Abstract
Background. Sleep-disordered breathing (SDB) is a group of disorders that can affect the upper airway by increasing the chances of collapsibility during sleep.
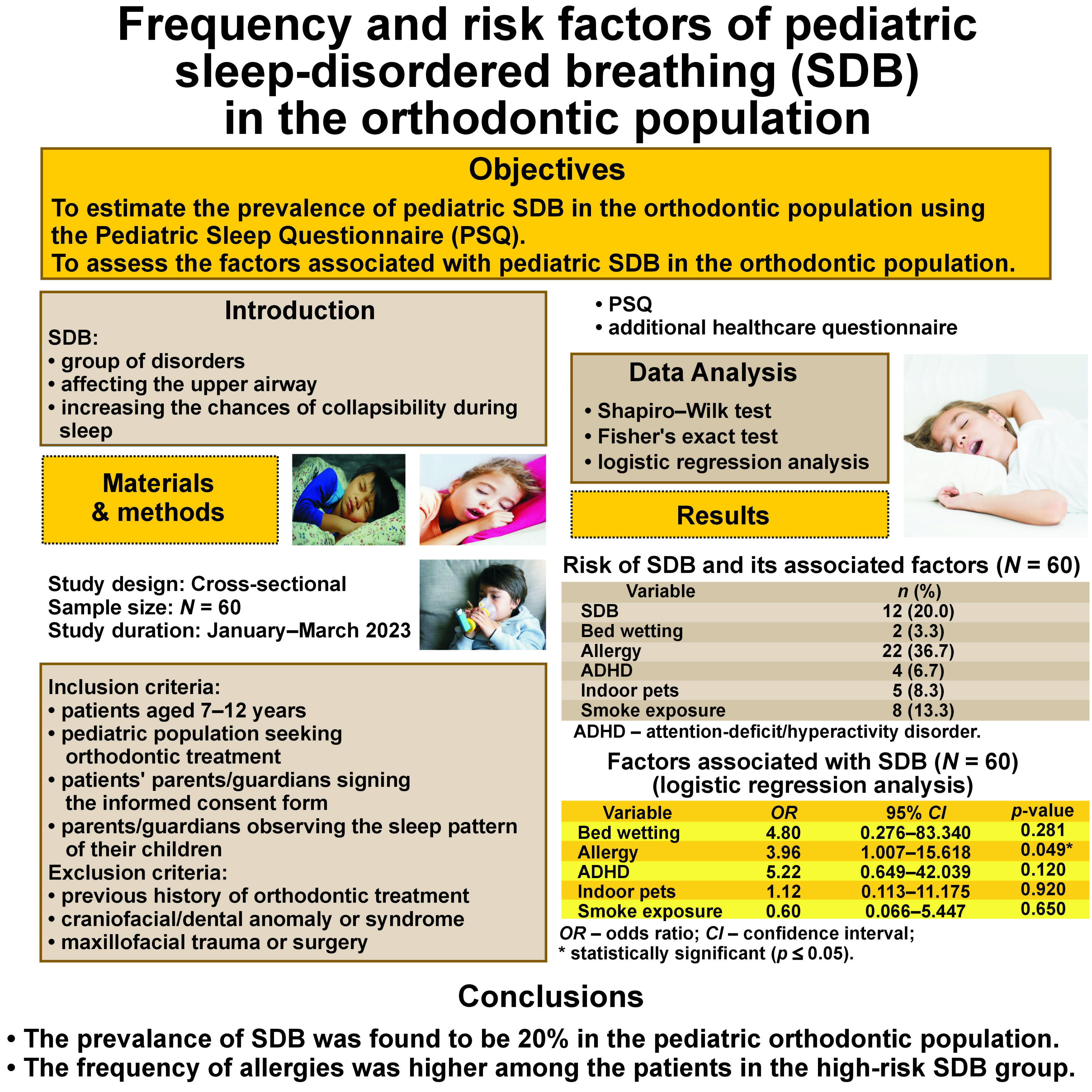
Objectives. The primary objective of the present study was to estimate the prevalence of pediatric SDB in the orthodontic population using the Pediatric Sleep Questionnaire (PSQ). The secondary objective was to assess the factors associated with pediatric SDB in the orthodontic population.
Material and methods. A cross-sectional study was conducted on a sample of 60 children aged 7–12 years who attended the orthodontic clinics at Aga Khan University Hospital, Karachi, Pakistan. The parents/guardians of the patients were asked to complete a validated PSQ and an additional health history questionnaire regarding the factors associated with SDB. The risk of SDB was reported for the pediatric orthodontic population. Logistic regression analysis was applied to assess the factors associated with SDB in the pediatric orthodontic population.
Results. A score suggestive of a high risk for SDB (≥33%) was found in 12 (20%) out of 60 patients. Patients with a history of allergies were 3.96 times more likely to have SDB (p = 0.049). In comparison with female patients, male patients had a higher susceptibility to SDB.
Conclusions. The prevalence of SDB was found to be 20% in the pediatric orthodontic population. The frequency of allergies was higher among the patients in the high-risk SDB group. Orthodontic practitioners are advised to incorporate routine SDB screening into their clinical practice, as there could be a specific subgroup of SDB patients that may go undetected in general pediatric clinics.
Keywords: pediatric, sleep, sleepiness, sleep disorders, Pediatric Sleep Questionnaire
Introduction
Sleep-disordered breathing (SDB) is a group of disorders that can affect the upper airway by increasing the chances of collapsibility during sleep.1 Sleep-disordered breathing can have numerous adverse effects on the quality of life (QoL), affecting both young individuals and adults.2 Interestingly, certain differences do exist between the pediatric and adult population when considering this condition.3 In the adult population, there appears to be a higher prevalence of the disease among males, indicating a gender difference.4 However, no definitive distinction is observed in the pediatric population.1 Additionally, there is a notable difference in etiology. In the adult population, obesity is recognized as the primary cause, while in the pediatric population, enlarged tonsils are considered to be the main culprit.5, 6 The evaluation of such features during a routine orthodontic examination emphasizes the crucial role of orthodontists/general practitioners in diagnosing patients and promptly referring them to the appropriate department.7
By virtue of physical and cephalometric inspections, multiple risk factors that contribute to the development of SDB have been identified.8 These factors include a constricted maxilla, a V-shaped arch form, a shortened mandible, macroglossia, and bidental arch constriction.9 A study conducted by Topaloglu-Ak et al. stated that sleep bruxism, temporomandibular disorders (TMDs) and untreated dental caries also had a negative impact on pediatric sleeping habits and characteristics.10
Polysomnography (PSG) takes precedence as the primary diagnostic modality for evaluating SDB.11 It involves electrooculography, electroencephalography, chin electromyography, the airflow measurement, monitoring the respiratory effort, tracking the oxygen saturation levels, and recording electrocardiography. Performing PSG in young patients requires specialized training in pediatrics to help children and their families adjust to the intensity of the monitoring. While PSG for children shares similarities with the procedure for adults, there are key differences to consider. Notably, these differences are most apparent in the positioning and placement of the monitoring leads due to the size disparity between children and adults. Furthermore, variations regard the identification of the sleep stages, the evaluation of the respiratory patterns and the assessment of the severity of sleep-related issues in adults vs. children.12 Nevertheless, there are certain drawbacks associated with PSG, including time requirements, high costs of initial installation, and the need for expert sleep physiologists to ensure accurate performance.13 Certain predictive tools, like the Berlin questionnaire (BQ), the STOP-BANG questionnaire, the Snoring, Trouble Breathing, and Un-Refreshed (STBUR) questionnaire, and the Pediatric Sleep Questionnaire (PSQ), were designed to minimize this burden.14
The PSQ is a reliable and validated tool to predict the risk of SDB in the pediatric population. This is a 22-unit questionnaire that covers various aspects, including but not limited to the snoring frequency, the observed apneas, difficulty in breathing, as well as inattentive and hyperactive behavior.11 This questionnaire serves as a screening tool to identify children who may need further investigation with PSG for a more detailed assessment.11 Early diagnosis can significantly influence the well-being of patients with SDB. Untreated SDB can potentially contribute to the development of attention-deficit/hyperactivity disorder (ADHD) and cardiovascular diseases (CVDs) characterized by arterial narrowing (atherosclerosis), ultimately leading to a diminished QoL.15, 16 Thus, screening for SDB and evaluating its risk in the orthodontic population can help orthodontists to make timely decisions, work on the factors associated with SDB and improve the overall treatment experience of these patients.
The aim of the present study was to assess the risk of SDB and the contributing factors associated with it in the pediatric orthodontic population using PSQ.
Material and methods
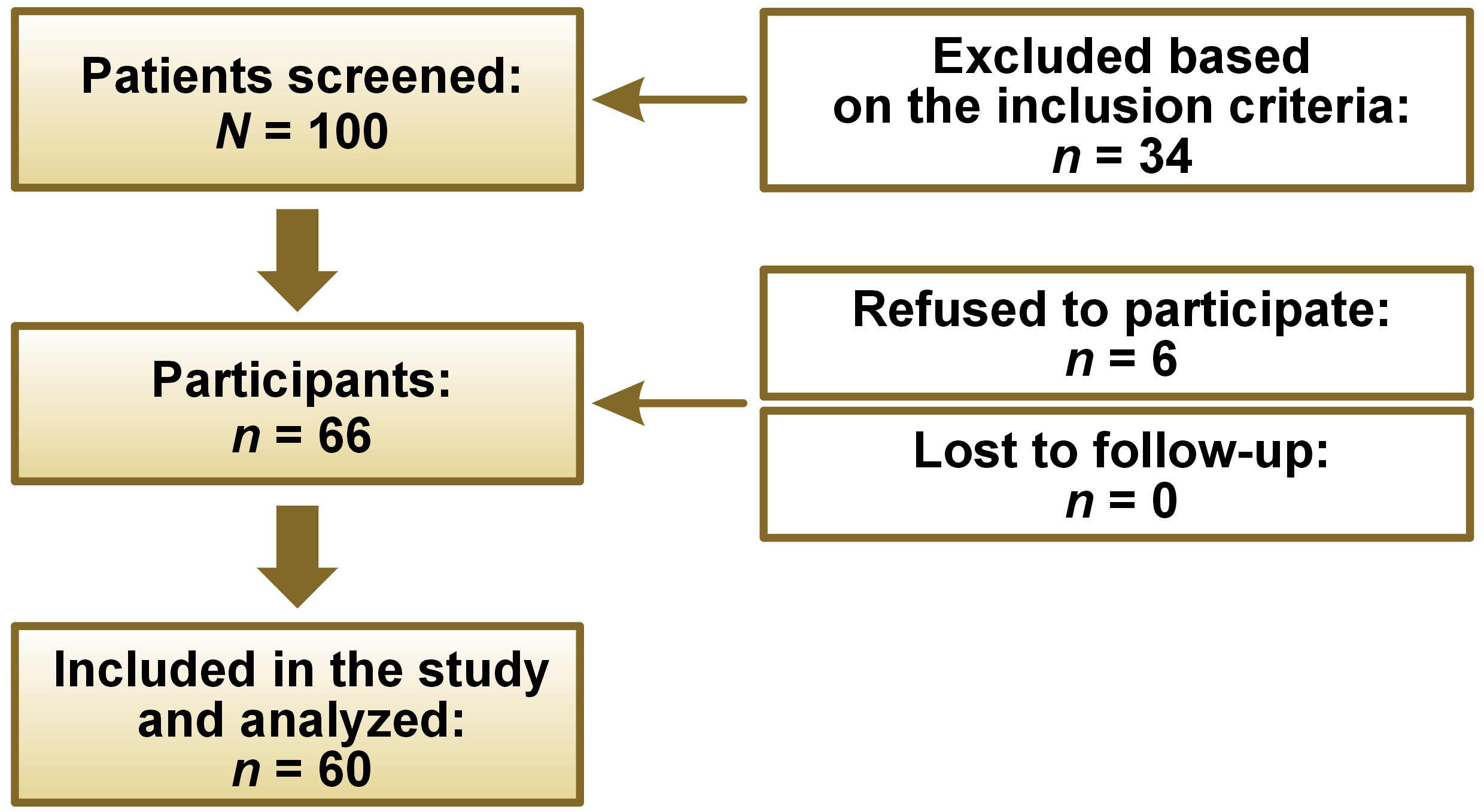
After attaining an approval from the institutional ethical review committee (ERC) (No. 2022-7818-22971), a questionnaire-based cross-sectional study was conducted at Aga Khan University Hospital, Karachi, Pakistan. The sample size was estimated with the OpenEpi® software, v. 3.01 (https://www.openepi.com/Menu/OE_Menu.htm) by using the findings of Sogut et al., who proclaimed an SDB prevalence of 3.3% in Turkish children.17 Consequently, keeping the absolute precision at 5% and a confidence interval (CI) of 95%, a sample size of 50 (N) was required. To address the sample attrition, we inflated the sample size by 20%, reaching the final sample size of 60 subjects in our study.
Data was collected from 60 patients seeking orthodontic consultation followed by orthodontic treatment, aged 7–12 years. The age range was determined by the research team, which consisted of a research assistant, 2 orthodontic residents and an orthodontist. The purpose of establishing this age range was to ensure inclusivity for the pediatric population seeking orthodontic care. The inclusion criteria were as follows: patients aged 7–12 years seeking orthodontic treatment; and patients’ parents/guardians signing an informed consent form and observing the sleep pattern of their children. Pediatric patients with the presence of any craniofacial/dental anomaly, syndrome, trauma, or a previous history of orthodontic treatment were excluded to keep the homogeneity of the sample and to avoid any kind of bias in the study.
In this cross-sectional study, during the first visit, all participants’ parents/guardians completed the appropriate informed consent forms, and the patients’ initial records were taken. After obtaining consent, the parents/guardians were asked to fill out PSQ (Table 1), assisted by an orthodontic team member. The PSQ used in the present study is a reliable questionnaire developed and validated by Chervin et al.11 It was applied to assess SDB. The PSQ comprises 22 questions with the responses assessed as follows: ‘yes’ = 1; ‘no’ = 0; and ‘don’t know’ = 00. It records the frequency of the observed apneas, snoring, daytime sleepiness, difficulty in breathing, and attentiveness in pediatric patients.11 The final score to confirm the presence of SDB has been recommended to be 0.33 (33% of positive responses), with higher percentages demonstrating a positive indication of SDB. The patients who scored 33% or higher in positive responses were classified as being at high risk for SDB.1, 11
The parents/guardians were also asked to fill out an additional health history questionnaire to assess the contributing environmental and health factors for SDB in the pediatric patients.1 This questionnaire consisted of questions confirming the presence or absence of asthma, environmental allergies, indoor pets, pre-term birth, nocturnal enuresis (NE), a family history of sleep apnea, and smoking environment.1 The treating practitioners ensured that patients identified as being at high risk for SDB received follow-up care and any necessary referrals.
Statistical analysis
Data analysis was conducted using IBM SPSS Statistics for Windows, v. 23.0 (IBM Corp., Armonk, USA) and the Stata software, v. 12.0 (StataCorp, College Station, USA). The normality of the data was assessed using the Shapiro–Wilk test, revealing a non-normal distribution. Descriptive statistics, such as median (Me) and interquartile range (IQR), were provided for the age of patients. The PSQ responses were collected from 60 patients and a positive score ≥33% was considered as a high risk for SDB. Frequencies were reported for the patients having a high risk for SDB and the factors associated with it. Furthermore, all individuals in the sample underwent additional health history questionnaire evaluations to assess their conditions. The results obtained from these evaluations were then compared between the patients categorized in the high-risk and low-risk SDB groups. Logistic regression analysis was applied to assess the factors associated with SDB. A statistical significance level of p ≤ 0.05 was adopted.
Results
The total sample size was 60, with a gender distribution of 30 males and 30 females (Figure 1). The age of the patients recruited in this study was 10.04 (8.00–11.33) years (Table 2).
A high risk for SDB (≥33%) was found in 12 (20%) out of the 60 patients. Evaluations from the additional health history questionnaire showed that 36.7% of the recruited population had environmental allergies (dust), 13.3% were exposed to smoke and 8.3% had indoor pets (Table 3). Among the patients who were at a high risk for SDB, 8 were males and 4 were females. In this group, 7 individuals had environmental allergies, whereas in the low-risk SDB group, 15 patients had environmental allergies (Table 4).
Logistic regression analysis was applied to assess the factors associated with SDB. It showed statistically significant results for the factor of allergy – the patients with a history of allergies were 3.96 times more likely to have SDB (p = 0.049). Moreover, the patients diagnosed with ADHD were found to have a 5.22 times greater likelihood of experiencing SDB as compared to individuals without ADHD (p = 0.120). In the case of the patients who wet the bed, the probability of having SDB was 4.80 greater in comparison with those who did not (p = 0.281). However, these results were not statistically significant (Table 5).
Discussion
This research was conducted to provide an insight into the risk of SDB in the Asian pediatric orthodontic population and its associated factors. Our study, based on a tertiary care hospital sample, yielded a risk of 20%. When looking into the literature globally, we found a recently conducted study from Alberta, Canada, reporting a prevalence of SDB of 10.8% in their orthodontic population.1 Additionally, 2 separate studies that focused on SDB in the orthodontic population reported a prevalence rate of 7.3% in their site-specific sample13 and 18% in their university-based sample.18 Utilizing the same objective questionnaire (PSQ), an SDB prevalence of 3.3% was observed in German preschool children.19 According to the findings of Graf et al., 53% of the children included in the study exhibited snoring, as reported by their parents.20 According to a meta-analysis conducted by Lumeng and Chervin, the prevalence rates of nocturnal childhood snoring ranged from 1.5% to 12.0%, with an average rate of 7.45% based on parent-reported data.21 A study conducted by Ancoli-Israel et al. compared the risk of SDB in the Caucasian and African-American populations.22 The study findings revealed that African-Americans exhibited a significantly higher risk of severe SDB, with a relative risk (RR) approx. twice as high (RR = 2.13) as that observed in Caucasians.22 Another study also reported a higher risk of SDB in African-Americans as compared to the Asian, Hispanic and European populations.23
Considering the prevalence at the regional level, children from Saudi Arabia had a 21% prevalence of SDB in the sample population.24 Kobayashi et al. conducted their study in Japan and reported the incidence of obstructive sleep apnea (OSA) in children to be 7.9%.25 Subsequently, authors from India used a modified STOP-BANG questionnaire and reported a 14% prevalence of OSA in Indian adolescents.26
One of the key competences of the clinician is the ability to predict the presence or absence of the disease in the general population. Investigators have pointed out that a convex facial profile, mandibular retrusion, tonsillar hypertrophy, and mouth breathing at an early age can lead to SDB.27 Further predictive factors reported by researchers include body adiposity and distal molar occlusion at the age of 6–8 years.27 In contrast to SDB in adults, which is predominantly associated with obesity, the pediatric population shows associations with adenotonsillar hypertrophy, allergies, frequent colds, and habitual mouth breathing.28 Our results are in agreement with the previous studies showing higher chances for SDB, especially in male patients with environmental allergies. Allergic reactions can lead to breathing irregularities through multiple pathways, including (1) inflammatory cytokines, such as histamine, which cause disturbance in the sleep/wake cycle; (2) nasal congestion, which is related to snoring and ultimately affects sleep quality; and (3) the dysfunction of the 2 important autonomic nervous system reflexes, namely the trigeminocardiac reflex and the nasotrigeminal reflex. Both are important in terms of sleep regulation and impairment.29
Among the other indicators described above, NE is also considered an important characteristic predictive of SDB. One study conducted in Taiwan concluded a positive association between environmental allergies, SDB and childhood NE.30 A prospective epidemiological study on 4,318 children reported that 33.1% of the children with SDB had NE.18 This is in accordance with our results, which showed 4.8 times higher chances for SDB among children with NE. Furthermore, work conducted by Lai et al. validated the findings of previous studies by reporting that children with allergic rhinitis had a higher incidence and risk of NE, especially males.31 Another critical factor causing OSA is an increased body mass index (BMI). Barone et al. conducted a study to assess the association of overweight and NE with SDB.32 They reported a non-significant association. However, when viewed separately, these characteristics (overweight and NE) showed a high probability of being present in children with OSA.32 In our study, we found an insignificant association of these factors with the risk of SDB.
The PSQ used in this study is the most widely used questionnaire to detect the risk of SDB in children and adolescents. The accepted age range to use this tool is 2–18 years. This comprehensive questionnaire has a sensitivity and specificity of 0.87 and 0.85, respectively.11 The PSQ is considered a time-consuming questionnaire by some researchers, so to rectify this situation, Kadmon et al. developed a more concise questionnaire termed I’M SLEEPY.33 It consists of 8 questions and has a sensitivity and specificity of 82% and 50%, respectively.33 A systematic review and meta-analysis published in 2020 found that PSQ had the highest sensitivity (74%) for detecting symptoms of moderate pediatric obstructive sleep apnea syndrome (OSAS).34 In screening for mild and severe pediatric OSAS, PSQ and pulse oximetry (PO) demonstrated equal sensitivity. Therefore, PO can be an excellent tool, along with PSQ, to detect SDB effectively when PSG is impractical.34
A study by Dastan et al. stated that the volume of the upper airway was significantly smaller in individuals with the dolichofacial pattern.35 Orthodontic interventions, such as functional appliances, in the growing phase and surgical interventions in adults can help in reducing the effect of the abovementioned feature, i.e., the decreased volume of the upper airway, and improve the quality of sleep and the overall QoL of these subjects.36 Treatment options for SDB vary, depending on the severity of the condition and the age of the patient. Treatment modalities for SDB encompass a spectrum of approaches, including non-surgical options, like mandibular advancement splints and continuous positive airway pressure, as well as surgical options, such as adenoidectomy and maxillomandibular advancement.37, 38 One mode of treatment can be the expansion of the maxillary arch via different methods, i.e., mini-screw-assisted rapid maxillary expansion (MARPE) and surgically assisted rapid maxillary expansion (SARPE), which are usually advised in adolescent and adult patients, respectively.38 A relatively new technique called endoscopically assisted surgical expansion (EASE) for the treatment of OSA has been advised.39 The method is claimed to be less invasive and provides consistently better results as compared to the previously mentioned maxillary expansion modalities. It is a surgical procedure performed under general anesthesia requiring nasal endoscopy for visualizing the midpalatal osteotomy, along with the separation of the pterygomaxillary suture with the use of a piezoelectric blade. After osteotomy, a transpalatal distractor is used to expand the nasal floor until the patient’s symptoms are alleviated or 7 mm of expansion has been achieved. The removal of the appliance is advised to be performed under local anesthesia after 2 months of obtaining the desired results.39
It is important to refer to the American Association of Orthodontists (AAO) white paper for OSA, which clearly states the responsibility of an orthodontist to screen, diagnose and advise a proper referral to patients.40 It is recommended that orthodontists who wish to treat SDB patients get equipped with the latest knowledge, skills and training in this field. It is encouraging to know that no orthodontic treatment causes or worsens OSA; rather, some form of orthodontic treatment is regarded as beneficial. However, an interdisciplinary team approach would serve the patient’s interests best.
Given the nature of observational studies, including our own, it is important to acknowledge the potential risks of bias and the confounding factors. The limitations of this research are a relatively small sample size and being a single-center study; the results cannot be generalized to the Asian population. An additional limitation is the absence of an objective method, such as PSG, for evaluating SDB in the orthodontic population. However, our study focused on assessing the risk of SDB in this population. As a cost-effective alternative to PSG, we utilized PSQ, which is a practical choice, especially for individuals who are not deemed to be at high risk for SDB and where PSG initial installation costs might be prohibitive. Despite these limitations, diligent attempts were made to minimize these issues. We employed rigorous statistical controls to account for various factors, and utilized a validated and reliable tool to accurately measure symptoms of SDB within the orthodontic population.
The literature suggests that adults with sleep bruxism may experience a poor quality of sleep, potentially leading to TMDs.41, 42 Therefore, studies should be carried out to assess factors such as sleep bruxism, and their association with SDB and TMDs. Additional recommendations would be to have a larger sample size and to include other parameters that can predict SDB in a non-invasive and inexpensive way.
The discrepancies in health conditions observed between patients attending general pediatric clinics and orthodontic clinics suggest that orthodontists may come across a specific subset of individuals with a higher likelihood of SDB that could potentially go unnoticed in other healthcare settings. As a result, the proactive engagement of orthodontists in screening for SDB can play a vital role in identifying the progression of the disorder in a timely manner, enabling a comprehensive and collaborative approach to diagnosis and treatment for patients who might otherwise remain undiagnosed.
Conclusions
The prevalence of SDB was found to be 20% in the pediatric orthodontic population. The frequency of allergies was higher among patients in the high-risk SDB group.
Orthodontic practitioners are advised to incorporate routine SDB screening into their clinical practice, as there could be a specific subgroup of SDB patients that may go undetected in general pediatric clinics.
Ethics approval and consent to participate
The study was approved by the institutional ethical review committee (ERC) (No. 2022-7818-22971) at Aga Khan University Hospital, Karachi, Pakistan. The patients’ parents/guardians signed an informed consent form before the commencement of the study.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.