Abstract
Background. Host response to periodontal pathogens present in microbial plaque is characterized by the expression of various inflammatory and immune mediators known as biomarkers. There is a paucity of literature addressing the impact of non-surgical periodontal therapy (NSPT) on serum biomarkers, such as mannose-binding lectin (MBL), sirtuin 1 (SIRT-1) and C-reactive protein (CRP), in non-smokers and smokers with stage III periodontitis.
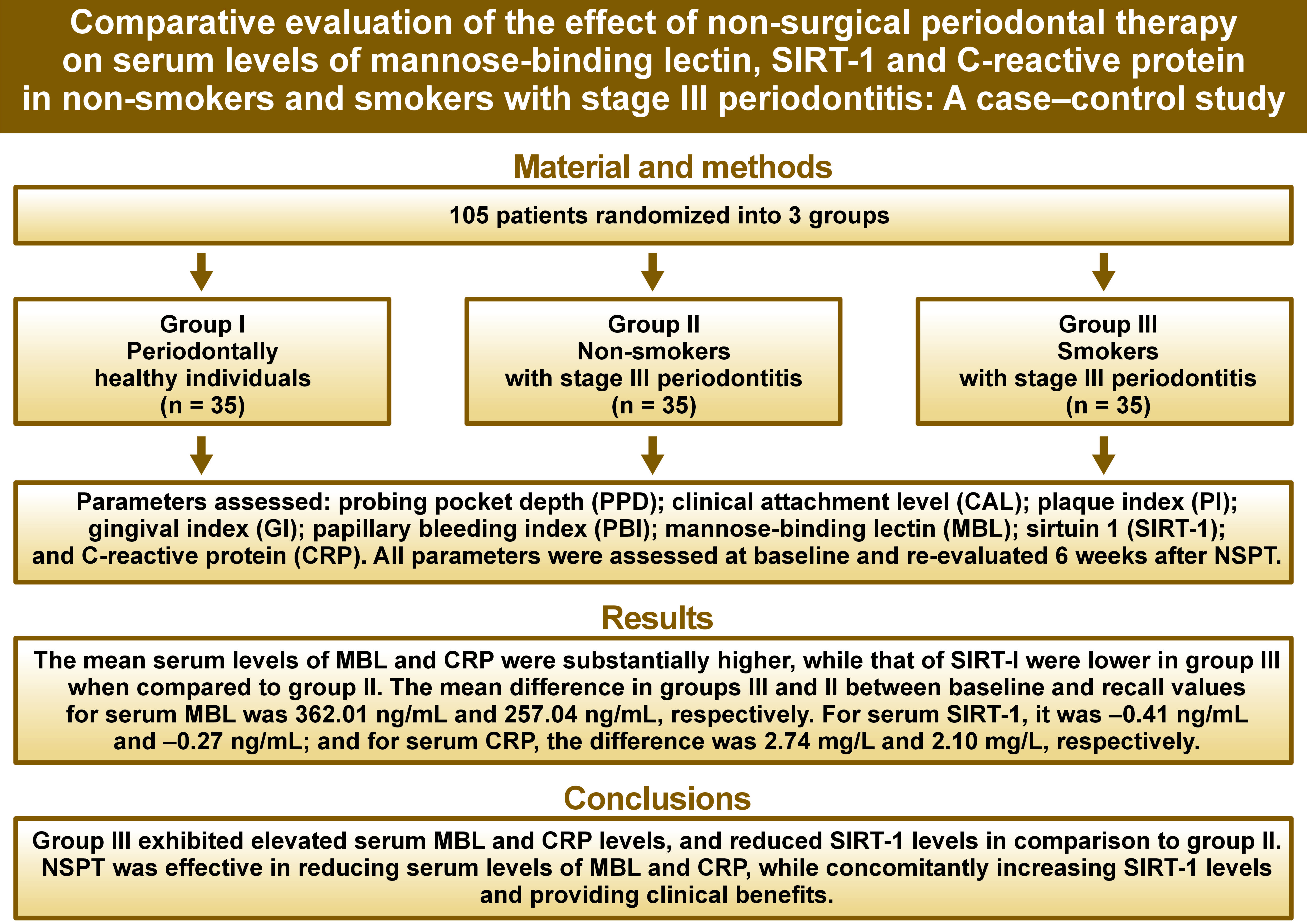
Objectives. The study aimed to evaluate and compare the effect of NSPT on serum levels of MBL, SIRT-1 and CRP in non-smokers and smokers with stage III periodontitis.
Material and methods. A total of 105 patients were equally divided into 3 groups, as follows: group I – periodontally healthy individuals; group II – non-smokers with stage III periodontitis; group III – smokers with stage III periodontitis. Probing pocket depth (PPD), clinical attachment level (CAL), plaque index (PI), gingival index (GI), and papillary bleeding index (PBI) were recorded, and serum MBL, SIRT-1 and CRP levels were analyzed using enzyme-linked immunosorbent assay (ELISA). The patients underwent NSPT, and all parameters were re-evaluated 6 weeks after the procedure.
Results. The mean change in MBL levels across the 3 groups from baseline to recall was significant. Conversely, SIRT-1 and CRP levels exhibited non-significant differences from baseline to recall, with p-values of 0.172 and 0.548, respectively. The mean differences in MBL and SIRT-1 levels between groups I and III at baseline (p < 0.0001 and p = 0.041 for MBL and SIRT-1, respectively) as well as in MBL between groups II and III at recall (p < 0.0001) were statistically significant.
Conclusions. A positive association of serum MBL levels and CRP levels as well as a negative association of SIRT-1 with the severity of periodontal disease may serve as a valuable, precise and feasible method of identifying individuals at risk of developing periodontal disease.
Keywords: smokers, periodontal diseases, non-surgical periodontal therapy
Introduction
Periodontal diseases are a group of microbial infections associated with a chronic inflammatory state. If left untreated, these infections cause extensive destruction of the adjacent tissues, ultimately resulting in tooth loss. Additionally, they are atypical in nature, with the associated inflammation being driven on by a complex biofilm of symbiotic and common pathogenic bacteria and their byproducts.1 An increase in the periodontal pathogens disrupts the homeostasis, thereby affecting the susceptibility of the periodontal tissues.2 The body’s reaction to periodontal pathogens in microbial plaque is characterized by an expression of various inflammatory and immune mediators, known as biomarkers, which have been studied extensively over the recent past.3, 4 Thus, the monitoring of biomarkers in various biological samples, such as gingival crevicular fluid (GCF), saliva and/or serum in patients with periodontal disease can provide important conclusions into the pathogenesis of the disease. Similarly, the observation of the precocity of the peri-implant environment can facilitate the detection of an early peri-implant condition.5, 6
Systemic alterations that ensue following an inflammatory response are referred to as the acute phase response,7 despite the fact that they accompany both acute and chronic inflammation. Mannose-binding lectin (MBL), C-reactive protein (CRP), plasminogen activator inhibitor-1 (PAI-1), complement proteins C3, C4 and C9, and fibrinogen are few examples of acute phase proteins that have both pro-inflammatory and anti-inflammatory properties. Acute phase reactants constitute an early, non-specific response to bacterial, viral or parasitic infection, mechanical or thermal trauma, ischemic necrosis, or malignant growth. The plasma concentration of these proteins undergoes a 25% increase or decrease in response to inflammation.8 Mannose-binding lectin, a weak acute phase protein, is an important constituent of the innate immune system and one of the proteins of the complement system. Mannose-binding lectin functions as an opsonin and stimulates the traditional complement route.7 The classical and lectin pathways are activated by the binding of complement-associated pattern recognition molecules to immune complexes (classical pathway) or carbohydrate subunits exposed on microbial or damaged host cells.9 Mannose-binding lectin primarily recognizes and binds to specific polysaccharide groups that are present on the surface of microorganisms.10 Interestingly, many of the periodontal pathogens also have mannan-containing polysaccharides on the cell surface, which can be a potential target for MBL binding. Following its binding to microorganisms, MBL activates the lectin pathway of the complement system.11 Since inflammation is a central pathology in periodontal diseases, the estimation of MBL levels could be of importance for correlating these values pre- and post-non-surgical periodontal therapy (NSPT) and enable a better understanding of this biomarker.
Dental stem cell proliferation and osteoblast differentiation are key cellular processes involved in periodontal diseases. Preliminary studies have shown that sirtuin 1 (SIRT-1) regulates cellular differentiation and controls metabolic pathways in a wide variety of tissues.12 Furthermore, it has been demonstrated that the levels of SIRT-1 influence the osteoblastic differentiation of human periodontal ligament stem cells (PDLSCs),13 as well as the management of inflammation and oxidative stress. However, the role of SIRT-1 in periodontal disease activity and the impact of NSPT on SIRT-1 levels remains unclear.
Increased levels of CRP, an acute-phase inflammatory protein, have been observed in both acute and chronic conditions. C-reactive protein can activate the classical complement system as well as phagocytic cells via Fc receptors, facilitating the clearance of cellular debris, injured or dead cells, and foreign pathogens. The concentration of CRP closely reflects the progression of the acute-phase response to inflammation or tissue necrosis, and hence could serve as a useful biomarker for many disease processes.7
Smoking is a substantial risk factor for the progression of periodontal disease, which greatly enhances the likelihood of developing severe and chronic diseases.14 Furthermore, it can negatively affect the regenerative healing response by suppressing vascular growth, inhibiting fibroblast proliferation and adhesion, and reducing collagen production.15
Non-surgical periodontal therapy is universally regarded as the gold standard for treating periodontal disease, despite its evolution over time. The goal of eliminating pathogenic microbes may be overly ambitious.16 However, a reduction in periodontal inflammation, attributable to a decreased microbial load, results in favorable clinical outcomes. There is a paucity of literature regarding the impact of NSPT on serum biomarkers, such as MBL, SIRT-1 and CRP. The present study was designed to evaluate and compare the effect of NSPT on serum levels of MBL, SIRT-1 and CRP in non-smokers and smokers with stage III periodontitis.
Material and methods
This case–control study was conducted from July 2021 to February 2023 in the Department of Periodontology at Ranjeet Deshmukh Dental College and Research Centre (Nagpur, India), following the approval of the Institutional Ethics Committee of Ranjeet Deshmukh Dental College and Research Centre, and in accordance with the Helsinki Declaration of 1975, as revised in 2013. This clinical study was registered with the Clinical Trial Registry-India (CTRI) (registration No. CTRI/2021/01/030833), the main registry of the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). Informed consent was obtained from patients who were willing to participate in the trial after the objectives and procedures were outlined to them.
Sample size estimation
Caribé et al. observed the influence of non-surgical treatment of periodontal disease on serum concentration of SIRT-1 and MBL before and after intervention in the periodontitis group and the healthy group.17 The effect size for these 2 parameters ranged between 0.10 and 0.55. The same 2 parameters, along with CRP, were measured before and after the intervention in these groups. According to the effect size of 0.53, the estimated sample size per group that can detect the effect with 95% confidence and 80% power is 28. However, considering 20% loss to follow-up, a sample of 105 (35 patients per group) was found appropriate.
Inclusion and exclusion criteria
The present study included 105 patients, comprising both males and females, divided equally into 3 groups and exhibiting good general health, generalized stage III periodontitis, and the presence of at least 15 natural teeth excluding third molars. Stage III periodontitis was diagnosed based on the current classification proposed by the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) in 2018.18 The study population was divided into 3 groups, each with specific inclusion criteria, as follows:
– group I: periodontally healthy patients with no history of smoking;
– group II: patients diagnosed with stage III periodontitis exhibiting probing pocket depth (PPD) ≥6 mm, interdental clinical attachment level (CAL) ≥5 mm and radiographic bone destruction extending almost up to the middle third of the root; no history of smoking;
– group III: patients diagnosed with stage III periodontitis exhibiting PPD ≥ 6 mm, interdental CAL ≥ 5 mm and radiographic bone destruction extending to or beyond the middle third of the root; and current smokers with a history of smoking more than 10 cigarettes per day.
Individuals who had undergone periodontal treatment within the past 6 months, those with any systemic disease, patients who had or were on immunosuppressive therapy, those with a history of antibiotic intake within the past 6 months or anti-inflammatory drug intake within the last 3 months, as well as pregnant and lactating women, were excluded from the study.
Clinical examination
Prior to the commencement of the clinical trial, the examiners were calibrated to ensure precise measurement recordings. A single examiner (RK) was responsible for taking a comprehensive case history and performing an intraoral examination using a manual periodontal probe (PCPUNC 15; Hu-Friedy, Chicago, USA). The intraoral examination consisted of clinical and radiological evaluations. Verbal interrogation was used to determine smoking status of the individuals. Periodontal parameters were charted for each patient. These included the plaque index (PI),19 the gingival index (GI),20 the papillary bleeding index (PBI),21 PPD, and CAL. All participants underwent NSPT, which included scaling and root planing performed by a single operator (AP) along with oral hygiene instructions. The parameters were recorded at the conclusion of the observation period. For each periodontal parameter, the intraclass correlation coefficient (ICC) was calculated as a measure of reliability of observations of different parameters using a two-way mixed effects model. The ICC in the groups ranged from 0.92 to 0.99 (p < 0.0001), demonstrating high intraobserver reliability.
Assessment of biochemical parameters
Fresh blood was drawn from the antecubital fossa via venipuncture using a 20-gauge needle. A total of 10 mL of venous blood was collected from each subject and transferred to sterile test tubes. The samples were then permitted to clot at room temperature for 20 min. The clot was removed by centrifuging at 2,000–3,000 rpm for 20 min. The collected serum was transferred to Eppendorf tubes (airtight plastic vials) using a clean pipette and stored at −20°C in a deep freezer until the final assay. The samples were analyzed for MBL, CRP and SIRT-1 levels using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Krishgen Biosystems, Mumbai, India) according to the manufacturer’s instructions. Briefly, serum samples were diluted with the provided dilution buffer, and the levels of MBL, CRP and SIRT-1 were determined using a sandwich ELISA technique. Blood samples were collected at both time points, that is, at baseline and at the 6th week following the intervention.
Statistical analysis
Continuous demographic, clinical and biochemical parameters were expressed as mean and standard deviation (M ±SD). The distribution of subjects according to their sex was presented in terms of frequency and percentage (n (%)). The statistical analysis of continuous parameters across groups was conducted using one-way analysis of variance (ANOVA). The parameters exhibiting significant differences in means across the 3 groups were subjected to further comparison through the implementation of the Tukey’s post hoc test. The distribution of subjects by sex was analyzed using Pearson’s χ2 test. The within-group comparison of clinical and biochemical parameters from baseline to recall was performed using the paired t-test. The statistical significance in clinical and biochemical parameters between groups II and III was determined by employing a t-test for independent samples. Furthermore, a one-way ANOVA was employed to compare the parameters at the study’s conclusion (recall) across the 3 groups. The post-hoc analysis was carried out using Tukey’s post-hoc test. All analyses were performed with the IBM SPSS Statistics for Windows software, v. 26.0 (IBM Corp., Armonk, USA). The significance level was set at 5%.
Results
A total of 105 patients attending the outpatient Department of Periodontology were included in the study. The demographic, clinical and biochemical parameters are depicted in Table 1. The mean age for groups II and III was found to differ insignificantly, whereas the mean age for group I was significantly lower compared to the other groups. The mean age of subjects in group I was 24.60 ±3.56 years, and it was 47.85 ±9.33 years in group II, and 45.08 ±7.89 years in group III. The distribution of sexes across groups also differed significantly (p < 0.0001). The mean biochemical parametric values of MBL, SIRT-1 and CRP were 809.22 ±109.41 ng/mL, 0.99 ±0.56 ng/mL and 2.61 ±0.76 mg/L, respectively, for group I. For groups II and III, the values for MBL were 978.75 ±275.39 ng/mL and 1,206.92 ±458.40 ng/mL, respectively. For SIRT-1, the values in groups II and III were 0.95 ±0.41 ng/mL and 0.71 ±0.46 ng/mL, respectively. Similarly, for CRP, the mean biochemical parametric values were determined to be 4.70 ±0.89 mg/L and 5.51 ±0.78 mg/L, respectively.
The PI, PPD and CAL values were significantly higher, while the GI and PBI scores were lower in group III. The clinical attachment level in group II exhibited a mean of 7.80 ±1.48 mm at baseline, which was significantly lower than that in group III (8.45 ±0.63 mm; p = 0.020). A comparison of clinical parameters within groups II and III between 2 time points demonstrated statistically significant differences (Table 2).
At baseline, a statistically significant difference was observed in the mean PPD, CAL, PI, GI, and PBI values across the 3 groups (p < 0.0001). The mean PI for group I was the lowest (0.71 ±0.29), while for group III, it was the highest (2.63 ±0.49). Six weeks after NSPT, a significant improvement was noted in all the periodontal clinical parameters (p < 0.0001). There was a significant decrease in CAL values in group II and group III from baseline to recall (Table 2). Additionally, a reduction in PPD, PI, GI, and PBI levels was observed in smokers and non-smokers after NSPT. The mean change in MBL levels across the 3 groups from baseline to recall was significant. Conversely, SIRT-1 and CRP levels did not demonstrate a statistically significant difference from baseline to recall, with p-values of 0.172 and 0.548, respectively. A comparison of the CAL values between groups II and III revealed no statistically significant differences. (Table 3).
Table 4 presents the pairwise comparison of biochemical parameters between the 3 groups at 2 time points. The mean difference in MBL between groups I and II at recall (87.51 ng/mL (95% confidence interval (CI): 23.19, 151.84)) was statistically significant (p = 0.005). Furthermore, the difference between groups II and III was significant (−123.21 ng/mL (95% CI: −187.53, −58.88)), with a p-value <0.0001. The results of the pairwise analysis for SIRT-1 indicated that the mean difference at baseline between groups I and III was 0.28 ng/mL (95% CI: 0.01, 0.56), which was significant (p = 0.041). For CRP, significant differences were observed at baseline among all groups, yet no significant differences were noted at recall.
Discussion
Periodontitis is defined as the dynamic interplay among pathogenic microbes and the host inflammatory response, which promotes the destruction of connective tissue and alveolar bone.22 Clinical parameters and radiographs that measure alveolar bone levels only provide information about past periodontal tissue destruction and do not elucidate current nor predict future disease activity due to their low sensitivity and positive predictive value. Recently, molecular determinants including enzymes, cytokines, receptors, and other proteins, have been used as potential biomarkers to establish a more biologically-based diagnostic approach and explain associations between periodontitis and systemic diseases.23
Groups II and III were characterized by a higher average age and a comparatively poor level of oral hygiene. Group III exhibited a preponderance of male participants, attributable to social and religious inhibitions concerning smoking among female subjects. The group demonstrated a higher degree of tissue destruction, as evidenced by the values of PPD and CAL, which are indicative of an increased severity of the disease. The patients in group III also exhibited lower GI and PBI and greater PI values, which is consistent with the findings of the previous studies.14, 24, 25 Nicotine has been shown to stimulate the sympathetic ganglia, leading to the production of neurotransmitters such as catecholamines. These neurotransmitters activate alpha receptors on blood vessels, resulting in vasoconstriction and further enhancing periodontal tissue breakdown. Smokers exhibit a reduced manifestation of gingivitis and diminished gingival blood flow when compared to non-smokers.26 Thus, smoking reduces the clinical signs of inflammation, including bleeding on probing, while concealing gingival inflammation.
In the current study, a significant improvement in the clinical parameters (PPD, CAL, PBI, PI, and GI) was observed 6 weeks after NSPT, which is consistent with the findings reported in previous literature.27 Clinical studies have demonstrated that NSPT reduces the overall number of gingival sites that exhibit bleeding on probing, allowing the oral microbiota to shift from gram-negative to gram-positive bacteria.27
The present study is one of the few trials evaluating periodontal parameters and correlating them with the serum levels of MBL, SIRT-1 and CRP in smokers and non-smokers with stage III periodontitis, as well as determining the influence of NSPT on these biomarkers. It highlights the role of MBL, SIRT-1 and CRP in regulating inflammation and oxidative stress among smokers and non-smokers with severe periodontitis.
The enhanced severity of the periodontal disease, as manifested through various clinical parameters, is consistent with the results of the current study. The study indicates that systemic MBL levels were significantly higher in groups III and II than in group I. This rise can be attributed to an increased inflammatory burden. The findings corroborate the results of previous studies.28 However, Maffei et al. did not demonstrate elevated serum MBL levels in periodontitis when compared with healthy controls.29 Instead, they identified higher plasma MBL levels in smokers,29 which is consistent with the results of the present study. In this study, the mean MBL levels were higher in group III (844.91 ±111.49 ng/mL) than in group II (721.71 ±118.32 ng/mL). Similarly, Louropoulou et al. reported an increase in serum MBL levels in patients with periodontitis, including those with deficient MBL production.30 The possible explanations for these discrepancies may be found in the inclusion of smokers in the control group in the aforementioned study. Additionally, some authors have identified MBL deficiency inadvertently with serum MBL levels. Six weeks after the intervention, the results demonstrated a significant decrease in MBL concentrations compared to the baseline levels, as well as a significant mean difference across the 3 groups. This finding points to anti-inflammatory benefits of NSPT on serum MBL levels.
Sirtuin 1 is the most studied member of the sirtuin family. Recent investigations have identified considerable shifts in the levels of several sirtuin family proteins in periodontitis.31 In the present study, the levels of SIRT-1 were found to be the highest in group I, and further decreased in groups II and III. It has been previously demonstrated that mitochondrial oxidative stress was caused by a decrease in SIRT-1 expression, which can be linked to the oxidative stress seen in smokers.32 Thus, smoking might have decreased SIRT-1 levels to activate lung fibroblasts by promoting mitochondrial oxidative stress, which dysregulated lipid metabolism due to impaired autophagy flux. Additionally, an increase in serum levels of SIRT-1 after NSPT was evident in groups II and III. This phenomenon can be attributed to a reduction in the severity of periodontitis due to decreased oxidative stress. This finding aligns with the studies suggesting that NSPT leads to elevated serum concentrations of SIRT-1 in individuals with periodontitis, irrespective of their smoking status.
The concentration of CRP is the greatest in serum, with certain infectious diseases raising its levels up to 1,000-fold. When the stimuli are removed, CRP levels drop exponentially over 18–20 h, a timeframe that closely aligns with the CRP half-life.33 Thus, this study analyzed serum CRP levels to ensure the attainment of more accurate results. C-reactive protein, an acute phase marker of inflammation, was significantly increased in group II and III participants with stage III periodontitis, as compared to group I. At baseline, greater CRP levels were observed in group III when compared to group II. The mean difference between the 2 groups was −0.81 mg/L (p < 0.0001). The results of the present study indicate an improvement in CRP levels after NSPT, which could be attributed to the beneficial effects of NSPT that ultimately reduce the inflammatory burden within the periodontium. The available literature on the above aspects also supports this understanding. Mechanical periodontal treatment has been shown to reduce serum CRP and markers of systemic inflammation.34 In contrast to the present study, Ide et al. conducted a study to determine whether treatment of periodontal disease would result in a decrease in circulating acute phase proteins.35 However, the authors did not observe a reduction in circulating CRP following NSPT.35 A potential rationale for the persistent elevation of CRP, even subsequent to scaling and root planning, is that this procedure may prove inadequate in fully controlling the progression of periodontal disease in subjects with periodontitis. The complete elimination of microorganisms and deposits from deep inaccessible pockets may require surgical intervention and/or the use of antimicrobial agents.
Results of the present study indicate a direct correlation between MBL and CRP levels, as well as an inverse relationship between MBL and CRP values with SIRT-1.
Limitations
The limitations associated with this case–control study include a relatively small sample size with an unequal age and sex distribution. Due to the limited follow-up, timely changes in serum biomarker levels cannot be identified. Longitudinal investigations are required to examine potential clinical benefits and to identify the phased alterations that will enable us to draw definitive and consistent conclusions. The sample was representative of a severe periodontitis population, exhibiting high levels of clinical disease, bleeding and dental plaque. Consequently, the findings of this study may only be applicable to patients with milder forms of periodontitis.
Conclusions
Non-surgical periodontal therapy is an effective modality for the reduction in clinical parameters of PPD and PBI, along with a gain in CAL. It also leads to a decrease in serum concentrations of MBL and CRP and an increase in SIRT-1 levels in smokers and non-smokers diagnosed with stage III periodontitis. A positive association of serum MBL levels, CRP levels, and a negative association of SIRT-1 with the severity of periodontal disease may be a valuable, precise and feasible method for identifying individuals at risk of developing periodontal disease. In addition, these markers could facilitate the confirmation of a diagnosis and the assessment of periodontitis severity. Additionally, they may aid in determining the prognosis of the smoking-associated inflammatory burden on the periodontium. These can be used as predictive biomarkers for disease development and as diagnostic markers in the evaluation of inflammation within periodontal tissues. Further clinical trials assessing the periodontal status in patients with periodontitis are needed to establish the links between MBL, SIRT-1 and CRP and the severity of periodontal disease.
Trial registration
This clinical study was registered using the Clinical Trial Registry-India (CTRI), the main registry of the WHO International Clinical Trials Registry Platform (ICTRP) (registration No. CTRI/2021/01/030833).
Ethics approval and consent to participate
This clinical study received the approval of the Institutional Ethics Committee of Ranjeet Deshmukh Dental College and Research Centre, and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from patients who were willing to participate in the trial after the objectives and procedures were outlined to them.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.