Abstract
Background. The immediate placement of the implant into a fresh extraction socket site with immediate provisionalisation is considered to be a predictable and acceptable procedure. However, there are mixed results regarding the advantages of the immediate provisionalization of dental implants, using a biomaterial in the jump space (JS).
Objectives. This randomized controlled trial (RCT) aimed to evaluate the use of a concentrated growth factor (CGF)-enriched bone graft in the JS of immediate implant placement with provisionalization (IIPP) in the maxillary esthetic zone.
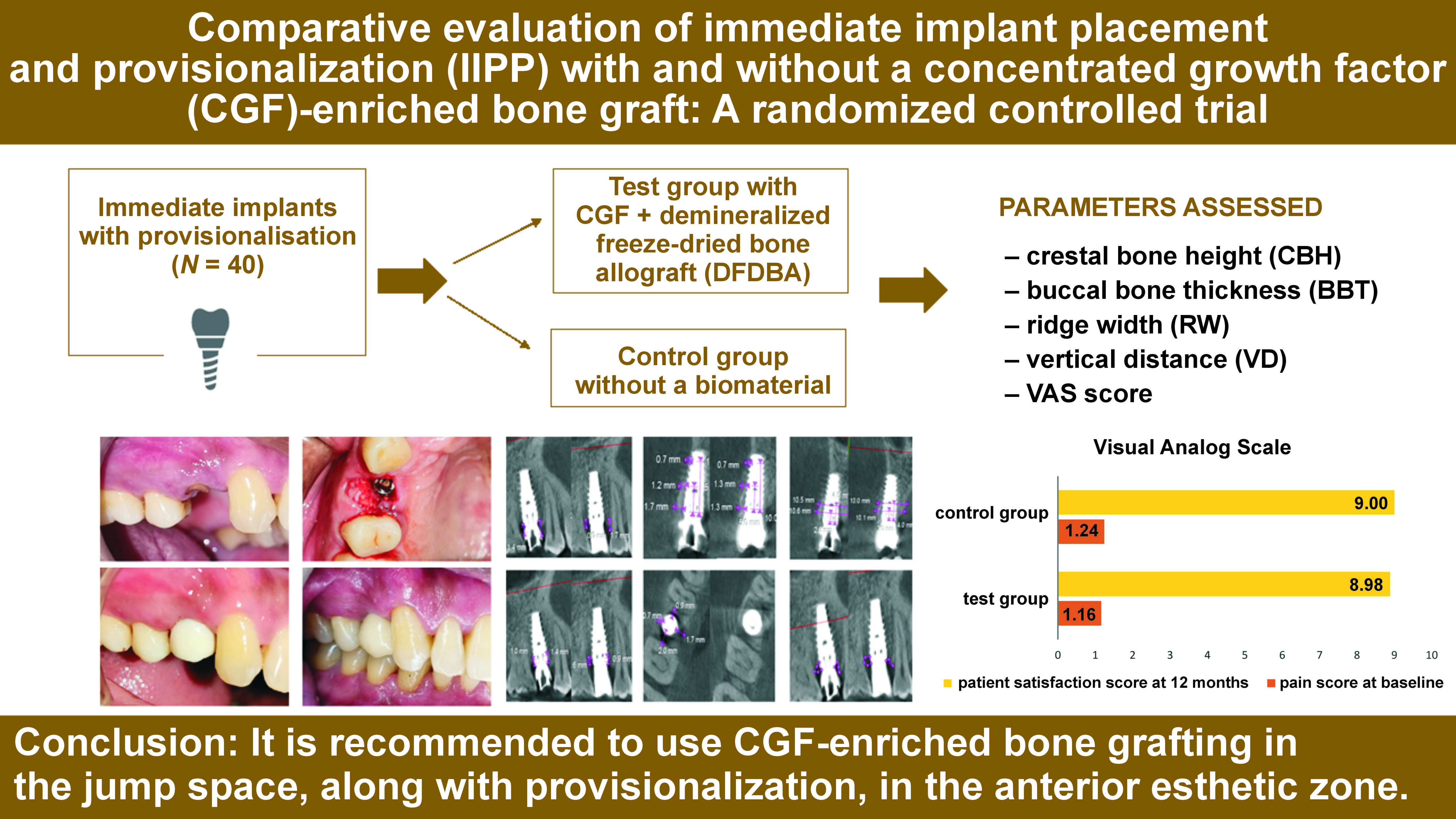
Material and methods. Forty immediate dental implants were placed with and without a CGF-enriched bone graft in the test and control groups, respectively, along with provisional restorations. The clinical evaluation of the modified plaque index (mPI), the modified sulcular bleeding index (mSBI), the probing depth (PD), the gingival thickness (GT), and the Testori implant esthetic score (TS) was done at baseline, and at 6 and 12 months postoperatively. The assessment of the crestal bone height (CBH), the buccal bone thickness (BBT), the ridge width (RW), the vertical distance (VD), JS, and the radiolucent area (RA) was carried out using cone-beam computed tomography (CBCT) at baseline and 12 months postoperatively. The visual analog scale (VAS) was used to assess pain and patient satisfaction.
Results. Highly significant differences were observed with regard to the change in RW at 4 mm from the crest (9.80 ±0.89 mm), VD-distal (1.35 ±0.43 mm), JS-mesial (0.38 ±0.34 mm), JS-distal (0.25 ±0.34 mm), JS-buccal (0.42 ±0.39 mm), RA-mesial (0.63 ±0.48 mm2), and RA-buccal (0.19 ±0.47 mm2) in the test group as compared to the control group at 12 months. The intergroup comparison for TS showed a statistically significant difference (p < 0.05).
Conclusions. It is recommended to use CGF-enriched bone grafting in JS, along with provisionalization, in the anterior esthetic zone.
Keywords: dental implants, allografts, cone-beam computed tomography, randomized controlled trial
Introduction
One of the most effective treatment options for a failing tooth is its replacement with an implant. The long-term goal has changed from implant survival to better maintenance of both the soft and hard tissues. The practice of placing implants immediately after extraction in conjunction with prompt provisionalization is increasingly widespread.1 The timing of implant insertion and provisionalization may have an impact on the peri-implant soft and hard tissues, which can affect the esthetic and patient-centered outcome. Wöhrle’s immediate implant placement with provisionalization (IIPP) contributes eminently to maximizing the esthetic success by retaining the osseous and gingival structure, which is necessary for providing a temporary restoration. When a treatment strategy of flapless extraction and implant placement was combined with bone grafting, connective tissue grafting and the attachment of an immediate provisional crown, the least amount of variation in results was observed.2
Bone morphogenetic proteins (BMPs) are known to have osteoinductive properties, and the demineralized freeze-dried bone allograft (DFDBA) contains BMPs 2, 4 and 7. The DFDBA typically breaks down quickly, allowing the formation of new bone. The biological cascade that the BMPs are a part of involves chemotaxis, matrix attachment, cell proliferation, and cell differentiation into cartilage, bone, and marrow.3 The main benefit of allografts is that, despite lacking vital cells, they may have mechanical properties similar to those of autogenous bone, and may contain the collagenous matrix and proteins seen in natural bone.
The process of osseointegration has been enhanced by a number of methods, including changing the implant’s topography, surface morphology, roughness and energy, strain hardening, and chemical composition, as well as the presence of impurities, the thickness of the titanium oxide layer, and the presence of non-metal and metal composites.4 The regulation of healing following implant placement is another strategy for hastening osseointegration. Herein lies the role of bioactive molecules known as growth factors (GFs). A natural source of GFs – platelets – contain insulin-like GF (IGF), platelet-derived GF (PDGF), transforming GF (TGF) -1 and -2, fibroblast GF (FGF), vascular endothelial GF (VEGF), and other GFs that promote angiogenesis, matrix remodeling and cell proliferation. Concentrated growth factor (CGF), introduced by Sacco in the year 2006, is obtained by centrifuging venous blood, which concentrates the platelets in a gel layer made of a fibrin matrix that is rich in GFs and leukocytes at alternating revolutions per minute, and yields a richer and denser fibrin matrix as compared to other autologous platelet concentrates. Concentrated growth factor releases GFs for at least 13 days and has demonstrated the stimulation of bone repair around implants in vitro.5 A recent study by Guarnieri et al. compared the expression of pro-inflammatory cytokines in peri-implant crevicular fluid at two-piece/bone level vs. one-piece/tissue level single implants after at least 5 years of loading.6 They reported that the two-piece implants presented a more profound pro-inflammatory condition, with higher levels of interleukins and higher crestal bone loss as compared to the one-piece implants.6 A similar long-term implant function study found that the amount of early marginal bone remodeling could not be considered as an indicator of the subsequent onset of periimplantitis, whereas high levels of active matrix metalloproteinase-8 (aMMP-8) 6 months after loading could have a distinct ability to predict the same.7 A recent study has demonstrated the improvement of the quality of life (QoL) of a Parkinson’s patient after implant insertion, with an acceptable 12-month implant survival rate.8
There is a dearth of literature and in vivo research examining alterations in the soft and hard tissues when employing a CGF-enriched bone graft in comparison with the spontaneous healing of the jump space (JS), despite the fact that numerous studies have supported the usage of diverse graft materials in IIPP. Hence, this randomized controlled clinical trial prospectively evaluated the clinical and radiographic effects of IIPP with and without a CGF-enriched bone graft on the soft and hard tissues, using cone beam computed tomography (CBCT).
Material and methods
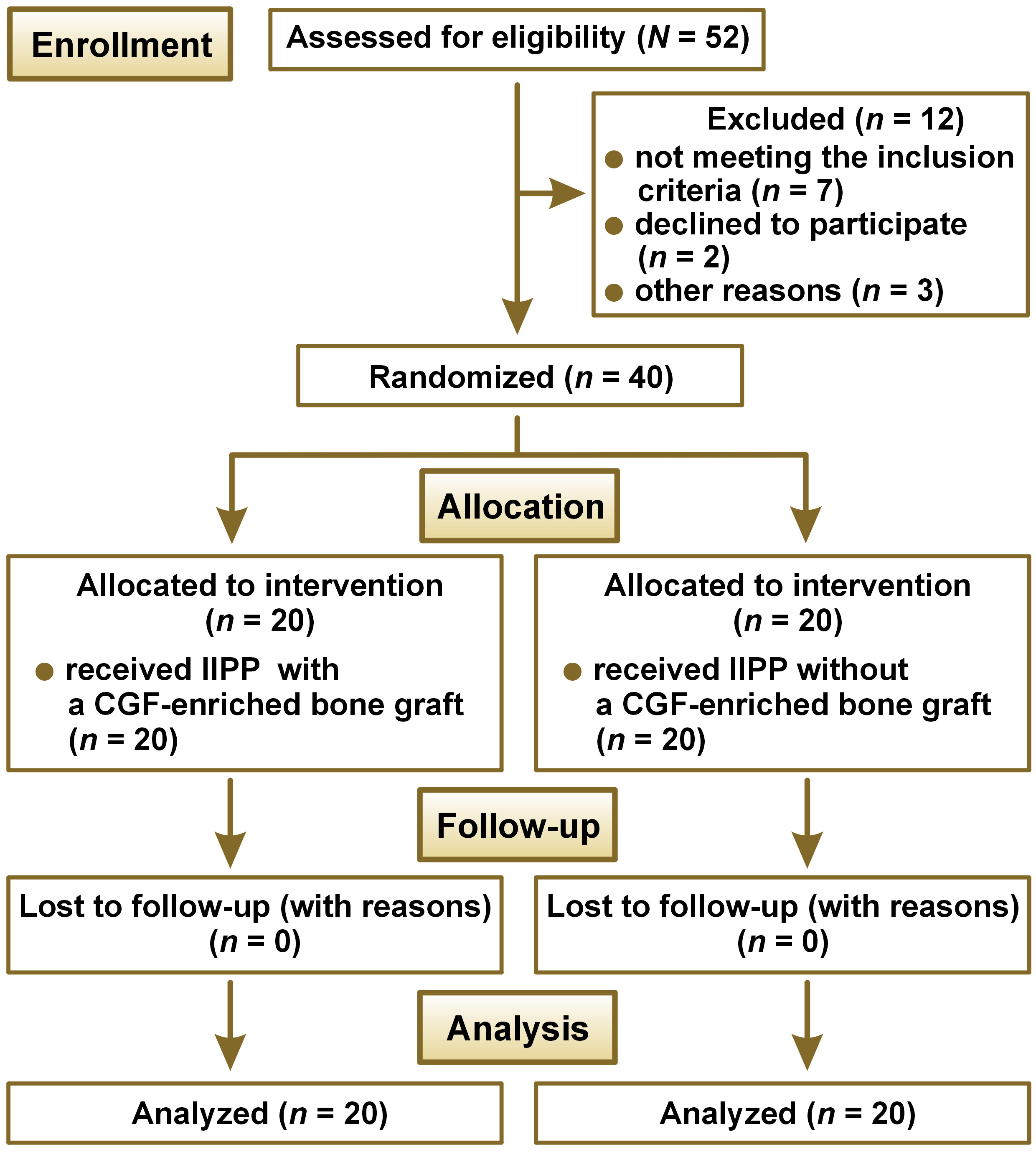
This comparative study was conducted as a double-blind randomized controlled clinical trial in accordance with the World Medical Association (WMA) Declaration of Helsinki and the CONSORT (Consolidated Standards of Reporting Trials) guidelines, after obtaining written informed consent from the participants. The study flowchart is depicted in Figure 1. After receiving approval from the institutional Research Ethics Committee at VSPM Dental College and Research Centre, Nagpur, India (No. of approval: IEC/VSPMDCRC/02/2020), this trial was registered with the Clinical Trial Registry – India under the number CTRI/2021/01/030848.
The study population comprised patients requiring immediate implant placement in the anterior esthetic area and meeting the inclusion criteria: systemically healthy patients; an unrestorable tooth; the presence of the adjacent teeth and the opposing natural tooth; patients with a healthy and stable soft-tissue architecture of the site receiving intervention; intact alveolar bone extraction socket walls; the presence of bone apical to the root apex and palatal to the socket; the sites at which torque ≥35 N·cm was obtained at the time of implant insertion; and JS-buccal of more than 1.5 mm. The reasons for the exclusion of patients from the study were as follows: general contraindications to implant surgery; patients with a history of irradiation in the head and neck area within the last 6 months; patients treated or under treatment with intravenous amino-bisphosphonates; pregnant or lactating women; smokers or patients with poor oral hygiene; parafunctional habits and severe maxillo-mandibular discrepancies; and an active pathology of the adjacent teeth.
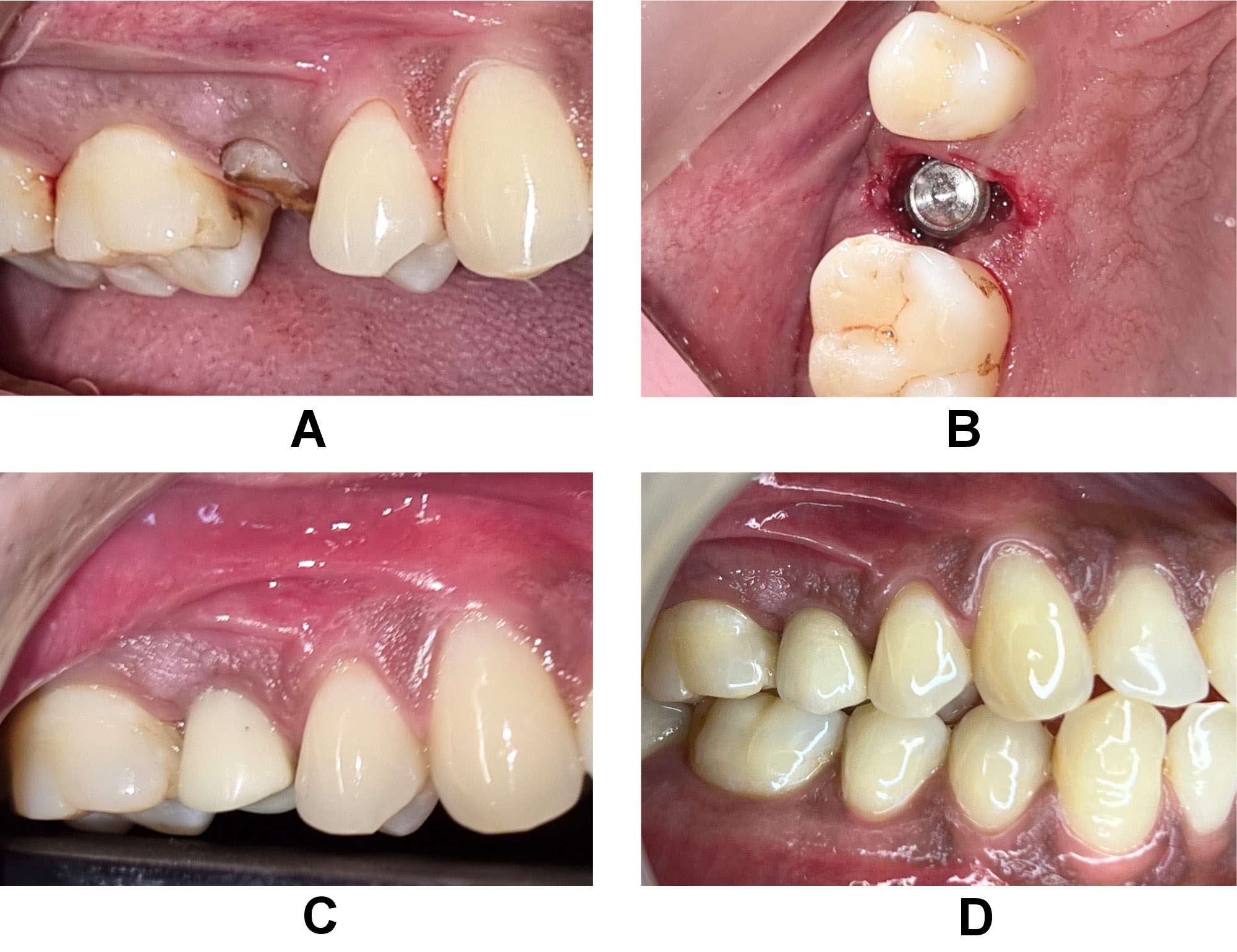
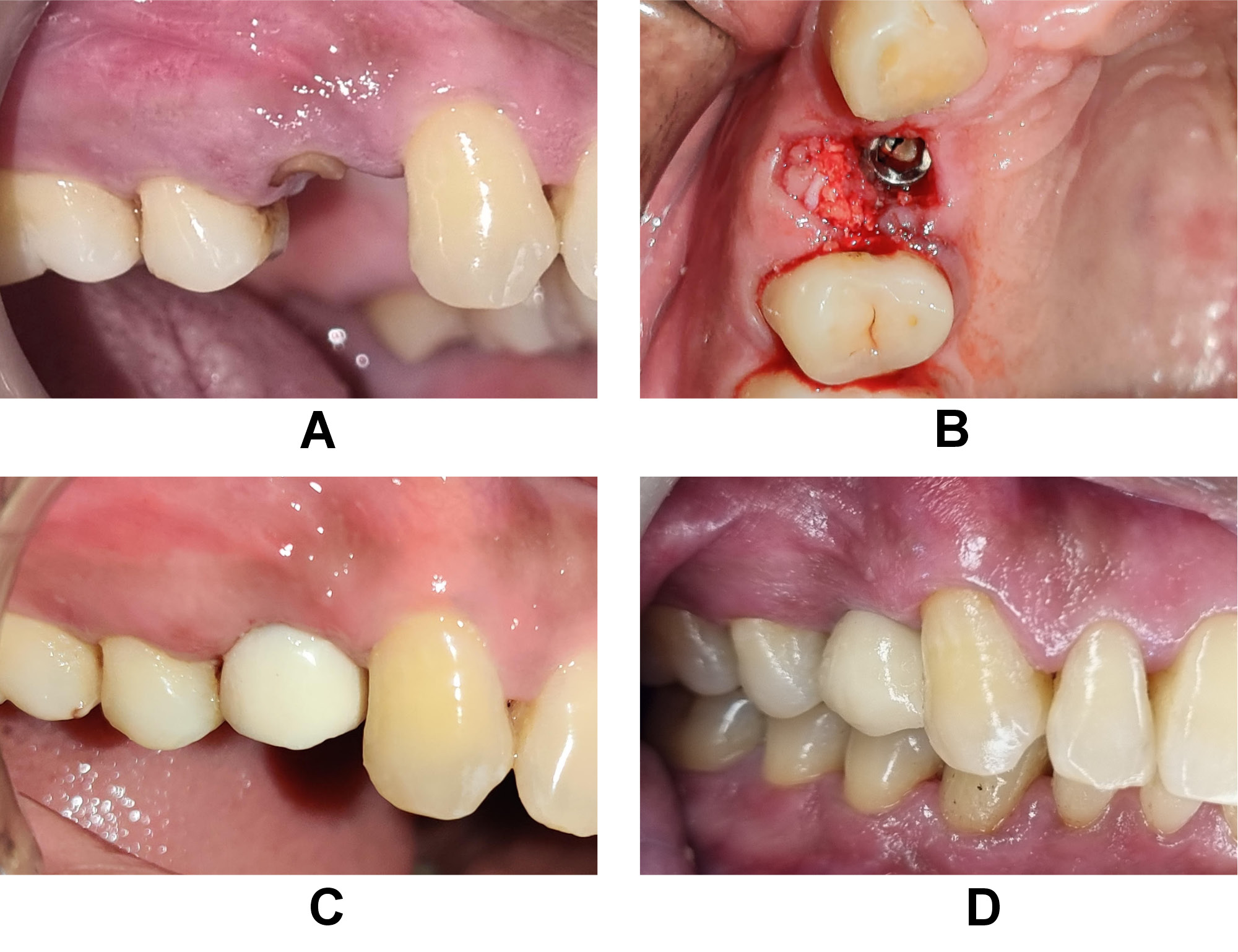
The bone graft used was particulate DFDBA (500–1,040 μm) that was procured from the Tissue Bank of Tata Memorial Hospital and Research Centre, Mumbai, India. The protocol applied for the preparation of CGF9 consisted in using 10 mL of intravenous blood, which was collected in 2 glass-coated plastic tubes with no anticoagulant addition and subjected to centrifugation (R-4C; Remi Lab World, Mumbai, India); it yielded 4 layers from bottom to top: the red blood cell (RBC) layer; the GF and stem cell layer (CGF); the buffy coat layer; and the serum layer. The jump space in the control group was left for spontaneous healing without any grafting material (Figure 2). The CGF layer was separated using sterile surgical scissors, and then mixed with the bone graft material before placement over the target site in the test group (Figure 3).
Referring to the study by Kabi et al.,10 and considering an effect size of 1.0, a sample of 17 sites per group was established to obtain the desired effect with the 95% confidence and 80% power of the test. Further, considering a 15% loss to follow-up, the effective sample size was determined as 40 sites. All sites were randomly allocated with an allocation ratio 1:1 to one of the 2 groups by means of computer-generated random numbers at the time of surgery, using the ‘blockrand’ library from the R programming tool (https://www.r-project.org). The patient and the primary outcome assessor were blinded, as the CBCT images were just given codes, with no reference to patients or groups.
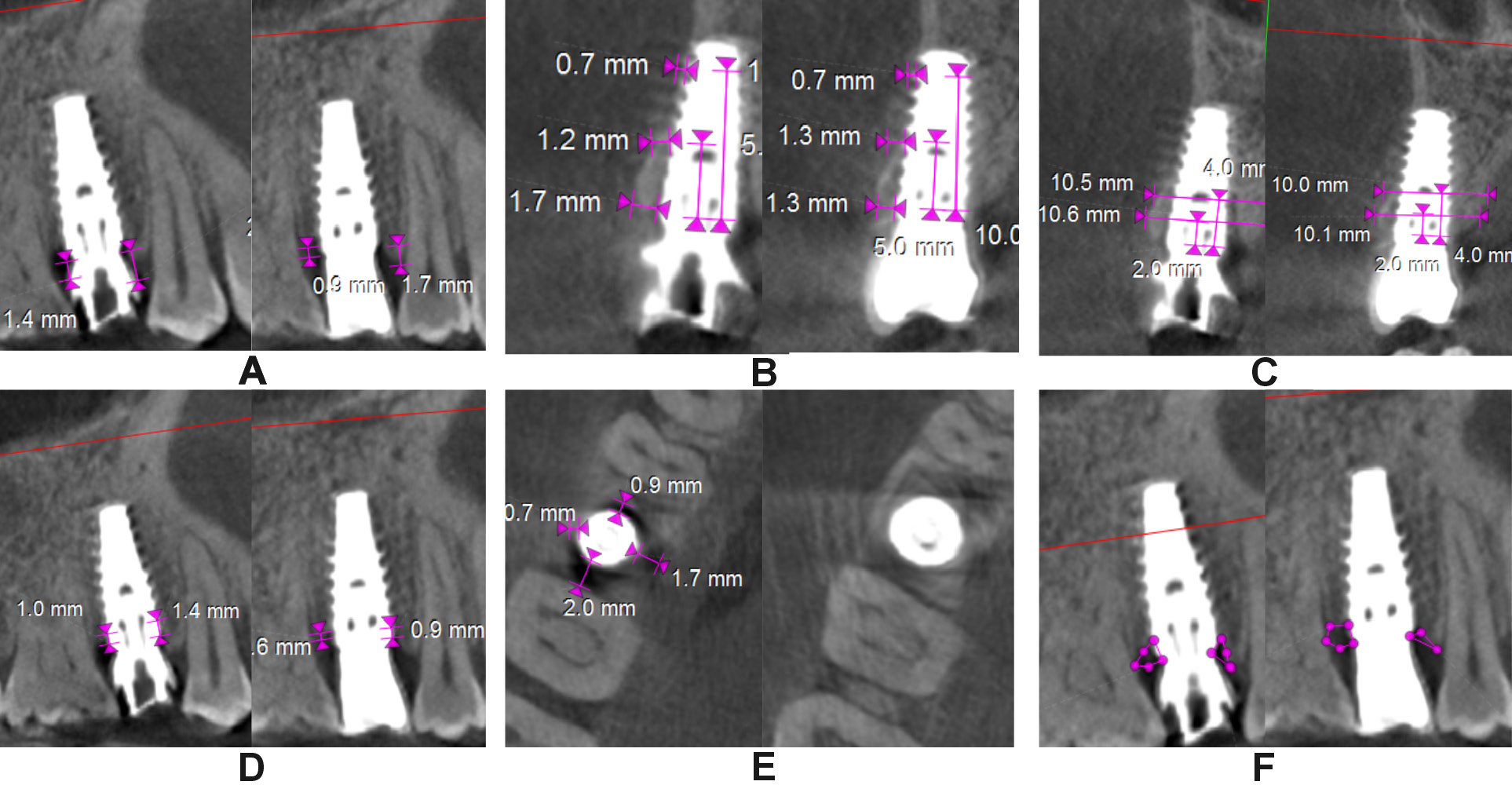
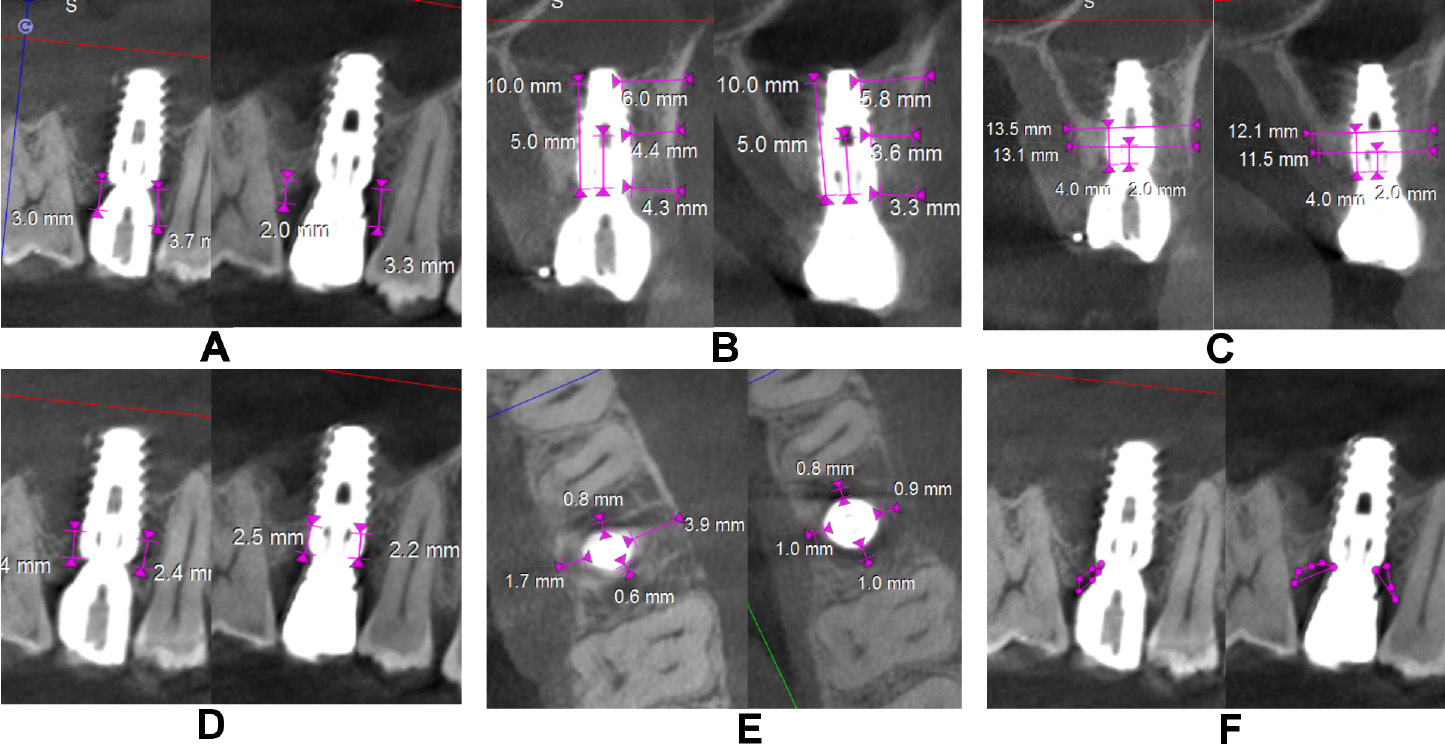
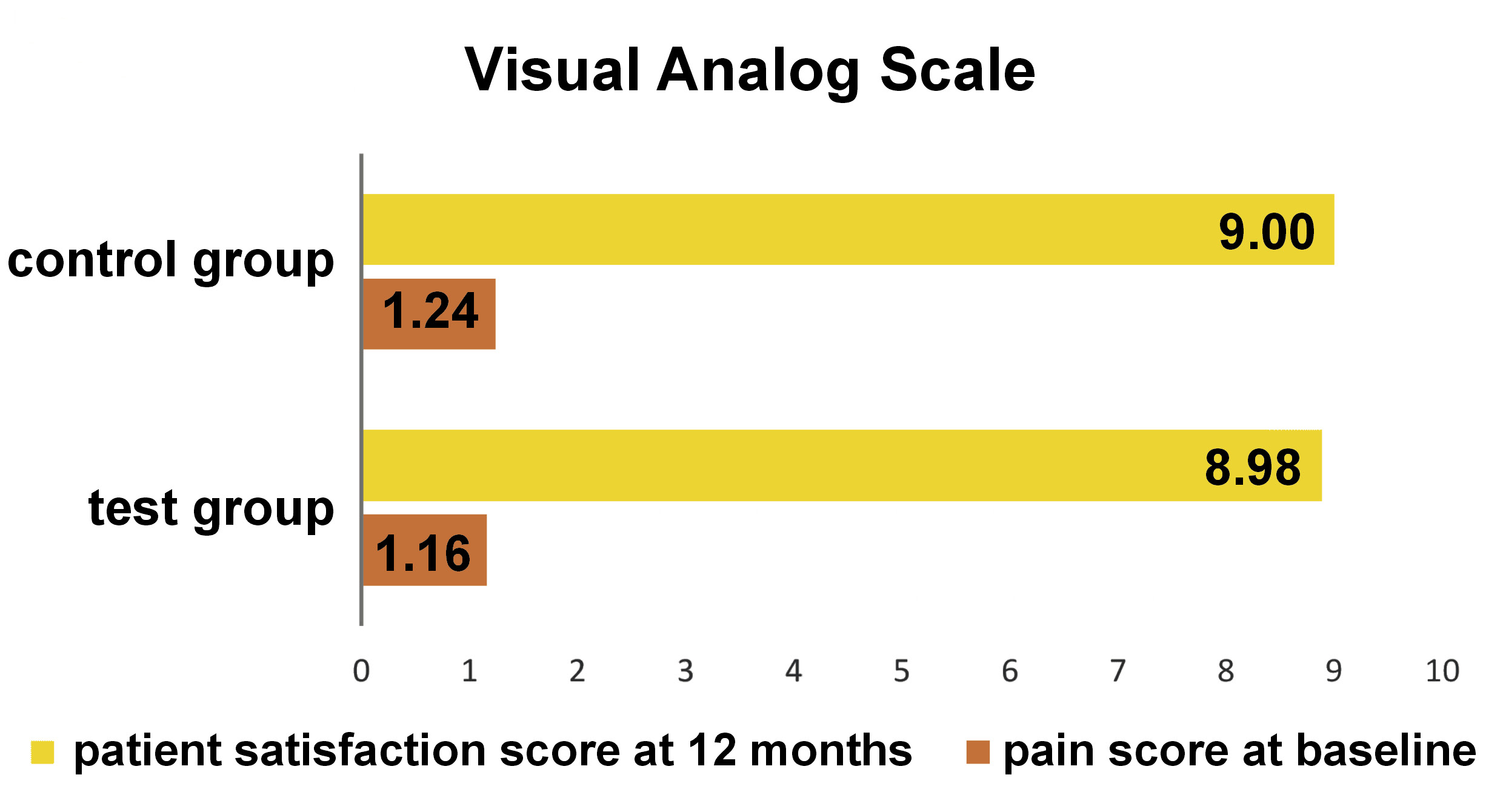
The clinical parameters assessed at baseline, and at 6 and 12 months were the modified plaque index (mPI),11 the modified sulcular bleeding index (mSBI),12 the probing depth/implant sulcus depth (PD),13 the gingival thickness (GT), and the Testori implant esthetic score (TS).14 Using CBCT, the following radiographic parameters were assessed on the day of surgery after implant placement and at 12 months (Figure 4 and Figure 5): the crestal bone height (CBH) – the distance between the implant shoulder to the most coronal point of the interproximal crestal bone height in the sagittal view15; the buccal bone thickness (BBT), measured at the crest, 5 mm from the crest and 10 mm from the crest in the coronal view16; the ridge width (RW) – the buccolingual dimension of the osseous ridge in the coronal view; the vertical distance (VD) – the distance between the first radiographic bone implant contact and the first implant thread on the mesial and distal sides, and the amount of bone loss on the mesial and distal sides in the sagittal view17; JS – the perpendicular distance from the most coronal point of the mesial, distal, buccal, and palatal bone crest to the implant platform in the axial view; and the radiolucent area (RA) – the area between the implant shoulder and the bone crest in the sagittal and coronal views.18 Discomfort/pain was assessed on the day of implant surgery, and patient satisfaction at 12 months postoperatively – both using the visual analog scale (VAS) (Figure 6).
Surgical protocol
After 1 week of phase I therapy, atraumatic extraction was done without the elevation of the mucoperiosteal flap followed by a thorough degranulation of any soft tissue remnants, ensuring the integrity of the buccal bone plate. A presurgical radiograph performed with the use of radiovisiography and a clinical assessment of the intended implant site were used to estimate the necessary implant sizes. Following the manufacturer’s instructions, the implant surgical drills from the Adin implant kit (Adin Dental Implant Systems Ltd., Afula, Israel) were used to drill 2–3 mm apical to the extraction socket. The Adin Touareg™ S implants were placed in. A calibrated-torque hand ratchet was used to secure the implant in place, at least 3 mm apical to the gingival margin and at the level of the alveolar crest. According to randomization, in the control group, IIPP without any biomaterial was conducted, while in the test group, JS was filled with a bone graft material enriched with CGF. For the test group, DFDBA and CGF were mixed together extraorally and the mix was allowed to settle for 2–3 min, which made the graft material slightly adherent to CGF, and then this CGF-enriched bone graft was placed into the space between the implant and the bone socket. The screw-retained provisional crowns were kept out of occlusal and eccentric contacts.
All patients were given appropriate oral hygiene and post-surgery instructions. The participants were administered a capsule of amoxicillin trihydrate (500 mg) 3 times a day for 5 days. A tablet of aceclofenac (100 mg) and a tablet of paracetamol (325 mg) were prescribed to control postsurgical discomfort if needed. The patients were advised to use a chlorhexidine mouthwash (10 mL twice daily) for 15 days, and to abstain from chewing tough or sticky food stuff. The patients were advised to undergo CBCT (Orthophos SL 3D, FOV (field of view): 5×5 cm (85 Kv,7 mA); Dentsply Sirona, Charlotte, USA) within 24 h of implant placement. Such a FOV enables the clinician to obtain scans with high definition and finer details of the structures for precise evaluation. The scan image analysis was performed with the imaging software 3 DIEMME, v. 4.2 (Bioimaging Technologies, Figino Serenza, Italy). After 4–5 months, the interim crowns were replaced with the final prostheses.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, v. 26.0 (IBM Corp., Armonk, USA), and the statistical significance level was set at 5%. The clinical and radiographic parameters at the end of study, i.e., after 12 months, were compared between the groups after adjusting to the baseline values, using the one-way analysis of covariance (ANCOVA). The within group comparisons of these parameters between the baseline and 12 months were performed using the paired t test. The change in the parameters from baseline to 6 months and 12 months, as well as from 6 months to 12 months, was evaluated; the mean change was compared between the groups with the use of the t test for independent samples.
Results
No implants were lost during the course of the study. During the follow-up period, neither the implants nor the bone graft material experienced any biological or mechanical issues. The demographic and implant characteristics of the 2 groups were comparable (Table 1).
The mPI values exhibited a substantial decline, i.e., 1.53 ±0.51, 0.63 ±0.50 and 0.37 ±0.50 in the test group, and 1.38 ±0.50, 0.67 ±0.58 and 0.33 ±0.48 in the control group at baseline, and at 6 and 12 months, respectively. The average PD values were 2.26 ±0.49 mm, 2.63 ±0.37 mm and 2.72 ±0.42 mm in the test group, and 2.26 ±0.50 mm, 2.57 ±0.36 mm and 2.77 ±0.29 mm in the control group at baseline, and at 6 and 12 months, respectively. The GT parameter showed a decrease in the mean values from baseline to 6 months and 12 months, i.e., from 1.94 ±0.24 mm to 1.68 ±0.21 mm and 1.62 ±0.23 mm, and from 1.93 ±0.11 to 1.74 ±0.15 and 1.66 ±0.18 mm in both the test and control groups, respectively. The Testori score showed a statistically significant increase in the test group over time, with a mean score of 7.81 ±0.68 at baseline, and 8.44 ±1.15 at 6 and 12 months (Table 2). The intergroup comparison of the mean values of the clinical parameters at 12 months after adjusting to the respective baseline values did not show any significant differences except for TS, with 8.49 ±0.82 in the test group and 7.96 ±0.76 in the control group (p < 0.05) (Table 3).
In the control group, CBH-mesial (2.33 ±0.62 mm, 1.95 ±0.41 mm), CBH-midfacial (2.49 ±0.73 mm, 1.88 ±0.49 mm), CBH-distal (2.32 ±0.68 mm, 1.87 ±0.46 mm), BBT at the crest (1.46 ±0.53 mm, 1.18 ±0.41 mm), and RW at 4 mm from the crest (8.57 ±1.01 mm, 8.10 ±0.82 mm) showed a highly significant decrease from baseline to 12 months in contrast to the test group. Similarly, VD-mesial and VD-distal showed an increase in the mean value in the control group (p < 0.001). A highly significant decrease from baseline to 12 months in JS-mesial (1.23 ±0.44 mm to 0.36 ±0.32 mm, and 1.37 ±0.35 mm to 0.91 ±0.39 mm), JS-distal (1.45 ±0.55 mm to 0.29 ±0.41 mm, and 1.06 ±0.47 mm to 0.94 ±0.25 mm), JS-buccal (2.34 ±0.58 mm to 0.43 ±0.40 mm, and 2.20 ±0.30 mm to 1.45 ±0.38 mm), and JS-palatal (1.11 ±0.48 mm to 0.42 ±0.34 mm, and 1.20 ±0.63 to 0.82 ±0.42 mm) was noted in both the test and control groups, respectively, except for JS-distal in the control group. A significant increase (p < 0.05) in RA on all sides was observed in the control group and on the buccal side for the test group (Table 4).
The intergroup comparison for the mean change in the radiographic parameters after adjusting to the respective baseline values showed statistically significant differences in all radiographic parameters except for CBH-distal, BBT at 5 mm and 10 mm from the crest, VD-mesial, and RA-distal. Highly significant differences were observed with regard to the change in RW at 4 mm from the crest (9.80 ±0.89 mm), VD-distal (1.35 ±0.43 mm), JS-mesial (0.38 ±0.34 mm), JS-distal (0.25 ±0.34 mm), JS-buccal (0.42 ±0.39 mm), RA-mesial (0.63 ±0.48 mm2), and RA-buccal (0.19 ±0.47 mm2) in the test group as compared to the control group at 12 months (Table 5).
Discussion
The vascularity generated from the periodontal ligament (PDL) is disrupted during flapless tooth extraction, leaving 2 sources of blood supply behind. On the other hand, elevating a flap during surgical extraction damages the periosteum, another vascular source. Consequently, until angiogenesis takes place and the periosteal blood supply is restored, there is only one source of the blood flow left to the buccal bone (endosteal marrow). It is typical for the cortical and cancellous bone to make up the buccal bone for dental implants. Due to a reduced vascular supply, a thin facial bone, which has a higher proportion of the cortical bone than the cancellous bone, may be more prone to resorption. In contrast, a dense facial bone has a superior blood supply and the implant site is less likely to experience bone loss.19 Hence, flapless immediate implant placement is advocated over raising a flap in such cases.
Several studies have shown that the one-stage technique has some clinical advantages when compared to the two-stage method,17 comprising the following: the avoidance of a second surgical procedure; the lack of a micro-gap at the bone crest level, resulting in less crestal bone resorption; the prosthetic procedure is simplified and less chair time per patient is required; and the non-loaded, immediate-loading or delayed-loading protocol can be implemented.
A meta-analysis by Pitman et al. showed 0.87 mm less midfacial apical migration of the midfacial soft tissue when IIPP was done when compared to immediate implant placement alone, with the mean follow-up ranging from 12 to 60 months.20 Recession was not recorded in any of the cases in either the test or control groups, as all the treated sites in both groups received provisional restorations. A horizontal gap distance, i.e., JS of more than 1.5–2.0 mm, most likely requires the placement of a particulate bone substitute material, including an allograft covered with a membrane, for soft tissue in-growth, thus encouraging the osteogenic cells to engage in bone regeneration.3 Hence, the same was tested against the no bone graft group in IIPP in the present study.
In ideal clinical circumstances, such as a fully intact facial bone wall with a thick wall phenotype (>1 mm) and a thick gingival biotype, Buser et al. advises using immediate implant insertion and states that the anterior maxilla hardly ever exhibits a thick wall phenotype.21 The flattening of the orofacial soft-tissue profile and the recession of the facial mucosa are potential hazards. These recommendations are not supported by the findings of the current investigation, as the patients were not chosen for having thick facial bone walls, and thin gingival biotypes were also present. Despite the presence of these risk factors, the mid-term follow-up results were favorable.
The present study showed a statistically significant increase in the intragroup comparisons of PD around the immediate implants over time, from baseline to 12 months, for both groups, which is in accordance with the findings of Buser et al., where a slight increase in PD was seen, from 2.81 mm to 3.14 mm,22 as PD can be influenced by variations in the gingival anatomy, and the distance between the implant margin and the mucosal margin (DIM). The evaluation of TS by Bhutani et al. yielded a score of 7.37 for the test group, which was more than in the control group (6.77), but the difference between the groups was statistically insignificant.14 It is in contrast to the TS obtained in the current study, which may be due to the difference in follow-up timing. In the abovementioned study, the follow-ups were established only with the provisional restoration, at 3 and 6 months,14 whereas the end outcome in the present study is with the final prosthesis, with a longer follow-up. Also, in all cases in the current investigation, screw-retained provisional restorations were strictly used, while in a study by Bhutani et al., the provisional restorations were either screw- or cement-retained14; it could alter the results as well.
Clinical studies have also shown that there is a significant amount of spontaneous filling of JS at the immediate implant site. Despite these findings, a recent recommendation is that marginal gaps should be filled with a bone replacement graft in order to get superior esthetic results. The clinical impact of such grafting, however, is a matter of debate, and few studies have been conducted to evaluate the spontaneous filling of JS as compared to the use of bone grafting. The present study is the first to use a CGF-enriched bone graft and evaluate it against a spontaneous gap fill in immediately placed implants in the anterior maxilla with provisionalization.
Kabi et al. reported that the mean alveolar bone loss was significantly greater in the sites left unfilled when compared to the sites filled with an autogenous graft at 6 and 9 months post implant placement,10 which supports the results obtained in our study, where CBH showed a significant decrease from baseline to 12 months in the control group and the intergroup comparison also showed a statistically significant difference. The buccal bone thickness affects the degree of buccal plate resorption after immediate implant placement. In this study, the value of BBT at the crest in the control group showed a mean decrease from 1.46 ±0.53 mm to 1.18 ±0.41 mm, which is similar to the findings of Seyssens et al. – 0.59 mm (95% CI: 0.41–0.78; p < 0.001) or 54% less horizontal buccal bone resorption following immediate implant placement with socket grafting (IIP + SG) as compared to immediate implant placement alone.23 In the same study, a trend toward less distal papillary recession was found (MD (mean difference) = 0.60 mm; 95% CI: −0.08–1.28; p = 0.080) when SG was performed, while mesial papillae appeared not significantly affected by SG.23 This could be relevant with regard to the current study, where the change in the mean CBH-midfacial and CBH-distal was smaller in the test group than the control group, with a statistically significant difference between the groups; however, the change in the mean CBH-mesial over time remains statistically insignificant between the groups.
Patient-reported outcome measures are generally reliable, yet there are limited studies on that matter. The VAS was used to report patient discomfort/pain, as well as satisfaction, showing comparable scores for both groups. The emergence profile of the provisional restoration mechanically supports the soft tissue, preventing its collapse after tooth extraction. Clinical and histological studies that support the outcomes of our research show that an esthetic contour can be maintained both vertically and horizontally when the implant–socket gap is filled with a bone grafting material.24, 25, 26
In the present study, immediate provisionalisation was done in both groups. It provides benefits such as short treatment time, the elimination of a second surgery, which is required in the delayed-loading protocol, the protection of the gingival papillae, an immediate esthetic effect, and high patient satisfaction.27
A similar study was performed by Amam et al.28 The authors compared radiologically the amount of bone gain and bone reduction by using tricalcium phosphate and calcium sulfate grafts mixed with advanced platelet-rich fibrin (A-PRF) in 18 maxillary sinus augmentation cases. They found no statistically significant differences between the 2 groups at a 6-month follow-up. However, a sufficient amount of bone was obtained when A-PRF was added to the 2 different bone grafts.28
It has been hypothesized that placing an implant right away after extraction and adding a graft material offers a scaffold on which blood clots can organize, aiding in maintaining the tissue volume. After a tooth is extracted, the soft tissue is mechanically supported by a temporary restoration and its emergence profile, which prevents collapsing. The evaluation period of the current study is, however, brief, and long-term follow-up results are required. The findings indicate a limited presence of intact facial bone walls around the premolars in the anterior maxilla. Volumetric tissue changes that occur during the healing of the treated site could be measured on study casts for more accurate values, and parameters like the bone density and the implant stability quotient (ISQ) should also be taken into account in future research.
Conclusions
The effects of providing a provisional restoration with or without bone grafting were the primary focus of this research. It is evident that this clinical approach is essential to reduce the degree of facial contour alterations that may result from immediate implant placement. Additionally, it plays a crucial role in shaping both clinicians’ and patients’ perception of esthetic outcomes.
The results of the present clinical trial indicate that crestal bone resorption occurred in both groups. However, a significantly reduced resorption was observed in the test group, which is attributed to the augmentation of JS. This was reflected and confirmed by a marked reduction in JS and RA in the test group, along with a significantly lesser reduction in BBT, RW and VD as compared to the control group.
Immediate implant placement with provisionalization in the maxillary esthetic zone – both with and without the CGF-enriched DFDBA – demonstrated high patient satisfaction, particularly concerning the level of pain experienced during the surgical procedure.
It is recommended to graft JS after IIPP in the anterior esthetic zone with a CGF-enriched bone graft. However, an extended evaluation period is needed to determine whether such an approach offers long-term benefits.
Trial registration
The trial was registered with the Clinical Trial Registry – India under the number CTRI/2021/01/030848.
Ethics approval and consent to participate
The research was approved by the institutional Research Ethics Committee at VSPM Dental College and Research Centre, Nagpur, India (No. of approval: IEC/VSPMDCRC/02/2020). Written informed consent was obtained from all study participants.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.