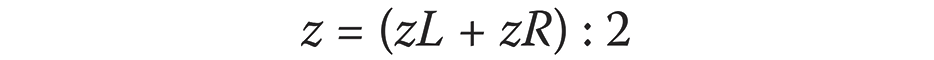Abstract
Background. Most dental implants are made of titanium (Ti) or, more recently, ceramic. Both of these materials are safe and biocompatible. Maintaining healthy bone around the dental implant platform is a key component of any modern implant therapy. Understanding the impact of various external factors on marginal bone preservation is essential for accurate diagnosis before any planned procedure.
Objectives. The aim of the present study was to assess the preservation of the alveolar bone near the implant platform with regard to varying parameters of gingival thickness over the implant site and different serum vitamin D3 levels.
Material and methods. The study analyzed the records of 72 patients who had a total of 115 dental implants inserted with the simultaneous preoperative determination of vitamin D3 levels and the soft tissue thickness between January 2022 and February 2023 at a private medical facility.
Results. Patients with vitamin D deficiency showed significantly higher levels of bone loss around implants as compared to those with normal vitamin D levels. Moreover, the thickness of the gingival tissue over the implant site had a significant effect on the preservation of the alveolar bone around the implant platform, regardless of the vitamin D3 status.
Conclusions. Monitoring the concentration of 25-hydroxycholecalciferol (25(OH)D), along with the radiological assessment of the gingival tissue thickness, may be a simple and reliable method to achieve predictable outcomes in dental implantology.
Keywords: crestal bone loss, vitamin D3 deficiency, thin mucosal tissue, thick mucosal tissue
Introduction
Successful osseointegration is a key criterion for successful implantological outcomes, and it is achieved through functional ankylosis. Titanium (Ti), which is used for dental implants, fuses with bone to form a functional unit known as the initial bone–implant contact (BIC).1, 2 Depending on its concentration, vitamin D can enhance3 or slow down4, 5 new bone formation.
Vitamin D is essential for maintaining bone mineralization by regulating Ca2+ and PO43− ion concentrations in blood.6 Vitamin D deficiency in both children and adults leads to decreased intestinal absorption of calcium (Ca), resulting in temporary decreases in the ionized Ca concentration in blood.5
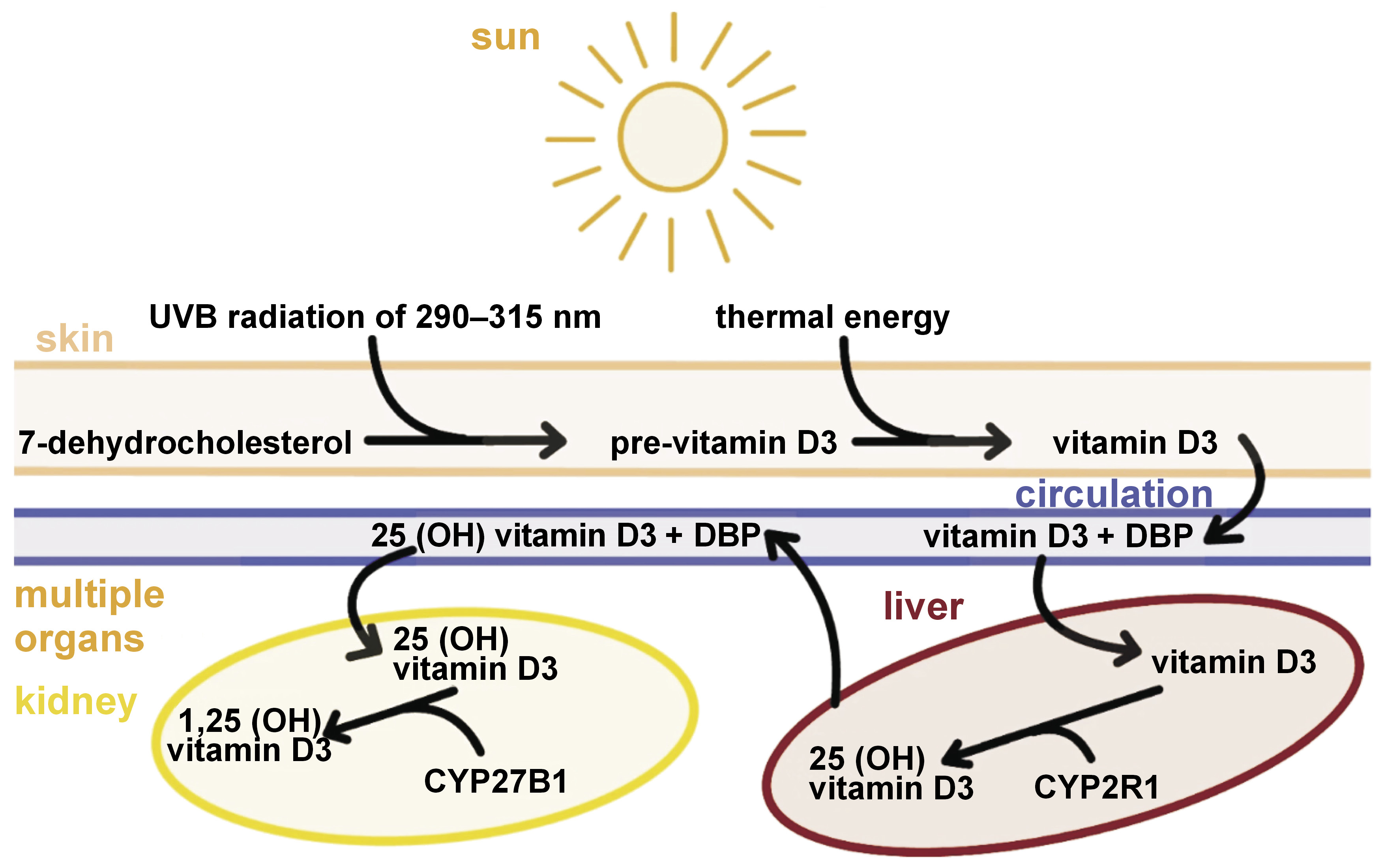
Vitamin D exists in 2 forms: vitamin D2, referred to as ergocalciferol, found in plant and fungal organisms; and vitamin D3, referred to as cholecalciferol, synthesized by animal organisms, including humans.7
Under the influence of ultraviolet B (UVB) radiation at a wavelength of 290–315 nm (characteristic of sunlight) and in the presence of the appropriate reductase, the B-ring of 7-dehydrocholesterol opens, and a non-permanent compound – pre-vitamin D3 – is formed. This, in turn, isomerizes to cholecalciferol at skin temperature (about 25°C), which then enters the bloodstream.7, 8 In the liver, 25-hydroxylase influences the formation of 25-hydroxycholecalciferol (25(OH)D, known as calcidiol). The 2nd stage takes place in the kidneys, where 1α-hydroxylase catalyzes the conversion of the compound to its active form, 1α,25-dihydroxycholecalciferol (1α,25(OH)2D, called calcitriol) (Figure 1).7
Calcitriol plays an important role in maintaining the health of the craniofacial bones and teeth. Calcitriol deficiency can lead to oral diseases, such as periodontitis, early tooth loss, osteoporotic fractures, impaired healing of wounds or fractures, and disrupted bone formation around dental implants.10
A sufficient level of vitamin D3 is crucial on the day of surgery, as it modulates the immune system, and influences the production of cathelicidin and defensin.9, 10 It also reduces the expression of proinflammatory cytokines and positively affects bone metabolism through osteosuppression by influencing osteoblasts and osteoclasts, as well as regulating continuous bone remodeling around implants after prosthetic restoration.10
The body supply of vitamin D can be assessed through laboratory tests based on the serum concentration of 25(OH)D (Table 1). This measurement indicates the available vitamin D in the bloodstream and is an established marker of the vitamin D status.
Another important factor in maintaining bone tissue health around the neck of the implant is the amount of gingival soft tissue. It has been suggested that a minimum of 3 mm of peri-implant mucosa is required for a stable epithelial connective tissue attachment to be formed.11
The transition from alveolar mucosa to peri-implant soft tissue after implant placement is a difficult and complex process. Płudowski et al. suggested that if insufficient gingival tissue is available, bone loss may occur to ensure the development of a proper biological width.12
The assessment of the gingival height can be invasive when using a needle, an endodontic tool or a periodontal probe, or non-invasive, including ultrasound or optical coherence tomography (OCT). Radiological methods, such as the paralleling technique with radiovisiography (RVG) or cone-beam computed tomography (CBCT) can also be used (Figure 2).
This retrospective study aimed to investigate the impact of dental implant procedures on the bone tissue under various complex combinations of factors, such as insufficient vitamin D levels with a thick soft tissue biotype or recommended vitamin D levels with a thin soft tissue biotype.
Material and methods
Population
The study analyzed records from the treatment histories of 72 patients who received a total of 115 dental implants between January 2022 and February 2023 at a private medical facility in Krakow, Poland. The mean age of the patients was 44.7 ±11.21 years.
Eligibility for the study
All eligible patients were adults in good general health, with no history of allergic reactions to drugs or dental materials, including Ti. Patient data was included if patients had completed all the required treatment and control steps. Criteria for inclusion in the study group embraced the absence of the need for simultaneous bone augmentation and the complete healing of the alveolar ridge with a clearly defined cortical plate, suitable for accurate measurements. Patients were included if the dental implant was placed at the bone level with the simultaneous preoperative assessment of vitamin D3 levels, and if healing occurred without complications. All 72 patients received Nobel Biocare Replace Select™ CC implants with the TiUnite surface (Nobel Biocare, Kloten, Switzerland). Restorations were screw-retained with various suprastructures (a stock metal abutment with a lithium disilicate supracrestal or zirconia framework, and lithium disilicate supracrestal restorations supported with a Ti base). All analyzed cases included interdental and partial deficiencies, with reconstruction types ranging from three-point bridges to single implant crowns. In all cases, the time interval between implant placement surgery and the exposure day was 4 months for the maxilla and 3 months for the mandible. In all cases, the emergence profile of the abutment maintained a consistent distance from the alveolar bone surrounding the implant platform.
Radiological evaluation of the bone tissue level around implants
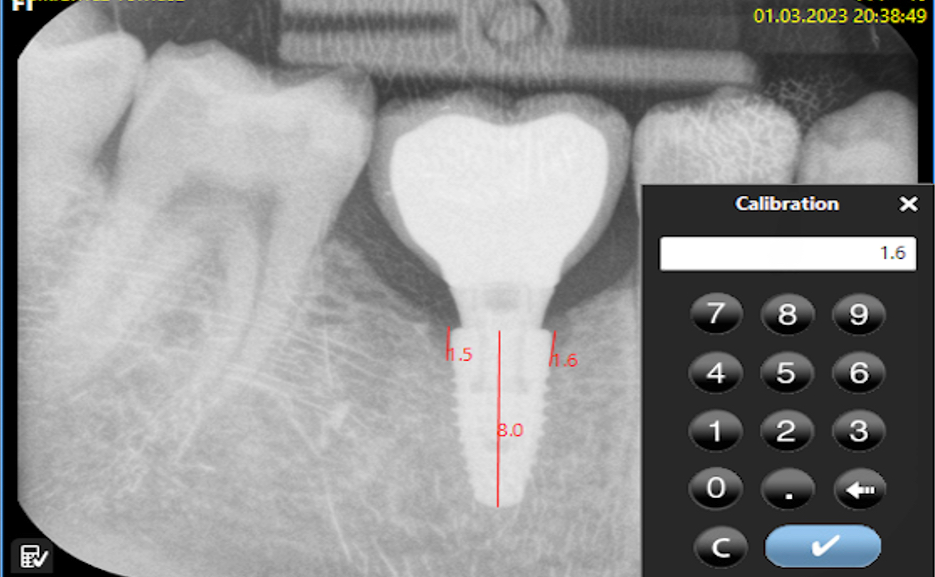
Bone loss was assessed using software designed for digital RVG analysis. Each evaluated image was calibrated against the implant length on the RVG and from medical records. The calculated value represented the arithmetic mean of the measurements taken on both sides of the implant, which were visible on the two-dimensional (2D) image. The calculations were made starting from the platform of the implant along its long axis (Figure 3 and Figure 4).
Bone loss was calculated as an average using the following formula (Equation 1):
where:
z – bone atrophy [mm];
zL – bone atrophy on the left side [mm]; and
zR – bone atrophy on the right side [mm].
Statistical analysis
The groups were compared using the Mann–Whitney U test after rejecting the assumptions for normality and homogeneity of variance. The significance level was set at 0.05. Statistical analysis was conducted using the R software, v.1.2.5042 (https://www.r-project.org).
Results
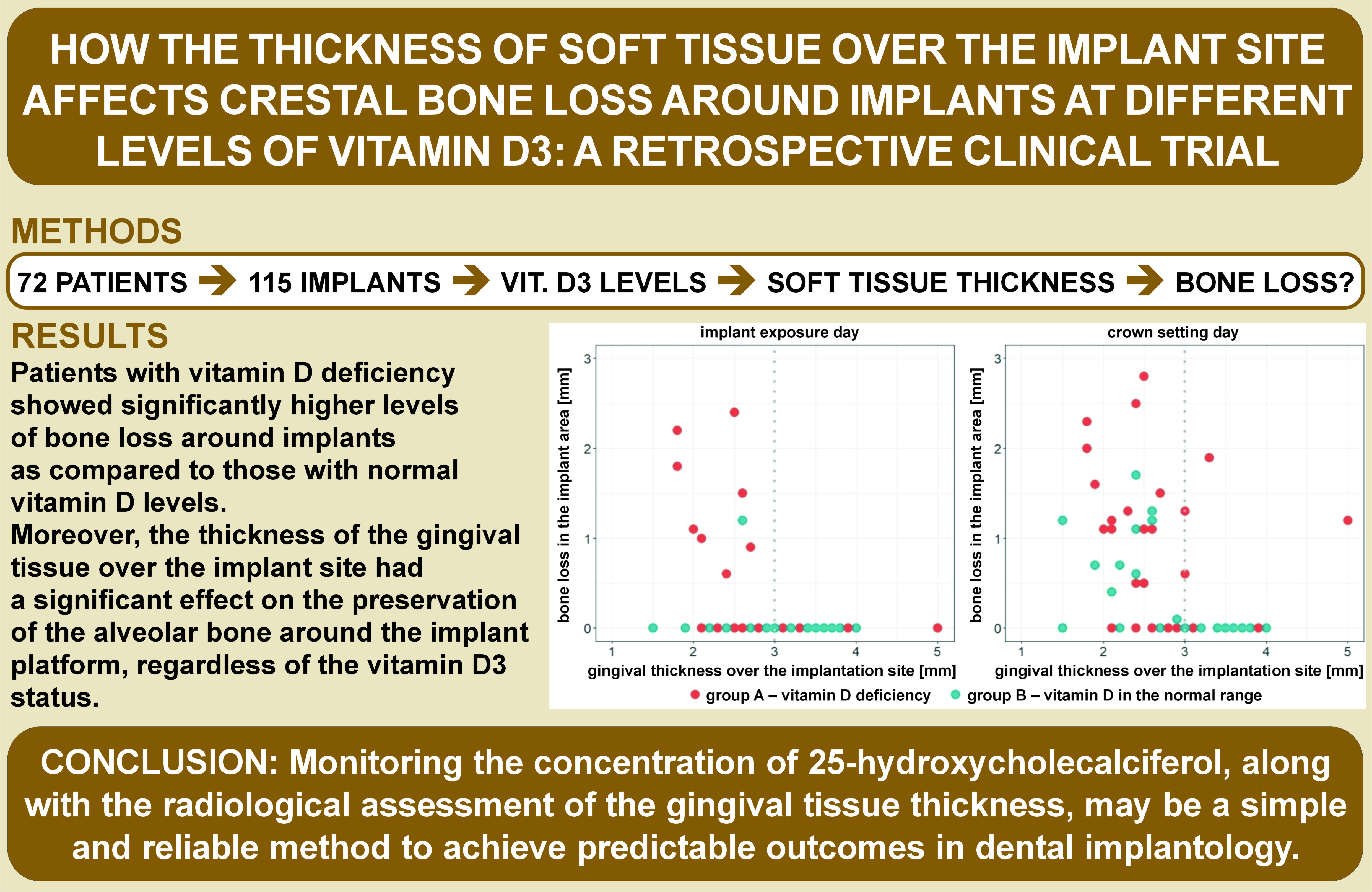
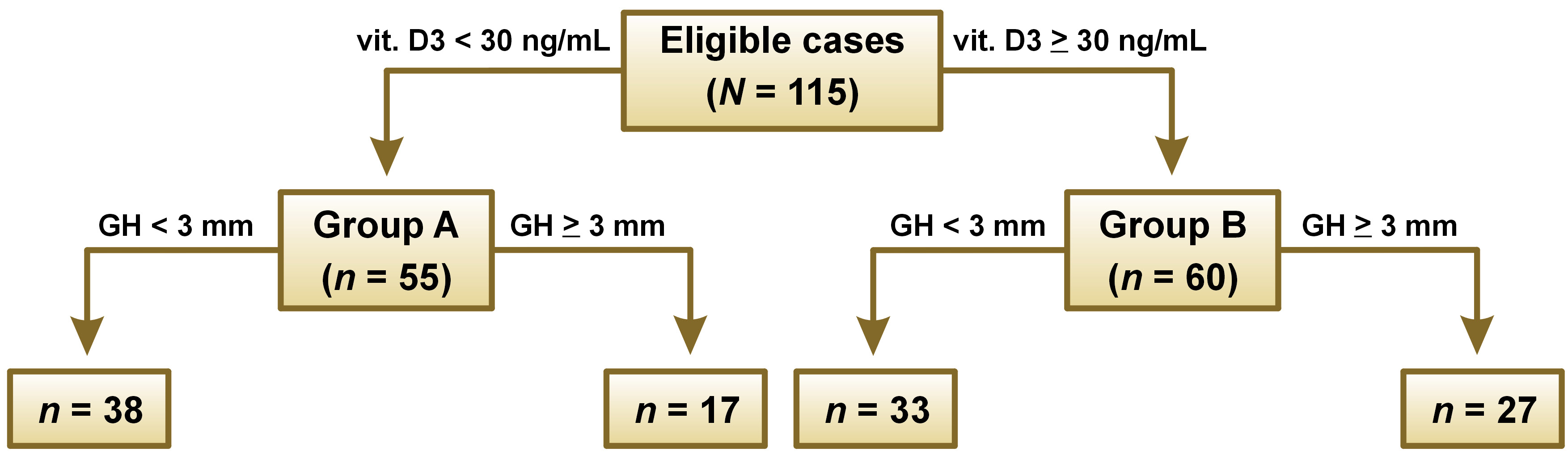
The cases (N = 115) were divided into 2 groups: group A (n = 55) were implants inserted with 25(OH)D levels below 30 ng/mL in blood serum, consisting of 17 cases with a gingival height of 3 mm or more and 38 cases with a gingival height of less than 3 mm; and group B (n = 60) were implants inserted with 25(OH)D levels equal to or above 30 ng/mL in blood serum, consisting of 27 cases with a gingival height of 3 mm or more and 33 cases with a gingival height of less than 3 mm (Figure 5).
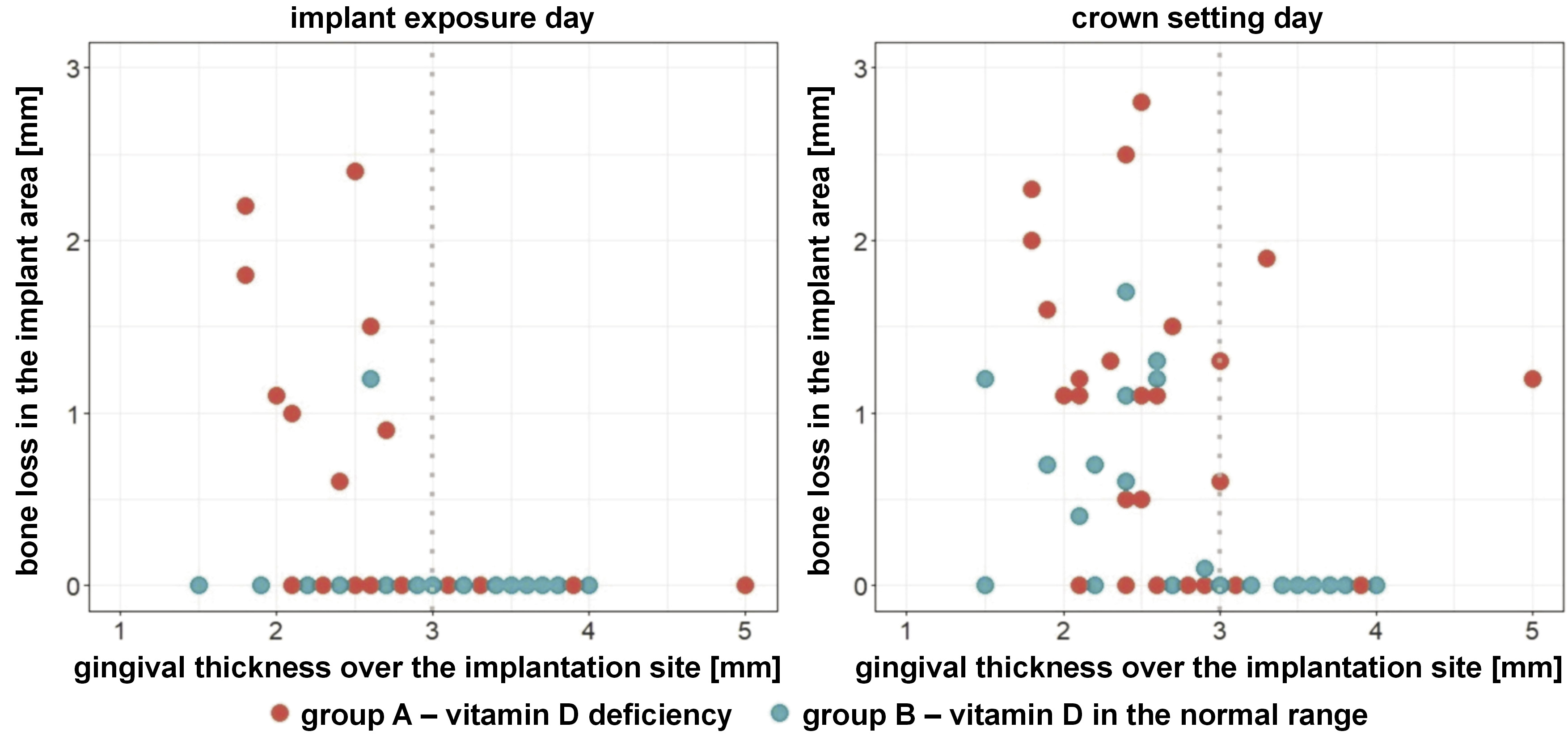
On the day of implant exposure, in group A (vitamin D3 deficiency), 8 cases of crestal bone loss around the implant platform were observed with a gingival thickness over the implant site of less than 3 mm, and no cases with a gingival thickness of 3 mm or more (p = 0.040). However, in group B (vitamin D3 in the normal range), only 1 case of crestal bone loss around the implant platform was observed with a gingival thickness over the implant site of less than 3 mm, and no cases with a gingival thickness of 3 mm or more (p = 0.366) (Figure 6).
Interestingly, on the day of prosthetic crown placement, in group A, 14 cases of bone atrophy around the implant platform were observed with a gingival thickness over the implant site of less than 3 mm, and 4 cases with a gingival thickness equal to or greater than 3 mm (p = 0.213). On the other hand, in group B, 9 cases of bone atrophy around the implant platform were observed with a gingival thickness over the implant site of less than 3 mm, and no cases with a gingival thickness equal to or greater than 3 mm (p < 0.001) (Figure 6).
Regardless of the day, no differences were detected based on gender (p = 0.173 and p = 0.390, respectively) or the implant region (p = 0.725 and p = 0.244, respectively).
Discussion
The stability of the crestal bone is one of the most desired features of successful implant treatment. Several factors contribute to bone loss, including improper bone healing around the implant, smoking, infection, inadequate keratinized gingiva in relation to the polished implant collar, overload, and a micro-gap.13
Among these factors, Berglundh and Lindhe highlighted the initial mucosal tissue thickness as a cause of crestal bone loss.14 In an animal experiment, they found that thin tissue could lead to crestal bone loss during the formation of the peri-implant seal. The implants installed in thick tissue were associated with significantly less bone loss.14 In another study, a mucosal tissue thickness of 2 mm or less was associated with bone loss of 1.38 mm, whereas implants placed in thicker tissue were associated with significantly less bone loss at 0.25 mm.15
Additionally, Linkevicius et al. examined the effect of the mucosal thickness on marginal bone loss in 19 patients with 46 implants in 2009.16 They placed 23 implants 2 mm supracrestally and 23 at the bone level. The mucosal thickness was classified as thin, medium or thick. Crestal bone changes were measured at the end of a 1-year follow-up. On average, thin, medium and thick mucosa showed bone loss of 1.35 mm, 0.32 mm and 0.12 mm, respectively.16 Thus, the pre-implantation thickness of the mucosa covering the edentulous alveolar ridge is very important for the subsequent stability of the peri-implant crestal bone and significantly determines the preservation of the bone tissue of the alveolar process surrounding the implant platform.
Another important factor is a vitamin D level in blood serum, which affects various stages of osteointegration. The vitamin D level has become an important field of knowledge in periodontology and implantology due to its role in the metabolism of the bone tissue and the immune system function.
Vitamin D levels typically decline with age and in individuals with periodontal diseases.17 Lower vitamin D3 levels have been associated with recurrent aphthous stomatitis in comparison with healthy individuals.18
Our study demonstrated a significant difference in the bone level at implant sites between the groups. We observed significantly greater bone loss around implants in vitamin D deficient patients as compared to those with normal levels, both on the implant exposure and crown setting days (p = 0.010 and p = 0.009, respectively), particularly when the gingival thickness was less than 3 mm. Similar findings were reported in a prospective study in the Indian population by Bhandage et al., showing that every increase by 1 ng/mL in vitamin D levels resulted in a 0.48-unit increase in the implant stability quotient (ISQ) value at 3 months and an increase by 0.62 units at 6 months.19
These results align with conclusions from a systematic review, which showed that vitamin D deficiency seemed to have an impact on marginal bone loss.20 It was also reported to negatively affect osseointegration in humans and animals.10 However, there is still little evidence that vitamin D supplementation enhances osseointegration in humans.10, 13, 21
Given the high prevalence of vitamin D deficiency, it seems appropriate to determine the blood levels of 25(OH)D before implantation.22 However, further research is needed to establish the beneficial effects of vitamin D supplementation on dental implant osseointegration.
Limitations
The height of the abutment and the diameter of the abutment were not measured, and the implant diameter was not taken into consideration. Vitamin D levels were measured only preoperatively.
Conclusions
We recommend that monitoring of the 25(OH)D concentration and the radiological assessment of the gingival tissue thickness is included in a short checklist when planning every dental implant procedure.
By adhering to the principles of thorough diagnosis, precise surgical procedures, appropriate material selection, and diligent postoperative care of dental restorations, along with assessing the soft tissue thickness and vitamin D3 levels, we anticipate more predictable long-term outcomes and treatment effectiveness.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Jagiellonian University Medical College, Krakow, Poland (No. of approval: 118.6120.160.2023). All patients involved in the study provided written informed consent for processing their data and using it for scientific research purposes.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.