Abstract
Background. Periodontitis is a chronic inflammatory condition caused by the bacterial infection of the gums that leads to tissue destruction, bone loss and tooth loss. Various risk factors, including smoking, age, diabetes, and obesity, contribute to its development and progression. Recent studies have revealed systemic effects of periodontitis, linking it to diabetes, atherosclerosis, stroke, and dementia.
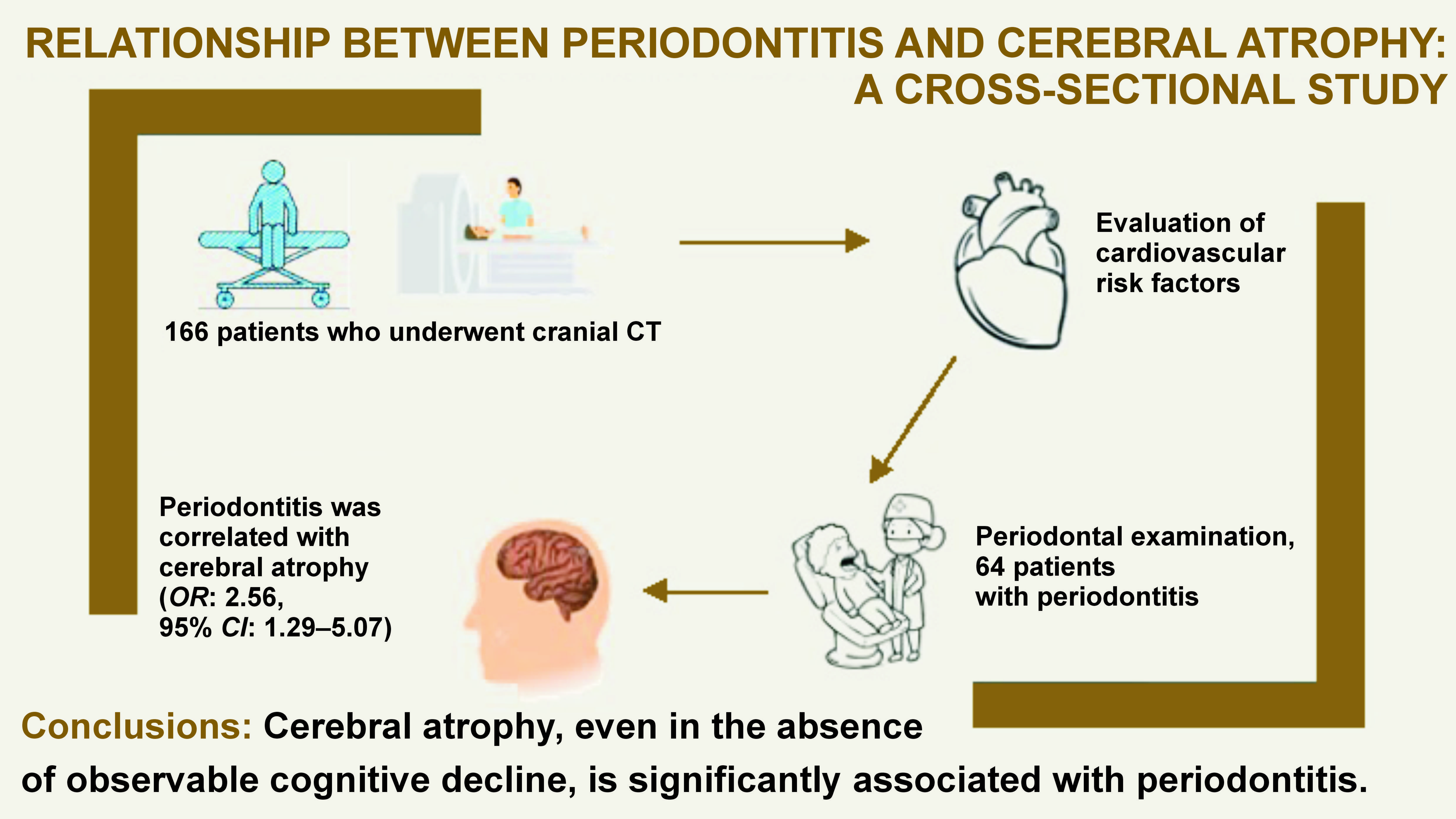
Objectives. The study aimed to assess the relationship between periodontitis and cerebral atrophy, an indirect marker of brain health.
Material and methods. A cross-sectional study was conducted to examine the association between cerebral atrophy and periodontitis. A total of 166 participants were included in the study. All individuals underwent computed tomography (CT) of the head and a full-mouth periodontal assessment to verify if they met the diagnostic criteria for periodontitis. They also underwent a complete neurological examination to rule out dementia.
Results. Sixty-four patients (38.6%) had periodontitis, 85 individuals (51.2%) had cerebral atrophy, and 43 patients presented with both conditions. The study sample included 89 females (53.6%), and the median age of the participants was 67 ±10 years. Patients diagnosed with periodontitis showed a higher grade of cerebral atrophy, as measured using the global cortical atrophy (GCA) scale. An independent association was identified between periodontitis and cerebral atrophy (odds ratio (OR): 2.56; 95% confidence interval (CI): 1.29–5.07).
Conclusions. Cerebral atrophy, even in the absence of cognitive decline, is significantly associated with periodontitis.
Keywords: dementia, periodontitis, cerebral atrophy
Introduction
Periodontitis is a chronic inflammatory condition in which bacteria commonly found in the mouth infect the gums, causing inflammation, tissue destruction, bone loss, and ultimately, the loss of the affected tooth.1 Several phases of periodontitis are defined, with the first being gingivitis, which is reversible and confined to the gums. While it was once accepted that bacteria were the sole factor in the development of this condition, in recent years, it has been shown that various personal2 and genetic factors also play a role. In fact, the presence and distribution of bacteria do not always correlate with the onset and progression of periodontitis.
Periodontitis, one of the most common diseases in human population, has been increasingly associated with other conditions such as diabetes, atherosclerosis, heart attacks, strokes, and dementia.3, 4 Although numerous epidemiological studies have been conducted to determine the prevalence of periodontitis, the results vary greatly,5, 6 likely due to the cross-sectional nature of most of the studies. According to recent research, the prevalence of periodontitis ranges from 8% to 47% of the population; furthermore, up to 10% of the population may be affected by advanced periodontitis.7 Periodontitis can become one of the top 5 most common diseases in some parts of the world, such as South America, with an incidence of up to 700 cases per person-year.8
Periodontitis is associated with numerous risk factors. Smoking has been linked to this condition, even when other confounding factors related to smoking are eliminated.9 Moreover, smokers have significantly greater bone loss and tooth mobility.9 On the other hand, factors such as age, diabetes and obesity have also been related to periodontitis.10, 11, 12 Diabetes is associated with a worse prognosis once the disease is established.13
Although periodontitis was initially considered a localized infection, recent studies indicate that this condition may contribute to the systemic circulation of various pro-inflammatory proteins, leading to a state of mild but chronic systemic inflammation. This chronic inflammation could be responsible for the relationship between several systemic pathologies and periodontitis. It has been demonstrated that these pathogenic bacteria are capable of colonizing other areas of the body by entering the bloodstream. Additionally, it has been hypothesized that they may significantly contribute to plaque formation in arteries, with bacterial DNA from these microorganisms being identified in such plaques.14 Various studies have shown that patients with periodontitis exhibit elevated levels of multiple blood markers of inflammation.14, 15
Other studies have shown that patients suffering from periodontitis have the capacity to produce antibodies in response to periodontal infection. Some of these antibodies, in addition to fighting the infection within the oral cavity, have the potential to cross-react, increasing the pro-inflammatory state of the system or even blocking protective molecules that usually try to prevent the formation of cholesterol plaque in the arterial walls. Consequently, numerous studies in recent decades have identified periodontitis as a potential risk factor for ischemic stroke.16, 17 It has also been demonstrated that patients with severe periodontitis are at an increased risk of hemorrhagic transformation once an ischemic stroke is established.18
Finally, various studies have shown a relationship between periodontitis, cognitive decline and dementia.19, 20 Cerebral atrophy, or cortical atrophy, is characterized by the widening of sulci, narrowing of gyri, decreased grey matter thickness, diminished white matter volume, and/or enlargement of the cerebral ventricles and subarachnoid spaces. Normal brain aging exhibits some of the same changes, although age-related atrophy is typically less rapid and less severe than that observed in individuals with neurodegenerative diseases.21 Importantly, cerebral atrophy has an impact on cognitive function, affecting memory, attention, language, and motor skills, even in the absence of dementia. Cerebral atrophy has been identified as an initial process of neurodegenerative diseases, such as Alzheimer’s. It may also result from other condition, potentially leading to cognitive decline.
To the best of our knowledge, no studies have investigated the effect of periodontitis on cerebral atrophy in the absence of dementia or neurodegenerative disease. The hypothesis of this study was that patients diagnosed with periodontitis have a higher degree of atrophy than those diagnosed with age-related atrophy. The aim of the study was to investigate whether there is an association between cerebral atrophy and periodontitis in the absence of cognitive decline.
Material and methods
A cross-sectional study was carried out from July 2021 to October 2023 at the University Clinical Hospital of Santiago de Compostela (Santiago de Compostela, Spain) and the Hospital Universitario Lucus Augusti (Lugo, Spain). The study was conducted in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.22 Patients aged ≥50 years who underwent computed tomography (CT) of the head as part of the care process were offered the opportunity to participate in this study. The exclusion criteria were: previous history of dementia, intracerebral tumor, malignancy, any other severe medical condition, or any other medical condition that could potentially be associated with cerebral atrophy, such as chronic renal failure; <10 teeth present, which would render the periodontal examination unreliable; those who, after anamnesis conducted by the principal investigator (neurologist), were deemed to have possible or probable dementia; and patients who did not consent to participate. The neurological criterion for possible or probable dementia was based on the latest international dementia diagnostic guidelines23 and relied on medical history, as well as at least 1 additional scale commonly used for cognitive testing: Montreal Cognitive Assessment (MoCA); or Mini-Mental State Examination (MMSE). The present study was conducted in accordance with the World Medical Association (WMA) Declaration of Helsinki (2013) and approved by the Research Ethics Committee of Santiago-Lugo (protocol No. 2022/18). All participants provided written consent.
Demographic data such as sex and age was collected. The medical history of the subjects was thoroughly reviewed to ascertain the presence or absence of various risk factors, including hypertension, type 2 diabetes, smoking, and alcohol consumption. The variables such as smoking, alcohol consumption, hypertension, periodontitis, and diabetes were dichotomous and recorded as “yes” or “no”.
Assessment of cerebral atrophy
The presence of cerebral atrophy was evaluated based on the head CT scans by a neurologist and a radiologist, who have experience measuring cerebral atrophy according to the global cortical atrophy (GCA) scale. A minimum of 13 brain regions were assessed separately in each hemisphere, with scores ranging from 0, indicating “normal volume”, to 3, representing “knife blade” atrophy. The sum of all regions’ scores constituted the final score.24 The 13 brain regions evaluated were as follows: sulcal dilatation in the frontal, parieto-occipital and temporal regions; ventricular dilatation in the frontal, parieto-occipital, temporal, and third ventricle regions. The evaluation of all regions was conducted bilaterally, with the exception of the third ventricle. The sum of the individual region scores, with a range of 0–3 points for each region, resulted in a total score of 0–39. The values equal or greater than 1 in each region were considered cerebral atrophy. The initial measurement of these scores was conducted by the radiologist, who was blinded to the clinical data, followed by the evaluation of the neurologist.
Assessment of periodontal disease
The diagnosis of periodontitis was made by a periodontist. A full-mouth periodontal examination was conducted on all subjects. Probing pocket depth (PPD) and clinical attachment level (CAL) were measured in all teeth, with the exception of third molars. The measurements were recorded at 6 sites per tooth (mesiobuccal, midbuccal, distobuccal, mesiolingual, distolingual, and midlingual). Slight periodontitis was defined as the presence of at least 2 interproximal sites with CAL ≥ 3 mm and at least 2 interproximal sites with PPD ≥ 4 mm (not on the same tooth) or 1 site with PPD ≥ 5 mm. Moderate periodontitis was defined as the presence of at least 2 interproximal sites with CAL ≥ 4 mm (not on the same tooth) or at least 2 interproximal sites with PPD ≥ 5 mm, also not on the same tooth. Severe periodontitis was defined as the presence of ≥2 interproximal sites with CAL ≥ 6 mm (not on the same tooth) and ≥1 interproximal site with PPD ≥ 5 mm.25 Total periodontitis comprised slight, moderate and severe cases. Bleeding on probing (BOP) and the presence of dental furcations were not assessed.
Statistical analysis
Categorical data was reported as percentages and compared using the χ2 test. The mean and standard deviation values (M ±SD) were calculated for the continuous variables, and a comparison was made using an independent t-test after confirming normality through the Kolmogorov–Smirnov test. Finally, a multivariate analysis was performed using logistic regression to ascertain the relationship between the variables and cerebral atrophy.
Results
A total of 231 patients were initially selected to participate in the study. Of these, 29 were excluded on the basis of the eligibility criteria. Nine participants were not included after anamnesis, conducted by the principal investigator, as they were considered to show signs of dementia. Twenty-seven individuals declined to participate or did not consent to undergo periodontal examination. The study sample comprised 166 patients who underwent cranial CT as part of their standard care regimen. Of these, 64 subjects (38.6%) had been diagnosed with periodontitis, while 85 individuals (51.2%) had been diagnosed with cerebral atrophy. Forty-three patients presented with both conditions. The study sample included 89 females (53.6%), and the median age of the participants was 67 ±10 years. With the exception of 1 patient, all subjects were diagnosed with hypertension and prescribed either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB) at the time of recruitment.
No statistically significant differences in sociodemographic characteristics were observed between patients with periodontitis and those without the condition. The study also revealed no significant differences for the vascular risk factors between the periodontitis and no periodontitis groups, with the exception of smoking (34.4% vs. 19.6%; p = 0.033). This finding is consistent with the established correlation between tobacco use and periodontal disease. The noted difference in the prevalence of diabetes, hypertension and alcohol consumption between the 2 groups was not statistically significant. The characteristics of the study population are summarized in Table 1.
The direct analysis of variables using the χ2 test demonstrated a relationship between cerebral atrophy and hypertension (p = 0.017) and periodontal disease (p < 0.001). The relationship between cerebral atrophy and type II diabetes was moderate but not significant (p = 0.056). The present study did not include any patients with type I diabetes.
The results of the multivariate logistic regression analysis are presented in Table 2. The logistic regression analysis demonstrated a statistically significant positive correlation between periodontitis and cerebral atrophy (odds ratio (OR): 2.56; 95% confidence interval (CI): 1.29–5.07). Hypertension and age were also related to cerebral atrophy, but to a lesser extent (OR: 1.94; 95% CI: 1.00–3.75 and OR: 1.04; 95% CI: 1.01–1.08, respectively).
To conclude, the data was examined using the Hosmer–Lemeshow test, yielding a p-value of 0.599. Additionally, the variability of the data was assessed by performing Nagelkerke’s R2, which resulted in an R2 value of 0.143.
Discussion
The findings of the present study suggest that periodontitis may contribute to cerebral atrophy, even in the absence of cognitive decline. In models implemented with logistic regression, we observed that periodontitis is strongly correlated with cerebral atrophy, even when accounting for confounding factors.
The relationship between periodontitis and various pathologies has been extensively examined in the literature. Numerous studies, including meta-analyses of epidemiological studies, have indicated that individuals diagnosed with periodontitis have a higher risk of developing coronary heart disease, ischemic stroke, dementia, and peripheral arterial disease when compared to those without periodontitis.17, 26, 27, 28, 29 In recent years, the elevation of periodontitis markers, such as the periodontal inflamed surface area (PISA), has also been linked to cardiac involvement in hypertensive patients.30 Although the direct relationship between cardiovascular diseases and periodontitis is not known, efforts have been made to understand the underlying mechanisms. A number of studies have investigated biomarkers such as pentraxin-3, C-reactive protein or paraoxonase-1 in patients with periodontitis, with the objective of understanding the heightened risk of atherosclerosis observed in these individuals.31, 32
Despite the paucity of research in this area, periodontal disease has also been linked to other neurological conditions such as multiple sclerosis and epilepsy. One article attempted to correlate the severity of seizures with oral hygiene practices. Additionally, a relationship between periodontitis and multiple sclerosis has been identified, which is consistent with the inflammatory nature of these diseases.33, 34
Despite the clear relationship between cerebral atrophy and cognitive decline, and numerous articles linking periodontitis with Alzheimer’s disease, only 1 paper was found relating periodontitis to cerebral atrophy. In the aforementioned study, 468 patients with periodontitis were analyzed, and the thickness and volume of the cerebral cortex were measured on magnetic resonance imagining, although this was not the study’s primary objective.35 Similar to our study, the article suggested a direct relationship between cerebral volume loss and periodontitis.
Other studies have correlated tooth loss with cognitive decline and cerebral volume loss.36, 37 However, they employed a methodology that differed from the present study. Some of them focused on patients with genetic Alzheimer’s disease in the prodromal phase, while others examined the correlation between tooth loss and cerebral atrophy, excluding the influence of periodontitis. Multiple studies have demonstrated a relationship between periodontitis and leukoaraiosis or small vessel brain disease. Additionally, other articles have linked periodontitis to ischemic stroke and other cardiovascular diseases.38, 39
The relationship between cerebral atrophy and cognitive decline is well-established. Although it is not always directly related to the degree of cognitive decline, it is an interesting factor to consider in the prevention of the condition. In the present study, we found that periodontitis is directly and independently related to the degree of cerebral atrophy, even in the absence of cognitive decline.
While the precise underlying mechanism through which periodontitis leads to cerebral atrophy remains unclear, it is likely that the continuous inflammatory state caused by this condition plays a pivotal role. Numerous studies have shown that the effects of inflammation are not limited to the oral cavity but can affect the entire body. In fact, the destruction of periodontal epithelium by pathogens enables the entry of endotoxins and exotoxins into the bloodstream, thereby increasing systemic inflammatory response, which can result in brain tissue damage. Other studies have shown the presence of periodontal pathogens in various tissues and organs of the cardiovascular system, including heart valves.40, 41, 42 One study sought the presence of various periodontitis-causing microorganisms in cerebrospinal fluid, but was unable to detect them.43
Limitations
Some limitations of the present study should be addressed. Firstly, the design of the study precluded the testing of causality regarding the association between periodontitis and cerebral atrophy. Secondly, the patients were recruited following a consultation with a neurologist. Patients who might have presented dementia or another condition directly affecting cerebral atrophy were excluded, which represents a selection bias that could influence the results. Thirdly, given the nature of the study, it was not possible to assess the severity of diabetes in each patient; it was simply considered a dichotomous variable. In future studies, it would be ideal to examine its influence using each patient’s glycated hemoglobin levels, for example. Lastly, the diagnosis of periodontitis was made based solely on PPD and CAL measurements. However, other indicators, such as BOP or PISA, may be of more importance when discussing systemic inflammation and the relationship between cerebral atrophy and periodontitis.
Future studies, especially prospective studies on healthy subjects with comprehensive periodontal exploration, are needed to confirm our results. However, this study, along with the recent investigations conducted on the relationship between systemic disease and periodontitis, underscore the importance for ongoing research in this area, focusing on the prevention and treatment of this condition.
Conclusions
Cerebral atrophy, even in the absence of observable cognitive decline, demonstrates a significant association with periodontitis. To substantiate this finding, further research is warranted, preferably through prospective studies, to establish and clarify the nature of this relationship.
Ethics approval and consent to participate
The study was conducted in accordance with the World Medical Association (WMA) Declaration of Helsinki (2013) and approved by the the Research Ethics Committee of Santiago-Lugo (protocol No. 2022/18). All participants provided written consent.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.