Abstract
Background. Periodontitis and peri-implantitis are chronic inflammatory diseases that lead to progressive connective tissue degradation and alveolar bone resorption. The presence of characteristic periodontitis-associated fibroblasts (PAFs) that display a remarkably high capacity for extracellular matrix (ECM) degradation was previously reported in periodontitis lesions.
Objectives. The aim of the study was to analyze collagen gel degradation in an experimental cell culture model using fibroblasts isolated from peri-implantitis lesions.
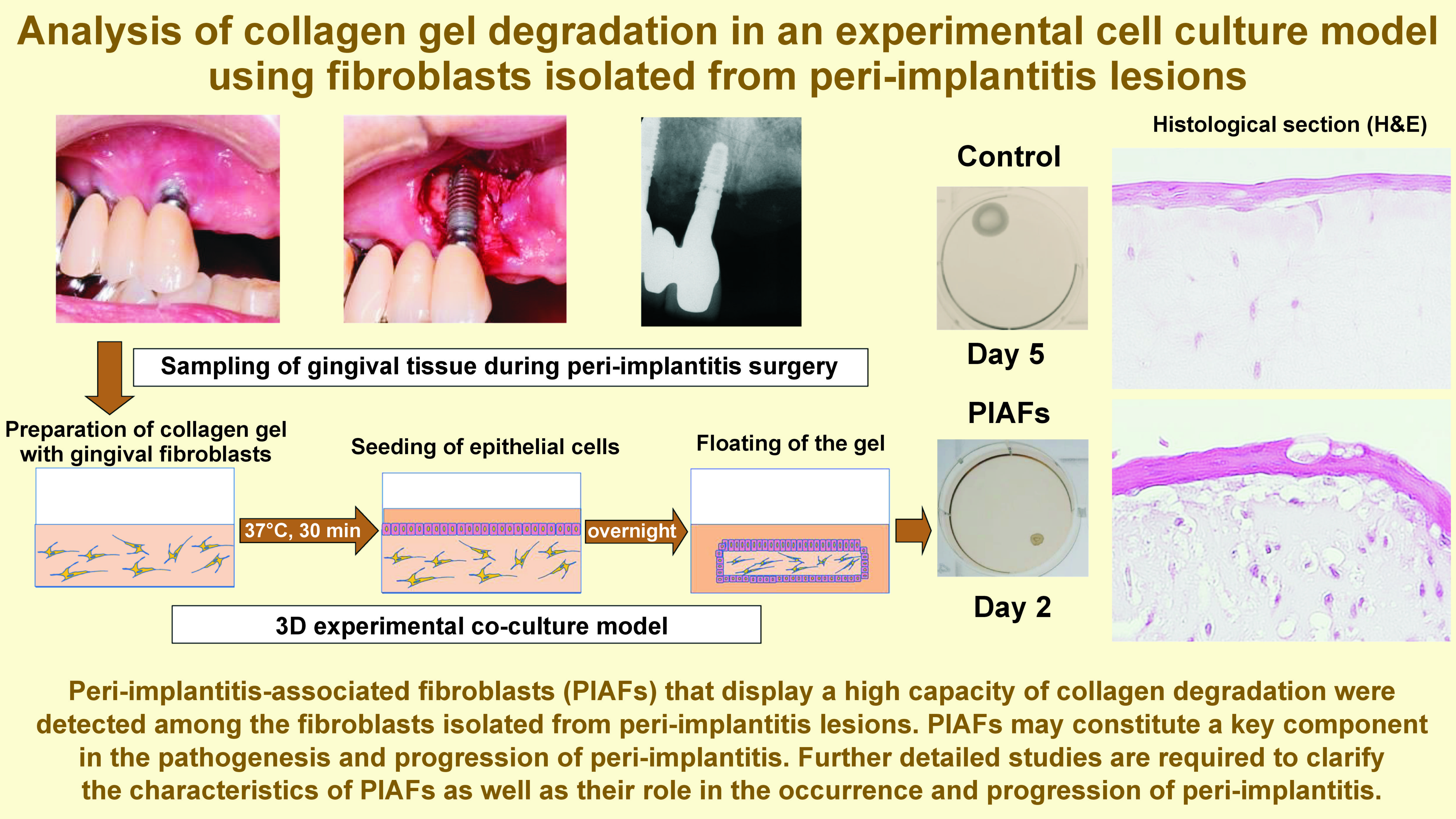
Material and methods. A patient-derived experimental cell culture model of periodontitis was developed. Gingival tissues were obtained during peri-implant, periodontal, and tooth extraction surgeries. Fibroblasts isolated from tissues affected by peri-implantitis, mixed in a three-dimensional (3D) collagen gel, were co-cultured with gingival epithelial cells. The degree of collagen gel degradation was analyzed using gel contraction, and a histologic examination was performed.
Results. In 5 examined cases, gel contraction was observed in the 3D co-culture model to considerably different degrees, which may demonstrate the presence of peri-implantitis-associated fibroblasts (PIAFs) that display a high capacity for collagen degradation. Histologically, the collagen gels with PIAFs showed numerous vacuoles adjacent to the cells when compared to gels with normal fibroblasts. The PIAFs from one case showed a rapid and significantly elevated level of collagen gel degradation in comparison to the PIAFs from the other cases.
Conclusions. The study revealed the presence of PIAFs among the fibroblasts isolated from peri-implantitis lesions, displaying a capacity for collagen degradation. Further detailed studies are required to clarify the characteristics of PIAFs as well as their role in the occurrence and progression of peri-implantitis.
Keywords: periodontitis, fibroblasts, peri-implantitis, epithelial cells, primary cells
Introduction
Implant therapy is a well-established method for the replacement of absent or lost teeth. Its popularity can be attributed to its high rates of fundamental long-term survival.1 Various studies have been conducted on the development of implant materials2, 3, 4 and treatment procedures for implant placement.5, 6, 7 However, the occurrence of peri-implantitis has become an increasingly prominent concern, affecting the stability of surrounding tissues and, consequently, the longevity of implants.8, 9 Definitive effective therapies have not been established, largely due to the difficulty of treatment procedures associated with the complicated structures of dental implants.10, 11, 12, 13 The pathological nature of peri-implantitis is similar to that of periodontitis, being closely associated with the accumulation of bacterial biofilm.14 However, the mechanism of the occurrence and progression of peri-implantitis remains unclear.
Recently, Ohshima et al. used an experimental three-dimensional (3D) culture model of periodontitis to report the presence of characteristic periodontitis-associated fibroblasts (PAFs) in the periodontitis lesion.15, 16, 17 These PAFs were found to highly degrade collagen gel. During connective tissue metabolism, activated fibroblasts are known to produce extracellular matrix (ECM) components as well as proteolytic enzymes, thereby contributing to ECM remodeling and degradation.18, 19 Ohshima et al. have isolated PAFs from the gingival tissue of periodontitis lesions in patients and consecutively characterized significantly different gene expression signatures of these PAFs compared to normal gingival fibroblasts derived from healthy individuals.15, 16, 17
In order to develop an experimental model of periodontitis, primary cultured gingival epithelial cells and periodontally-affected fibroblasts were co-cultured in collagen gels, which appeared to recapitulate epithelial cell–fibroblast interactions in the gingival connective tissue.15, 16, 20 A series of previous reports have demonstrated that PAFs display a remarkably higher capacity for ECM degradation compared to normal fibroblasts.15, 16, 17
Based on the findings gained through the identification and study of PAFs, we speculated that there would exist peri-implantitis-associated fibroblasts (PIAFs) that display a capacity for collagen degradation, similar to PAFs, in the peri-implantitis lesions. Thus, the purpose of the present study was to analyze the presence of fibroblasts with a high capacity for collagen degradation, isolated from peri-implantitis lesions. In the present study, we collected peri-implant tissues during peri-implantitis surgery in the clinic, isolated fibroblasts from the tissues, and investigated the collagen gel contraction in the experimental 3D cell co-culture model.
Material and methods
Cell culture
Gingival tissues were obtained during peri-implant, periodontal, and tooth extraction surgeries at the Department of Periodontics, Institute of Science Tokyo Hospital (formerly Tokyo Medical and Dental University Hospital) and at Dental Hospital, School of Dentistry, Nihon University (Tokyo, Japan). Informed consent was obtained from all patients. The patients with peri-implantitis who were included in the study were indicated for surgical treatment and exhibited moderate to severe peri-implantitis, with probing pocket depth ≥6 mm, bleeding on probing (BOP) and bone resorption. Exclusion criteria encompassed current smokers, patients taking medications that induce bone necrosis after periodontal surgery (such as bisphosphonates), individuals with mobile dental implants, patients with systemic/local diseases undergoing treatment, and pregnant women. The protocol of the study was approved by the Ethics Committee of Nihon University School of Dentistry (approval No. 2013-1, EP18D018) and the Faculty of Dentistry, Institute of Science Tokyo (approval No. D2014-141-02).
During peri-implant surgery, excessive marginal gingival tissues, diseased connective tissues of the inner surface of the gingival flaps, and/or diseased connective tissues from within the bone defects around implants were obtained.
Gingival fibroblasts and epithelial cells were isolated from the gingival and connective tissues. Cell cultures were performed as previously described.21, 22, 23 With regard to the PIAFs, briefly, the collected tissues were cut into small pieces and placed into 6-well plates. Fibroblast populations were established from each well, and subsequently pooled to form a single population.
Collagen gel co-culture assay
The experimental model, previously used for periodontitis, was employed in this study. The model involved the 3D co-culture of gingival fibroblasts (isolated from periodontitis lesions) and gingival epithelial cells.15, 16, 20
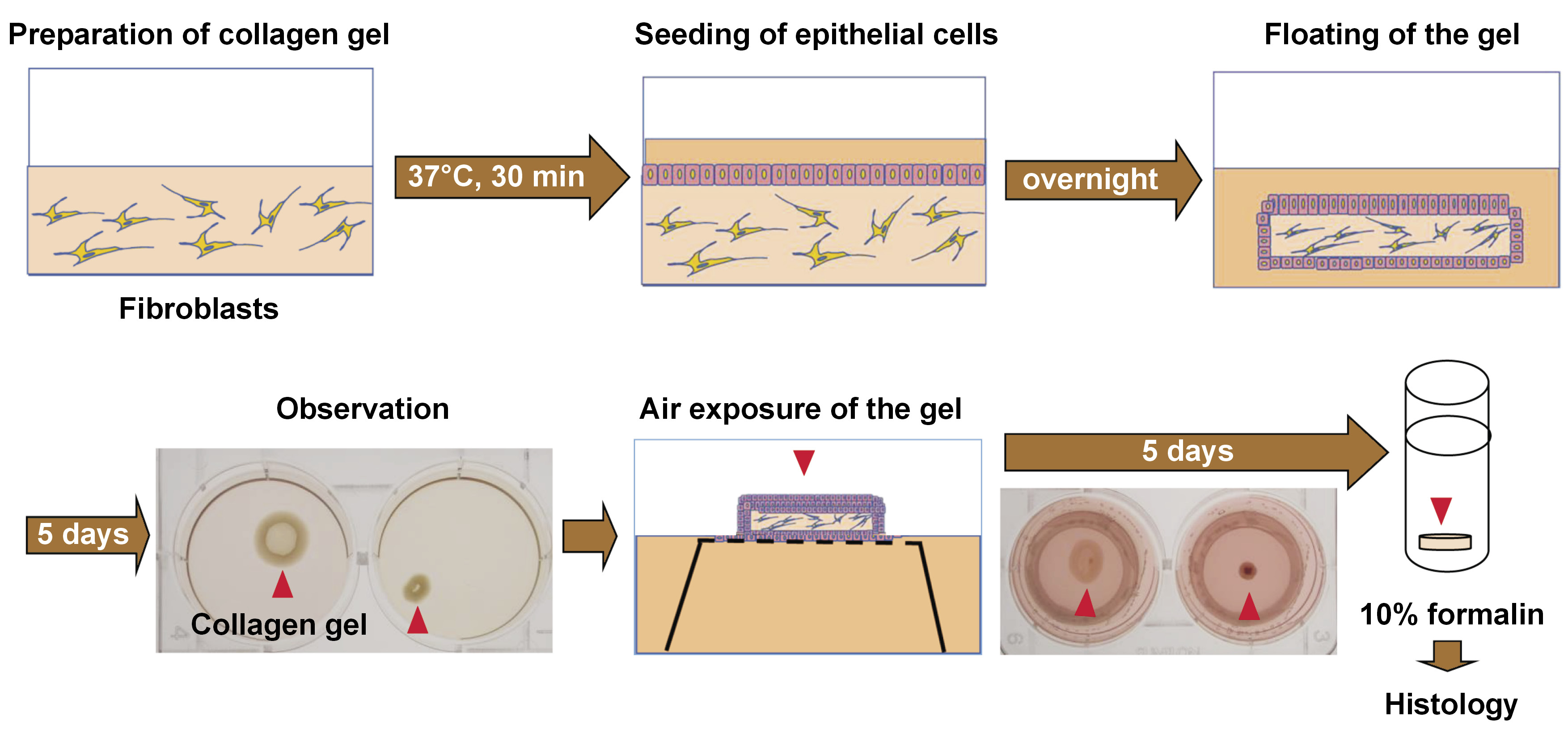
Briefly, as presented in Figure 1, collagen gels were prepared by mixing 0.5 mL of fibroblast cell suspension (2.5 × 105 cells) in fetal bovine serum (FBS), 2.3 mL of type I collagen (Cellmatrix Type I-A; Nitta Gelatin Inc., Osaka, Japan), 670 µL of 5× Dulbecco’s Modified Eagle Medium (DMEM), and 330 µL of reconstitution buffer, in accordance with the manufacturer’s instructions. The mixed solution (3.5 mL) was placed into each well of a 6-well plate and allowed to gelatinize in an incubator (MCO-18AC-PJ; Panasonic Corp., Ora, Japan) set at 37°C for 30 min. Subsequently, gingival epithelial cells (2.5 × 105/2 mL) were seeded onto the surface of each gel. The co-culture medium consisted of a mixture of 0.5 mL of DMEM supplemented with 10% FBS and 1.5 mL of EpiLife™ medium with Supplement S7 (Thermo Fisher Scientific, Waltham, USA).
The gels were cultured overnight and carefully separated from the edge of each well to generate a floating gel culture. The floated gels were then cultured in the medium for 5 days and evaluated.
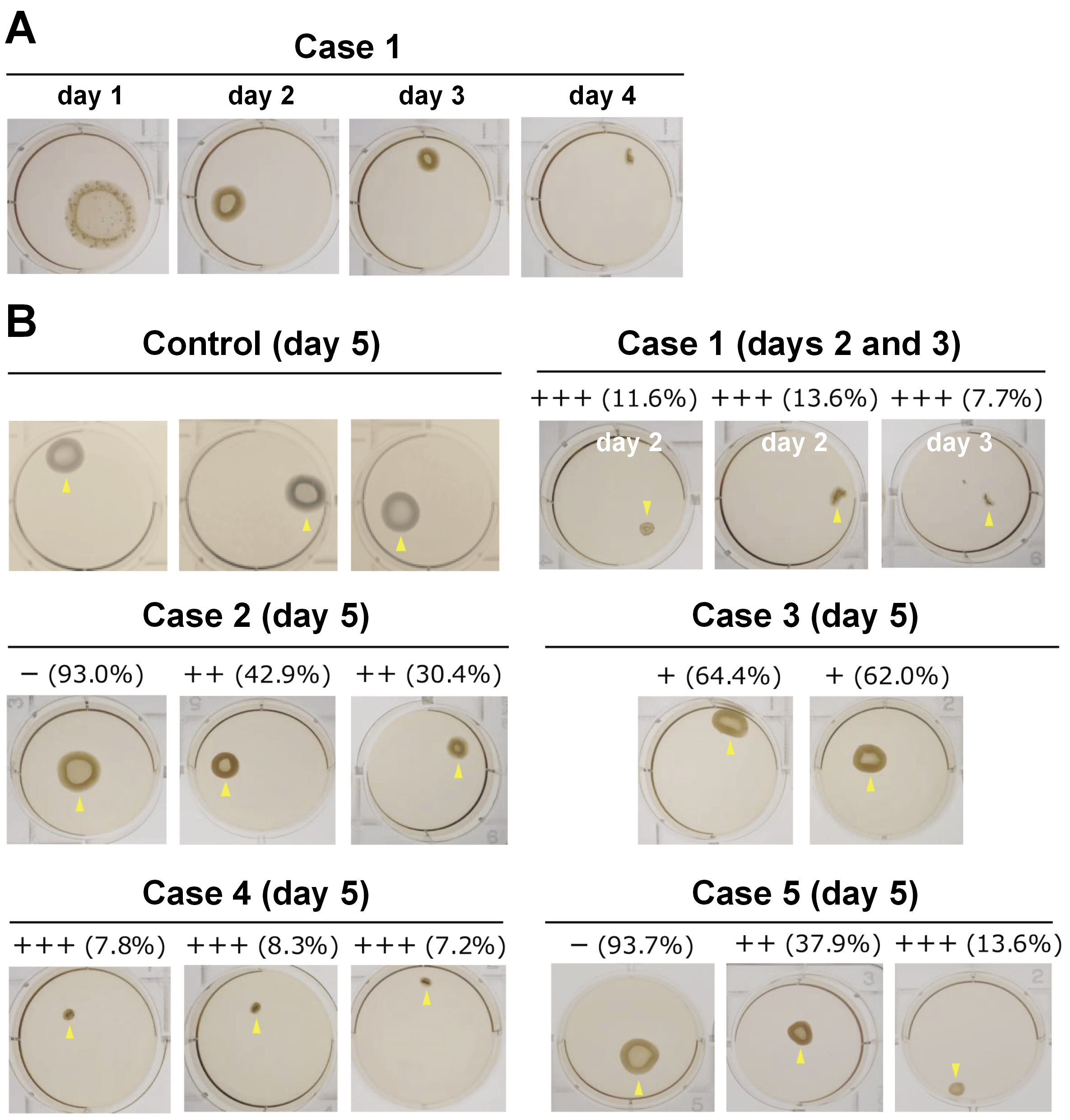
Gel contraction was observed and photographed for area measurement. The surface areas of gels were measured using the ImageJ software (NIH, Bethesda, USA), and the gel size rate (GSR) of each sample was expressed as a ratio of the individual sample gel area (with fibroblasts derived from a peri-implantitis lesion) to the average control gel area (with normal fibroblasts). The degree of gel contraction was determined using the GSR, as follows: none (−): GSR ≥ 80%; mild (+): 80% > GSR ≥ 50%; moderate (++): 50% > GSR ≥ 20%; and severe (+++): GSR < 20%.
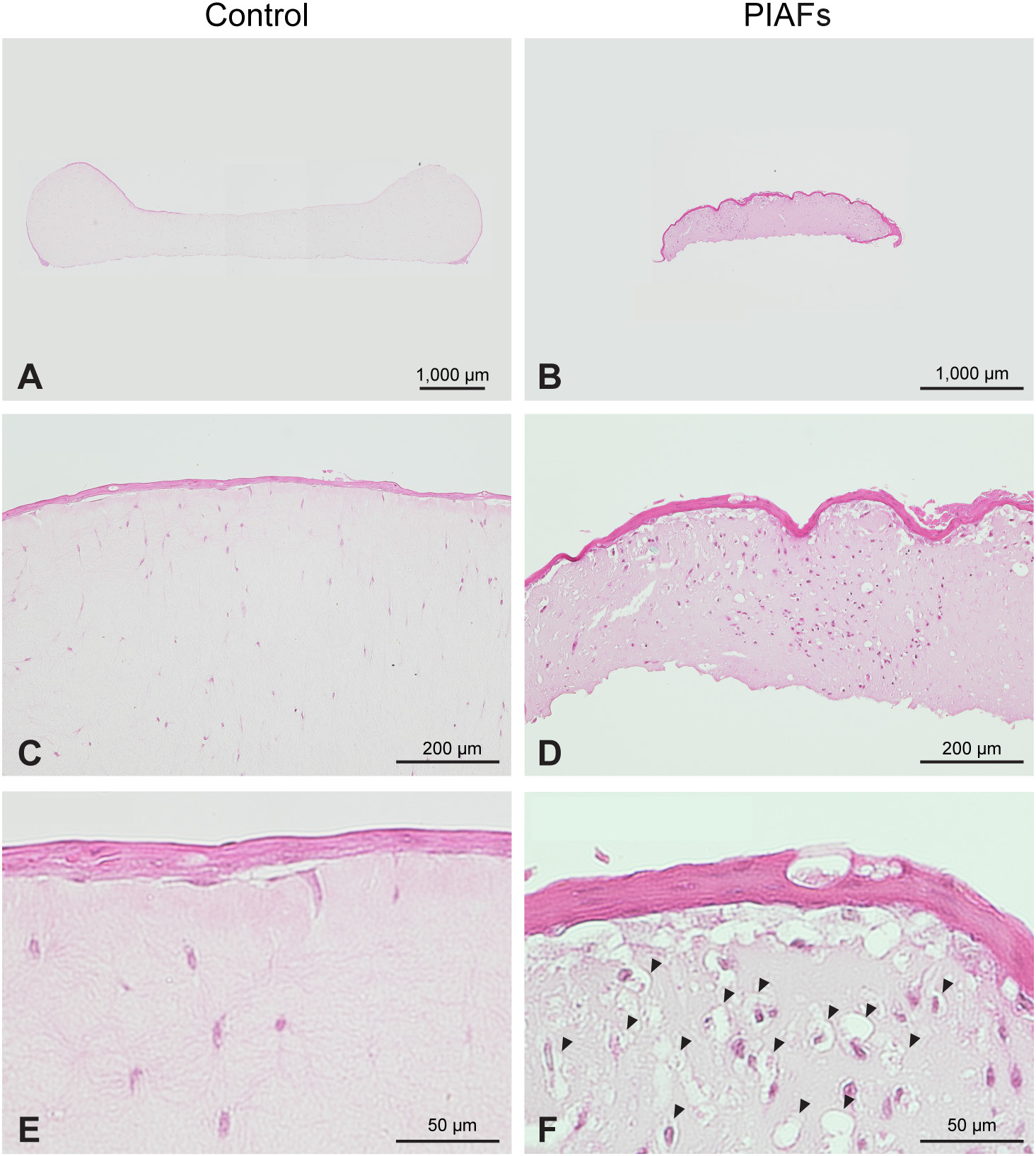
For histological analysis, some gels were cultured at the air-liquid interface to induce stratified epithelium.24 Cell inserts (Falcon® Cell Strainer; Corning, New York, USA) were adjusted and placed upside down in the wells. The floated gels were cultured on the turned-over inserts for an additional 5 days. After this period, the gels were fixed in 10% neutral buffered formalin solution and embedded in paraffin. Vertical sections were stained with hematoxylin and eosin (H&E) and examined under an optical microscope (ECLIPSE Ni-L; Nikon Corp., Tokyo, Japan) (Figure 1).
Results
Case demographics
The peri-implant tissues were obtained from peri-implantitis regions of 5 patients at the Department of Periodontics, Institute of Science Tokyo Hospital. The study sample included 2 males and 3 females with ages ranging from 56 to 83 years (mean (M): 69.2 ±10.6 years). The number of implants with peri-implantitis and the total number of existing implants were as follows: 4 and 7 for case 1, 1 and 1 for case 2, 5 and 8 for case 3, 3 and 5 for case 4, and 3 and 5 for case 5. Each patient exhibited localized peri-implantitis in the absence of generalized peri-implantitis. None of the patients had a history of implant loss. Cases 2 and 5 had hypertension, with case 2 receiving a calcium channel blocker medication. One patient (case 4) was being monitored without medication for aortic valve dysfunction. One individual (case 5) exhibited a thoracic aortic aneurysm, necessitating the administration of an anticoagulant.
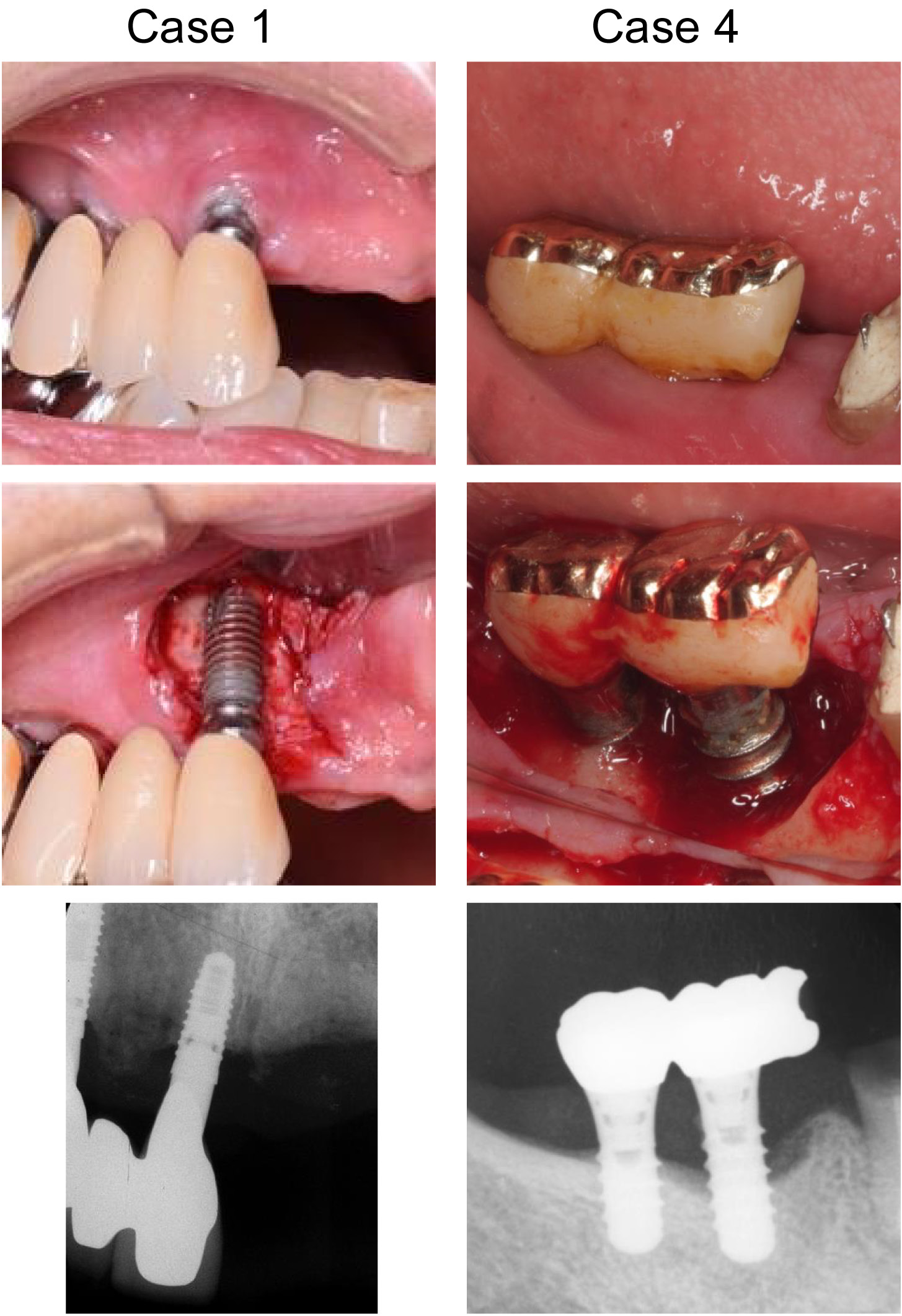
The demographic information of the participants and the characteristics/locations of the treated implants are presented in Table 1. The levels of peri-implantitis for those implants ranged from moderate to severe (4.2–8.7 mm of bone defect depth) in terms of bone resorption, with 6–10 mm of probing pocket depth (PPD), and BOP observed at all sites. Figure 2 presents the clinical photographs and radiographs of 2 cases (case 1 and case 4).
Affected fibroblasts were isolated from the peri-implant tissues obtained from the participants. Epithelial cells were obtained from 5 patients (systemically healthy females, aged 23–76 years) during peri-implant, periodontal and extraction surgeries. The control group consisted of normal fibroblasts obtained from 3 patients (2 males and 1 female, aged 23–27 years) during tooth extraction (FDI tooth positions 34, 38, 48). These patients were non-smokers, systemically healthy, and had no periodontal inflammation around the teeth. The above epithelial cells and normal fibroblasts were obtained from the Dental Hospital, School of Dentistry, Nihon University.
PIAF detection
Fibroblast growth was observed to be optimal in 4 cases and poor in 1 case. The 5 cases showed gel contraction to varying extents (Table 2, Figure 3), which may indicate the presence of PIAFs that display the capacity for collagen degradation. Severe (+++) gel contraction was detected in 3 cases (1, 4, 5) for which good cell growth was observed. In 2 of those cases (1 and 4), a significant number of populations showed severe (+++) gel contractions, with case 1 being associated with the most severe gel contractions. Interestingly, the gel contraction observed in case 1 was notably aggressive, with the fastest contraction speed noted in this study, surpassing the rates recorded in our prior investigations employing PAFs. Consequently, the gel evaluation for case 1 was conducted earlier, on days 2–4.
Histological observation of the gels
Figure 4 shows representative photomicrographs of gels with normal fibroblasts (Figure 4A,C,E) and PIAFs (case 4) (Figure 4B,D,F). The collagen gels with normal fibroblasts showed plain and weak staining with few cells (Figure 4C,E), while the gels with PIAFs demonstrated a significant contraction with a substantial cellular presence. This contraction can be attributed to the shrinkage of the gel and the presence of numerous vacuoles adjacent to the cells (Figure 4D,F), possibly occurring due to the degradation of the collagen matrix. The cell nuclei were considerably different, with spindle-shaped nuclei characteristic of normal fibroblasts and round-shaped nuclei predominating in PIAFs.
Discussion
In the present study, we analyzed fibroblasts isolated from peri-implantitis lesions in vitro using a 3D experimental collagen gel model co-cultured with epithelial cells. In the context of moderate to severe peri-implantitis regions, a range of collagen gel contraction degrees was observed. This may be attributable to the presence of characteristic fibroblasts that highly degrade collagen in peri-implantitis lesions. We have proposed to name these peri-implantitis lesion cells “peri-implantitis-associated fibroblasts” (PIAFs), similar to “periodontitis-associated fibroblasts” (PAFs) that we previously detected and reported in the periodontitis lesion.15, 16
Periodontitis-associated fibroblasts are frequently detected in severe periodontitis lesions.15, 16, 17, 20 These cells have a unique ability to significantly degrade collagen gel in a 3D experimental model co-cultured with epithelial cells. In order to induce collagen degradation with fibroblasts, it was determined that the interaction with primary gingival epithelial cells and primary gingival fibroblasts is important and necessary. Thus, this 3D experimental co-culture model was suitable for inducing interaction, and was employed using both clinical samples in the present study.
Enzymatic degradation is necessary for gel degradation.25 In our PAF study, we reported that the gene expression of matrix-degrading enzymes, such as matrix metalloproteinase (MMP)-1 and -3, was upregulated.15 The upregulation of transforming growth factor-beta (TGF-β) responsive genes, such as Smad7 and type I collagen α1 chain, confirmed the activity of TGF-β during the contraction of the gel. In addition, it was demonstrated that vascular endothelial growth factor (VGFT) receptor-1 (Flt-1) and hepatocyte growth factor (HGF) are involved in matrix degradation in the 3D culture system. Furthermore, PAFs were revealed to be important components in the pathogenesis of periodontitis.16, 20 Gel degradation was significantly suppressed by serine protease inhibition (aprotinin) and hydroxamate/MMP inhibition (marimastat),15 which is in line with previous reports.26
In addition, we investigated genes related to cancer-associated fibroblasts (CAFs),27, 28 since the migration of gingival epithelial cells to the apical region (epithelial downgrowth) during the progression of periodontitis exhibits similarities to the invasive process of cancer cells.29 Among the CAF-associated genes, HGF, COX-2, LAMA2, SFRP-1, SFRP-2, CXCL12, ITGA11, and ANGPTL2 were unregulated during the process of gel degradation in co-cultures. However, the expression levels of some genes such as MMP-2, PAI-I, CCL5, and syndecan-1 remained unaltered,15, 16, 30, 31 indicating the possibility of similar characteristics between CAFs and PAFs.
In the present study, the extent of the gel contraction exhibited significant variation among the cases, possibly due to the presence of different numbers of PIAFs, as well as differently affected PIAFs in each case. Case 2 received a calcium channel blocker, which may have affected gingival overgrowth, but the patient did not show generalized peri-implantitis, and the level of collagen gel contraction was generally not severe. Cases 1 and 4 demonstrated a high degree of collagen gel degradation potential. Notably, PIAFs in case 1 demonstrated the most significant propensity to rapidly and aggressively degrade collagen gel among all cases, even when compared to the levels that had been previously observed in studies using PAFs. In the present study, case 1 was systemically healthy. A thorough examination of the sampling sites in these 2 cases suggests the potential existence of PIAFs within the inner surface of periodontal flaps rather than in the diseased connective tissue within the bone defects. In preliminary experiments, the application of anti-HGF neutralizing antibodies, which have an inhibitory effect on collagen degradation in PAFs, to the co-culture with case 1 PIAFs, failed to inhibit collagen degradation. However, imatinib (anticancer drug) inhibited collagen degradation, suggesting that PIAFs in case 1 may differ from the typical PAFs. The aggressive nature of PIAFs in case 1 may be associated with the advanced status of peri-implantitis observed in that patient.
Initially, PAFs and PIAFs occur around the cervical area after the interaction of the inflammatory sulcular epithelium and gingival fibroblasts. Then, PAFs may gradually affect the periodontal ligament, resulting in attachment loss and disease progression in periodontitis. However, in the case of peri-implantitis, there is no connective tissue between implants and bone. Thus, if PIAFs are associated with the progression of peri-implantitis, PAFs/PIAFs may also directly affect bone. Further investigation is necessary to clarify the influence of PAFs/PIAFs on periodontal and peri-implant bone.
In the context of peri-implantitis, different characteristics of implant surfaces can elicit different biological responses in peri-implant fibroblasts and bone cells.32 Furthermore, in contrast to the case of periodontitis, the release of toxic titanium particles due to mechanical wear, chemical corrosion, microbial contamination, and implant surface treatment may impact peri-implant tissues.33 Consequently, titanium might contribute to collagen degradation associated with PIAFs, as compared to PAFs. Recently, other complications with dental implants, including the occurrence of cancer around implants, have been reported.34, 35, 36 It is conceivable that the aggressive characteristics of PIAFs might be associated with cancer occurrence.
The effective treatment of peri-implantitis represents a major clinical challenge.13 This is partially due to the lack of detailed information and understanding regarding the molecular and cellular pathogenesis of peri-implantitis, as well as its occurrence mechanism. This study demonstrated the presence of PIAFs with the capability of matrix degradation in peri-implantitis lesions, a finding which indicates that PIAFs may constitute a key component in the pathogenesis and progression of peri-implantitis. If the capacity for collagen degradation in PIAFs can be controlled, a new treatment strategy may be developed in peri-implant disease therapy. These points support the clinical relevance of the present study within the context of peri-implantitis treatment.
Limitations
There are some limitations to the present study. These include the small sample size, the presence of comorbidities, the disparity in ages between normal fibroblasts and PIAFs, the absence of immune cells in the experimental cell culture model, the assumption that collagen contraction is caused exclusively by degradation provoked by the cells, and the lack of more accurate assays to evaluate the collagen degradation. These points should be investigated through detailed studies. In this preliminary report, we conducted a semi-quantitative analysis of gel area measurement. In subsequent studies, analyses such as collagen zymography, gene expression, collagenase/MMP detection, and proteomics for collagen matrix degradation should be considered.
Conclusions
The fibroblasts isolated from peri-implantitis lesions showed various degrees of collagen gel contraction when co-cultured with epithelial cells using a 3D collagen gel model. This observation suggests the potential presence of PIAFs, which are capable of collagen degradation. In particular, aggressive types of PIAFs were detected, the characteristics of which may differ from those of PAFs. Further detailed studies are required to clarify the characteristics of PIAFs as well as their role in the occurrence and progression of peri-implantitis.
Ethics approval and consent to participate
The protocol of the study was approved by the Ethics Committee of Nihon University School of Dentistry (approval No. 2013-1, EP18D018) and the Faculty of Dentistry, Institute of Science Tokyo (approval No. D2014-141-02). Informed consent was obtained from all patients.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.