Abstract
Background. The composition of saliva serves as an indicator of the state of the oral cavity.
Objectives. The aim of the study was to examine the salivary levels of leptin, fibronectin (FN), matrix metalloproteinase (MMP)-1, and MMP-2 during orthodontic treatment.
Material and methods. A total of 50 patients scheduled for orthodontic treatment and 50 healthy volunteers were included in the study. The orthodontic patients underwent the placement of standard brackets (MINI MASTER® fixed braces; American Orthodontics) and self-ligating brackets (H4, 0.022” × 0.028”; Orthoclassic). Non-stimulated saliva samples were collected before orthodontic treatment, 24 min after the procedure, and 1 month after the procedure. The levels of MMP-1, MMP-2, FN, and leptin in the saliva of patients were measured before and after orthodontic treatment.
Results. The levels of salivary leptin were significantly elevated before orthodontic treatment (p < 0.001), and a subsequent rise was also observed during orthodontic tooth movement. Salivary FN levels were significantly increased before orthodontic treatment (p < 0.001), followed by a decrease during orthodontic tooth movement, remaining outside the normal range. The concentration of MMP-1 in saliva was significantly higher before orthodontic treatment (p < 0.001) and decreased during orthodontic tooth movement, though this change was not statistically significant. Similarly, the concentration of MMP-2 in saliva was significantly higher before orthodontic treatment, but its levels were even higher during orthodontic tooth movement. However, this increase was not statistically significant.
Conclusions. The levels of leptin in saliva were elevated during orthodontic treatment. Leptin stimulates wound healing and angiogenesis in the oral cavity. In addition, it serves as a mediator in the orthodontic movement of teeth.
Keywords: leptin, orthodontic treatment, MMP-1, fibronectin, MMP-2
Introduction
The oral cavity is a site of continuous reactions between food, gland secretions and bacteria. Saliva is the most important fluid in this environment. Its main constituents are water (99.5%), as well as organic (0.3%) and non-organic factors (0.2%).1 The composition of saliva reflects the state of the oral cavity and the entire organism.2, 3, 4, 5, 6 Orthodontic treatment alters the oral environment, stimulates the flow rate of saliva, its buffer capacity, and pH level.
During orthodontic treatment, continuous pressure is applied on the tooth, leading to alveolar bone remodeling. The tooth undergoes movement within the interior of the bone, thereby causing the attached periodontal system and alveolar processes to be dragged along. The most widely accepted theory for orthodontic tooth movement is the pressure-tension theory. It is characterized by blood flow changes caused by constant pressure, which in turn serve as indicators of tooth movement. Firstly, it creates periodontal ligament (PDL) pressure, and secondly, it induces PDL extension. On the pressure side, the blood flow ceases and it is possible to notice chemical changes in gingiva liquid. Regarding the tension aspect, an increase in blood flow and oxygen level is observed. The process of bone remodeling is initiated by chemical changes that prompt the release and differentiation of specific bone remodeling cells. The application of force initiates the previously described mechanisms within few minutes.
Tooth movements can be categorized into 3 phases, as follows: alterations in blood flow and PDL tension; generation or release of chemical mediators; and cell activation, which is responsible for bone remodeling and is a result of tooth movement. The movement of teeth triggers an acute inflammatory reaction in periodontal tissues and stimulates cells of PDL to produce enzymes and cytokines necessary in the remodeling of connective tissue.1 The remodeling of connective tissue is achieved by a complex process, in which matrix metalloproteinases (MMPs) play a major role.
Matrix metalloproteinases are enzymes that degrade the extracellular matrix (ECM) and components of the basement membrane. They are synthetized by connective tissue cells, including osteoblasts, odontoblasts and fibroblasts.7, 8, 9, 10, 11, 12 Matrix metalloproteinases circulate in gingival crevicular fluid (GCF) and saliva, and they can be found in the dentin of carious teeth and plaque.9, 10, 11, 12
Several types of MMPs were identified in saliva. The levels of MMP-1 and MMP-2 were measured. Matrix metalloproteinase-1 is responsible for the breakdown of the ECM, including collagen types I, II and III, while MMP-2 is a collagenase that destroys collagen type IV and regulates inflammatory and vascular processes.9, 10, 11, 12, 13, 14
In vivo, mechanical forces trigger the production of MMP-1 in the cells of the periodontal ligament.11 Matrix metalloproteinase-1 is able to break the molecules of interstitial collagen, initiating cell remodeling.11 Moreover, animal studies have demonstrated that the application of inhibitors of MMPs can delay the orthodontic tooth movement.15
The activity of MMPs is balanced by the catalysis of their proenzymes and the production of tissue inhibitors of metalloproteinases (TIMPs).13, 14, 15 These inhibitors are produced by B-cells and are found in the composition of GCF.13, 14, 15 The α2-macroglobulin, a non-specific inhibitor of MMP, is also found in GCF.9, 10, 11, 12, 13 The balance between MMPs and TIMPs determines the extent of tissue destruction.13, 14, 15
Matrix metalloproteinases influence the resolution of acute inflammation. In the absence of MMPs, a progression to chronic inflammation occurs, resulting in tissue damage. One of the roles of MMP-2 in inflammatory processes is massive leukocyte infiltration and heightened levels of pro-inflammatory cytokines observed in MMP-2-devoid animals.7, 8, 9, 10
Additionally, polypeptides, such as leptin and fibronectin (FN), take part in the remodeling of connective tissue that occurs in response to tooth movement.
Leptin is a polipeptide produced by adipocytes; however, it is also present in osteoblasts and muscles, the stomach, the brain, the salivary glands, and the placenta.16, 17, 18, 19, 20 The polypeptide regulates energetic balance through its influence on the hypothalamus. When the leptin level is low, there is an increase in glucocorticoid production, which in turn stimulates agouti-related protein (AgRP) neurons and boosts appetite. Leptin has an influence on metabolism and weight, thermogenesis, hematopoiesis and angiogenesis, mineralization of bones, and wound healing.16 Leptin was identified in both GCF and tooth pulp.21, 22, 23 Furthermore, an interrelation was observed between plasma or salivary leptin concentration and body mass index (BMI).16 Obese individuals exhibit an elevated concentration of leptin in the serum, which has been demonstrated to influence bone remodeling.24, 25 According to the latest surveys, leptin concentration in GCF decreases during orthodontic tooth movement.26, 27
Fibronectin is a glycoprotein that exists in 2 forms, a soluble form found in body fluids and an insoluble form found in cells. In cells, FN moderates cell–matrix attachments and facilitates cell migration and the differentiation of organs. Fibronectin is produced by osteoblasts and odontoblasts. It is involved in the interaction between the implant and the bio-matrix.28, 29, 30
The aim of the present study was to examine the levels of MMP-1, MMP-2, FN, and leptin in the saliva of patients before and after orthodontic treatment.
Material and methods
Patients
A total of 50 patients scheduled for orthodontic treatment to improve their dentofacial appearance and 50 healthy volunteers were included in the study. The orthodontic patients underwent the placement of standard brackets (MINI MASTER® fixed braces; American Orthodontics, Sheboygan, USA) and self-ligating brackets (H4, 0.022” × 0.028’’; Orthoclassic, McMinnville, USA). The nickel titanium wires were used. There were no statistical discrepancies regarding the crowding levels, which were classified as mild to moderate. No extractions were scheduled before orthodontic treatment. The estimated duration of treatment was 2.5–3 years. More precisely, approx. 20 visits were planned for standard brackets, while 30 visits were planned for self-ligating brackets. The inclusion criteria were good oral hygiene and good health. Subjects taking long-term medications and smokers were excluded from the study. The eligibility criteria were assessed during interviews with the subjects. Written informed consent was obtained from all participants. The study received the approval of the Ethics Committee of Medical University of Bialystok, Poland (approval No. R-I-002/41/2019).
The study group (31 females and 19 males) had a mean age of 31.4 ±1.9 years, while the control group (36 females and 14 males) had a mean age of 21.3 ±1.8 years. In the latter, dental examinations were performed before the collection of saliva.
Methods
Patients referred for orthodontic treatment were scheduled for scaling and sandblasting of teeth (removal of plaque and tartar above and below the gumline) 1 day before treatment. The subjects were instructed to abstain from eating and were allowed only to drink water for 2 h before the extraction of saliva. Patients were seated and whole saliva samples were collected in plastic tubes by the passive spitting method in order to obtain approx. 1–2 mL of sample. Non-stimulated saliva samples were collected prior to orthodontic treatment, 24 min after the procedure, and 1 month after the procedure. The collection periods were selected on the premise that periodontium metabolism and factor secretion occur immediately after orthodontic force application.
The samples were centrifuged at 10,000 g for 5 min, and the supernatants were frozen and stored at −80°C. One researcher performed interviews, examinations of the oral cavity and saliva collection. All methods were performed in accordance with the relevant guidelines and regulations.
For the measurement of MMP-1, MMP-2 and FN levels, a highly selective surface plasmon resonance imaging (SPRI) biosensor was used, as previously described in other studies.7, 8, 31 The SPRI biosensor measures the changes of the refractive index caused by molecules bound to the metal surface. Surface plasmon resonance (SPR) is generated at a thin metal surface when an analyte binds the ligand on the metal film, thereby causing a change in the interfacial architecture. The SPRI signal is dependent on the change in the wavelength and the angle of light polarization.
The main component of the biosensor for MMP-1 detection is an immobilized rabbit anti-human MMP-1 antibody, which binds the enzyme present in the sample. The analytical response signal of the biosensor is in the range of 0.05–20.00 ng/mL−1. The detection limit is 9 ng/mL−1, and the limit of quantification is 18 ng/mL−1.7, 8 For MMP-2 measurements, MMP-2-specific inhibitor ARP 101 was used as the receptor to bind the enzyme from the sample.31 The biosensor for FN used the specific reaction of rabbit anti-FN antibody. To evaluate the SPRI method, the levels of MMP-1, MMP-2 and FN were determined in the biological samples using enzyme-linked immunosorbent assay (ELISA). There was a strong correlation between the 2 methods. For example, for MMP-2, the correlation coefficient for healthy donors was 0.996, and for patients, it was 0.984.7, 8, 31
The quantitative measurement of leptin in saliva was performed using a leptin enzyme immunoassay or an ELISA kit.2
Statistical analysis
The normality of distribution was tested using the Shapiro–Wilk test. Non-Gaussian data was presented as a median (minimum–maximum) and analyzed using the non-parametric Kruskal–Wallis test. The differences were deemed statistically significant when p < 0.05. The calculations were performed using the GraphPad 7.04 Prism software (GraphPad Software, Boston, USA).
Results
Leptin
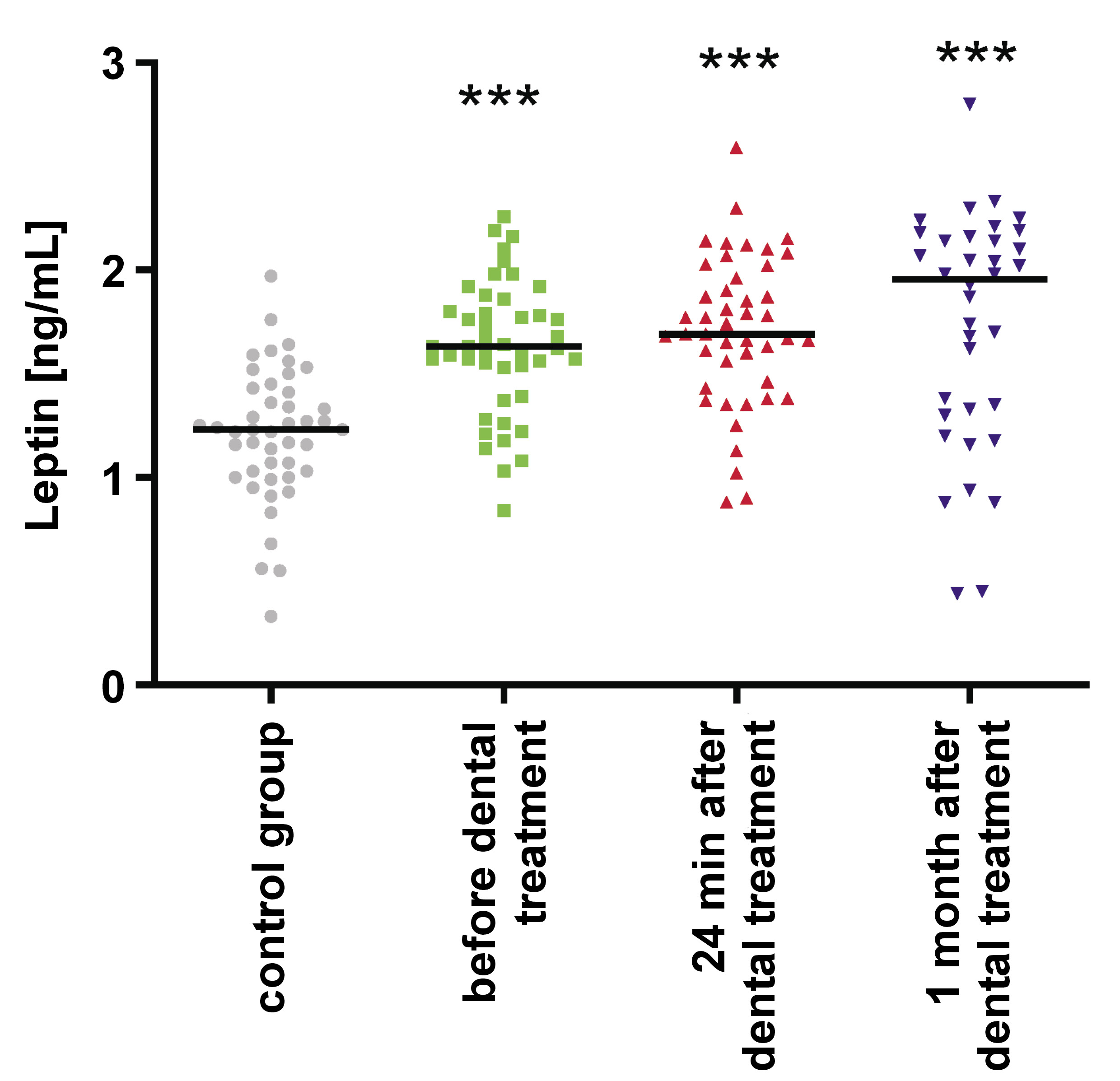
In the study group, salivary leptin levels were significantly higher before orthodontic treatment in comparison to controls (p < 0.001). Additionally, a notable increase in salivary leptin levels was noted during orthodontic tooth movement (Table 1, Figure 1). There was no correlation between salivary leptin levels and BMI between the study group and the control group.
Fibronectin
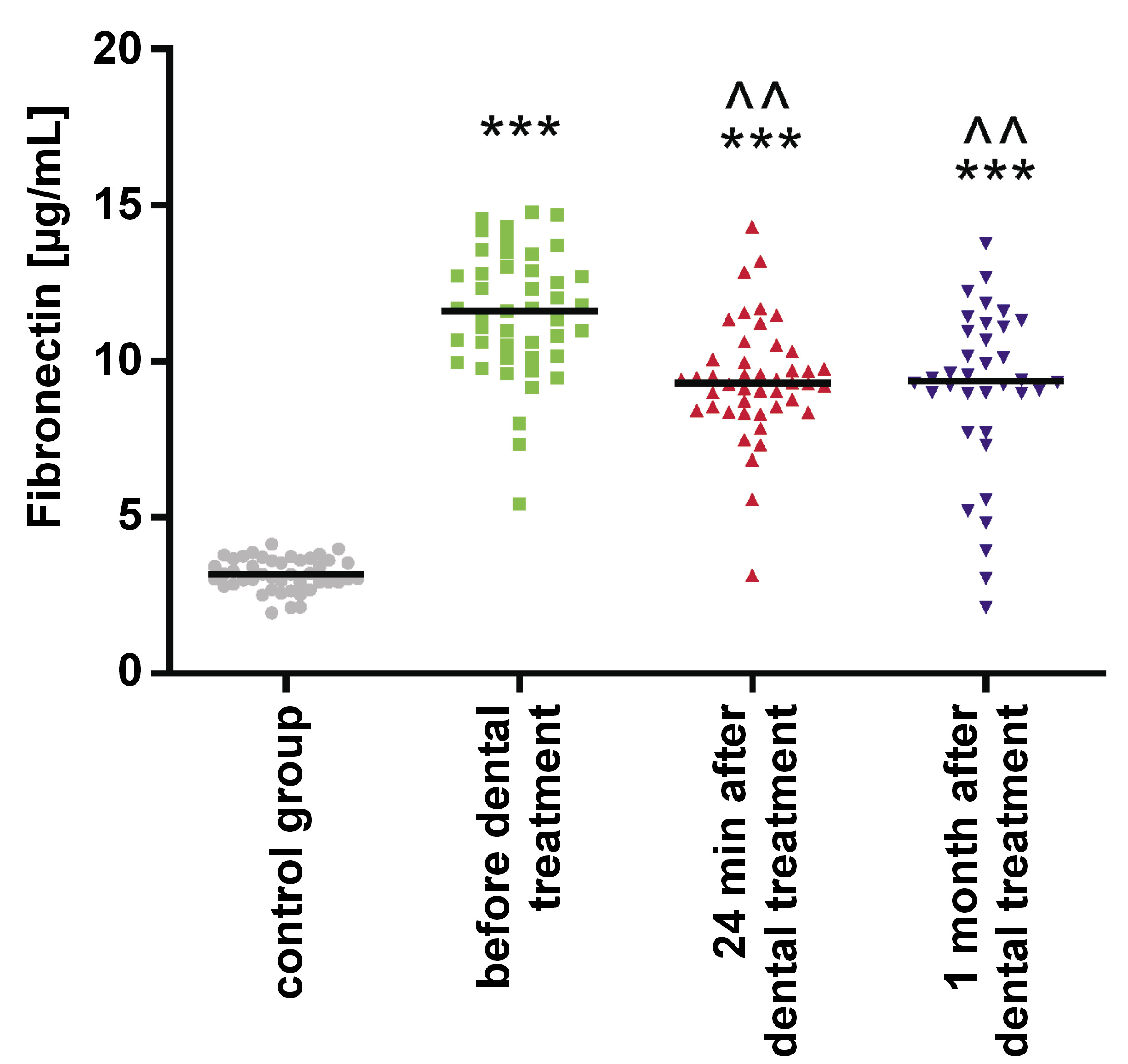
Salivary FN levels were significantly elevated in the study group before orthodontic treatment when compared to the control group (p < 0.001). They decreased during orthodontic tooth movement, without reaching the normal range (Table 2, Figure 2).
MMP-1
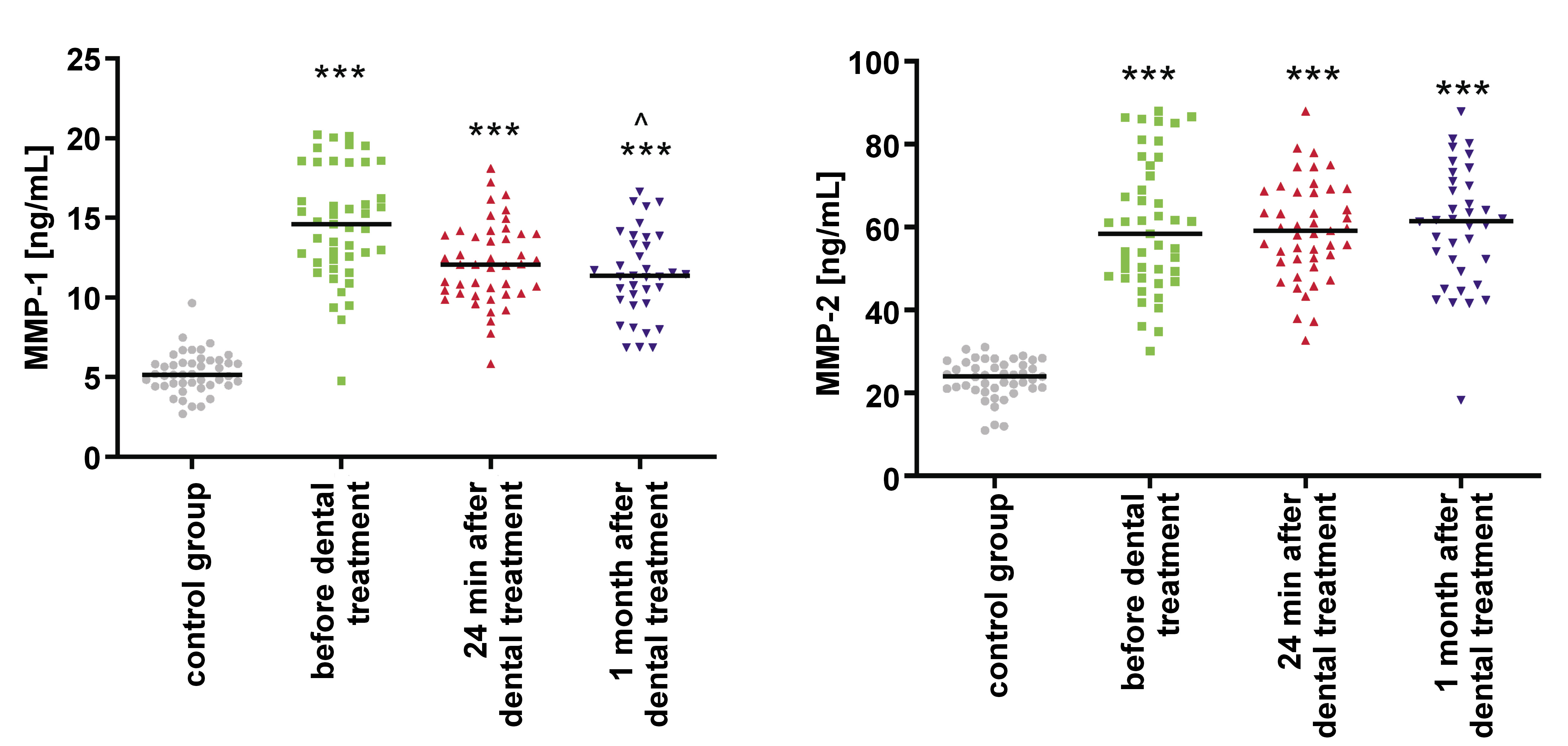
The concentration of MMP-1 in saliva was significantly higher in the study group before orthodontic treatment when compared to controls (p < 0.001). A decrease in the concentration was observed during orthodontic tooth movement; however, it was not statistically significant (Table 3, Figure 3).
MMP-2
In the study group, the concentration of MMP-2 in saliva was significantly higher before orthodontic treatment as compared to the control group (p < 0.001). Its levels further increased during orthodontic tooth movement, but the rise was not statistically significant (Table 3, Figure 3).
Discussion
The periodontal ligament is a membrane-like connective tissue that surrounds the root of a tooth. It is located between the hard tissues of the alveolar bone and the cementum of teeth, thereby anchoring the tooth to the alveolus.9, 10, 11, 12 During orthodontic treatment, forces compress the PDL fibres, leading to a reduction in the PDL space, which triggers local damage, repair and inflammation processes involving vascular activity, and the involvement of leukocytes and macrophages.9, 10, 11, 12
In the present study, a significant rise in the levels of leptin, FN, MMP-1, and MMP-2 was observed during orthodontic tooth movement. Cantarella et al. observed that the forces exerted during orthodontic treatment stimulate the production of MMP-1 and MMP-2.13 During the first hour of treatment, the levels of MMP-1 rise, returning to its normal range within the next 3 h. With regard to MMP-2, its concentration remains higher for 8 h in the compression areas; however, in the tension area during the same period, it returns to the normal range.13 Redlich et al. demonstrated higher MMP-1 activity in the compression side in the animal model.14
After the application of orthodontic forces, an increase in the concentration of pro-inflammatory cytokines is observed within the first 24 h.1 Subsequently, a new biological equilibrium is established.1, 9, 10 Similar observations were made in the current study. A paucity of human studies has focused on MMPs during orthodontic stress.9, 10, 11, 12, 13 Garlet et al. found higher MMP-1 mRNA at the resorption and apposition sides compared with the controls.32 This corresponds with the findings of the present study. As Takahashi et al. have stated: „At the resorption side, bone resorption takes place and extensive re-modelling of the PDL. At the apposition side, only bone apposition takes place and more limited PDL re-modelling”.33 In animal models, the MMP inhibitors delay orthodontic tooth movement.15 Most probably, MMP-2 controls inflammatory signaling, influencing the resolution of acute inflammation. In the absence of MMPs, a progression to chronic inflammation occurs, resulting in tissue damage.
In the context of periodontal health, elevated levels of leptin have been observed in GCF. This observation suggests a potential role for leptin as a protective factor, potentially stimulating the immune system, impacting the osteoblasts and enhancing bone formation.17, 18, 19 Although other researchers have noted a decrease in leptin concentration after orthodontic force application on teeth, our observations indicate an increase in salivary leptin levels along with tooth movement. Dilsiz et al., who evaluated the levels of leptin in GCF before and after the application of orthodontic force, also noted a decrease in GCF leptin levels, which contradicts the results of this study.27
In overweight and obese subjects, an elevated leptin concentration has been observed in the serum, which exerts an influence on bone metabolism.34 Jayachandran et al. observed that the duration of orthodontic treatment was greater in obese individuals.16 These subjects exhibited higher leptin levels in the serum and high mineral density of bones.16 In the present study, no statistically significant correlation was identified between BMI and leptin levels in the control group and in the study group before orthodontic intervention.
Another pattern of leptin levels was observed by Srinivasan et al.35 The researchers identified a biphasic change in leptin concentrations, with an apex 24 h after the application of orthodontic force, and the lowest level after 1 week.35
Most probably, leptin modulates processes of inflammation.36, 37, 38, 39, 40 Bozkurt et al. observed lower leptin levels in GCF in smokers.41 They showed that smoking damages the mechanism regulating leptin levels.41
Collagen and FN play a crucial role in tissue remodeling after orthodontic tooth movement.28, 29, 30 Fibronectin is involved in the migration and attachment of the junctional epithelium and wound healing. It also promotes the growth of periodontal ligament cells and protects gingival tissues.28, 29, 30 According to Huynh et al., “particles of FN found in GCF are the result of cleavage of FN by proteases such as MMPs during inflammation, wound healing and infections”, so fragments of FN “may be used as an indicator of oral cavity status”.42 Kapila et al. proved that “FN and specific FN fragments induce the expression of proteinases in periodontal ligament cells, causing tissue degradation during periodontal disease”.43 Higher levels of FN were detected during acute infections and chronic diseases, e.g., liver cirrhosis or hepatic carcinoma.28, 29, 30 Fibronectin, a non-specific salivary defense factor, has been shown to bind to bacteria and play a role in the formation of plaque.28, 29, 30 Salivary levels of FN in subjects with oral lichen planus are lower than in the general population.44 In the present study, we observed significantly higher salivary levels of FN before orthodontic treatment, likely attributable to an additional intervention performed 1 day earlier (scaling and sandblasting of teeth).7, 8 Levels of FN decreased during orthodontic tooth movement, both 24 min and 1 month after the application of braces.
The oral mucosa is particularly susceptible to mechanical injuries, e.g, during chewing and biting. In certain cases, ulceration may occur. Umeki et al. studied the role of leptin in the healing of the oral mucosa.45 The healing of the ulcer was expedited in the group that received leptin in comparison to the control group.45 Leptin stimulates angiogenesis and epithelial cells.46, 47, 48, 49 We speculate that the elevated salivary levels of leptin, FN, MMP-1, and MMP-2 in our patients before orthodontic treatment were caused by an additional intervention performed 1 day before, which involved scaling and sandblasting of teeth.
A notable limitation of this study is the fact that the force levels released by nickel titanium wires vary from subject to subject, even if the wires are from the same manufacturer and have equal diameters. Furthermore, the levels of orthodontic forces exerted by nickel titanium wires do not remain stable in the oral environment.50 However, we believe that these factors do not diminish the value of our study.
Conclusions
Our study revealed an increase in the levels of leptin in saliva during orthodontic treatment. Leptin stimulates wound healing and angiogenesis in the oral cavity. It also serves as a mediator of orthodontic movement of teeth. Additionally, we observed significantly higher salivary levels of leptin, FN, MMP-1, and MMP-2 before orthodontic treatment, likely attributable to scaling and sandblasting of teeth performed before orthodontic treatment. The concentration of FN and MMP-1 decreased during orthodontic tooth movement, 24 min and 1 month after the application of braces. In contrast, MMP-2 levels increased during orthodontic tooth movement; however, these changes were not statistically significant.
Ethics approval and consent to participate
Written informed consent was obtained from all participants. The study received the approval of the Ethics Committee of Medical University of Bialystok, Poland (approval No. R-I-002/41/2019).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.