ≥ 5/h, the sensitivity of ESS is rather low (approx. 50%).49 The present results suggest that while the daytime sleepiness scale exhibits poor OSA screening abilities, the fatigue scale may be more effective, at least for male subjects.
The results concerning anxiety are somewhat puzzling. The presence of anxiety led to an odds ratio (OR) of less than 1, meaning that the predicted probability of OSA decreases as anxiety increases. Chen et al. demonstrated that patients with OSA had significantly higher comorbidity rates for anxiety disorders.51 Additionally, a cross-sectional exploratory study which analyzed data from patients suffering from treatment-resistant depression showed that daytime fatigue may have the potential to mask an underlying sleep disorder in women.52 An extensive study on 2,251 participants from the Netherlands Sleep Registry showed that anxiety can serve as a bridge factor of self-reported SB not only to insomnia but also to depression, OSA risk, age, and sex.53 However, nothing in the present results suggests such findings. The PHQ-4 is a brief screening questionnaire whose definition of anxiety and depression relies on merely 2 questions for each condition. It would be too pretentious to draw any conclusions concerning OSA and anxiety/depression from the present results. The role of anxiety and depression in OSA is more complex than initially assumed.54
The USPSTF has indicated a need for more accurate studies on screening tools in a general adult primary care population, especially in persons with unrecognized or mild OSA symptoms.35 The inclusion of screening modes for fatigue, rather than for daytime sleepiness, could enable better screening for moderate to severe OSA, at least in male subjects.
Limitations
The data was collected from the public via an anonymous Internet survey. Subjects who chose to participate do not represent the general population. It is possible that subjects with a special interest in OSA constituted the majority of the participants, a factor that could have led to response bias. Secondly, the risk of moderate to severe OSA was determined through the STOP-Bang Questionnaire rather than through polysomnography, which is the gold standard for OSA diagnosis. Further studies, incorporating additional screening questionnaires (such as GOAL and/or Berlin) and encompassing larger populations, should be performed to better define sex-specific screening tools for OSA.
Conclusions
Fatigue, rather than daytime sleepiness, can serve as an effective indicator of the risk for moderate to severe OSA in males.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee at Tel Aviv University, Israel, approved all study procedures (approval No. 0005883-2 15/1/2023). Informed consent was obtained from all study participants.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.