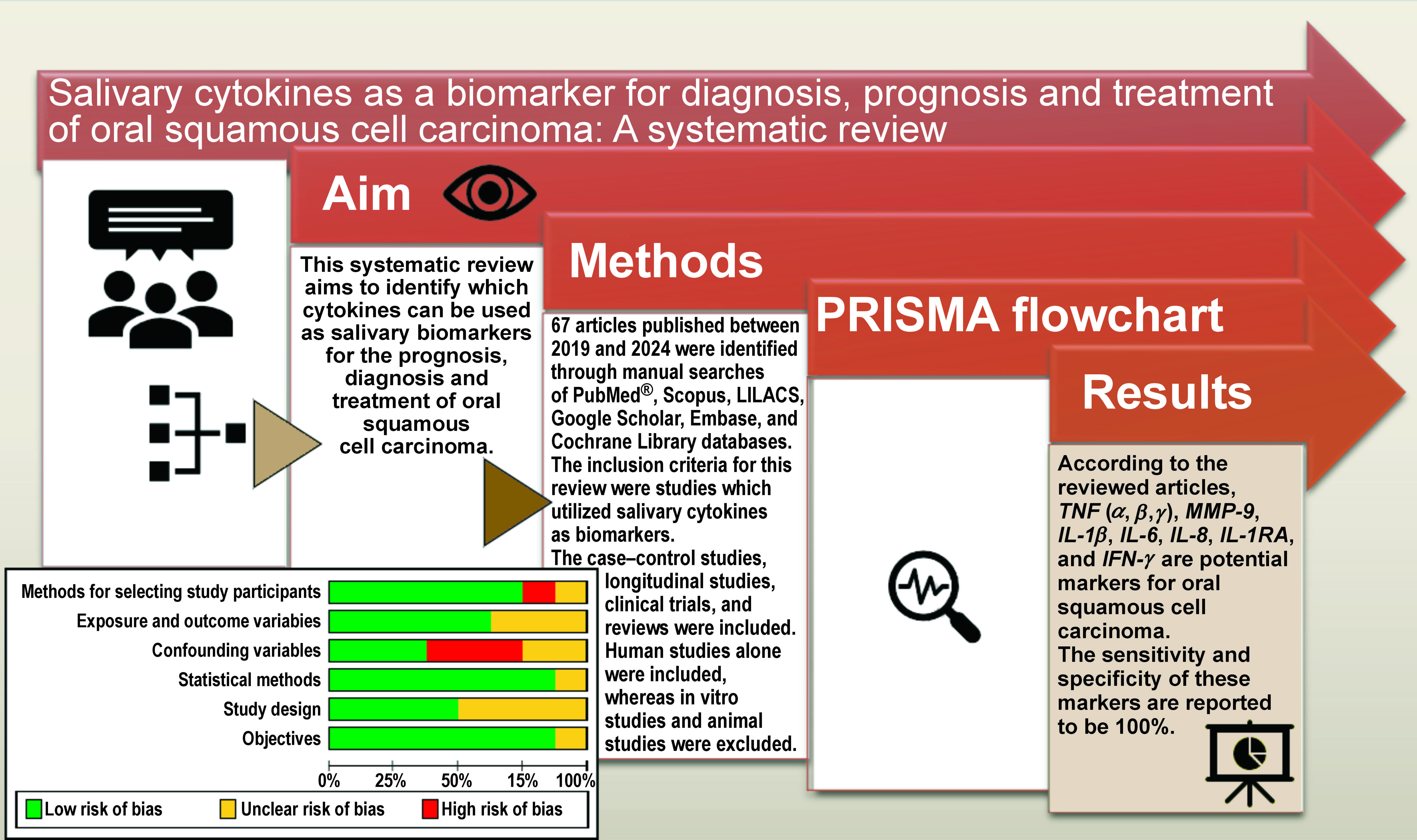
Abstract
Oropharyngeal squamous cell carcinoma and oral squamous cell carcinoma (OSCC) are the most common types of head and neck squamous cell carcinoma. Oral squamous cell carcinoma is the 6th most prevalent epithelial malignancy worldwide, and it is known for having significant morbidity and mortality rates. The present systematic review aims to identify which cytokines can be used as salivary biomarkers for the prognosis, diagnosis and treatment of OSCC. The early detection of the tumor and its precursor lesions is critical for improving the survival rate, reducing costs and enhancing the quality of life following treatment for OSCC. The conducted literature search yielded 65 articles; however, only 8 articles met the inclusion and exclusion criteria and were selected for a systematic review. Eighty percent of the articles were review articles, encompassing case–control studies and longitudinal studies. In 50% of the studies, diagnostic meta-analyses were conducted. According to the reviewed articles, interleukin-1β (IL-1β), IL-6, IL-8, IL-1 receptor antagonist (IL-1RA), interferon-γ (IFN-γ), tumor necrosis factor (TNF) (-α, -β, -γ), and matrix metalloproteinase-9 (MMP-9) are potential markers for OSCC, with a sensitivity and specificity of 100%.
Keywords: salivary cytokines, biomarkers, oral squamous cell carcinoma
Introduction
The mucosal epithelium lines the larynx, hypopharynx, oropharynx, nasopharynx, and oral cavity. It is in these regions that squamous cell carcinoma of the head and neck originates. Squamous cell carcinomas are the most prevalent histological manifestation of head and neck cancer, accounting for 90% of all head and neck malignancies.1 Oropharyngeal squamous cell carcinoma and oral squamous cell carcinoma (OSCC) are the most common types of head and neck squamous cell carcinoma.2 Oral squamous cell carcinoma is the 6th most prevalent epithelial malignancy worldwide, and is known for its significant morbidity and mortality rate. Consequently, growing worldwide public health concerns are related to OSCC.2
According to the World Health Organization (WHO), the five-year mortality rate for oral cancer is 45%.3 There is an 80–90% chance of survival if oral cancer is discovered in its early stages.4 Unfortunately, due to inadequate public education and screening techniques, these malignancies are difficult to detect in the early stages, which typically has a negative impact on prognosis and survival rate.5 A reliable early diagnosis of the tumor and its initial lesions is required for a decrease in treatment costs, an increase in the survival rate, and an enhancement of the quality of life following OSCC treatment.6
Comprehensive clinical examinations, costly biochemical analyses and invasive biopsies remain the gold standard for detecting oral malignancies.7, 8, 9 The discovery of biomarkers in biological matrices (blood, urine and saliva) may potentially help in the early identification of disease.10 Saliva is a biofluid made up of components that can be utilized as biomarkers, including cytokines, RNA and DNA molecules, circulatory cells, and microvesicles.11 In the last decade, research has focused on the examination of bodily fluids, also referred to as “liquid biopsy”, to identify biomarkers that could be predictive or diagnostic in OSCC.12 Notably, due to its accessibility, proximity to cancer cells non-invasiveness and cost-effectiveness, saliva has emerged as a promising biological specimen for early cancer detection.13 Consequently, the use of saliva for early detection of cancer is a promising strategy in the search for new diagnostic and therapeutic biomarkers.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33
Advances in the understanding of the molecular development of OSCC have facilitated the recognition of several potential biomarkers in unstimulated whole saliva, indicating changes in genomic and epigenetic processes, as well as metabolic or proteomic activity, in malignant cells.11, 34, 35 This knowledge encourages the prompt identification and diagnosis of OSCC.11 Cytokines are main intercellular protein mediators of the immune system.36 They are essential for growth, development and healing in addition to regulating immunological processes.37 Additionally, they facilitate communication between the body’s immune system and other systems. Cytokines are synthesized and secreted by cells throughout the body and central nervous system (CNS). While the secretion of cytokines often occurs concurrently with protein synthesis, some are produced and retained for rapid release within intracellular granules. The effects of cytokines are frequently redundant or synergistic, and they have a variety of target cells and modes of action.
The production of certain cytokines by OSCC cells has been demonstrated. It has been proposed that these cytokines play a part in the formation of tumors and in the process of angiogenesis.38 Moreover, these cytokines have been proven to be present in saliva. Consequently, salivary cytokines have the potential to serve as crucial biomarkers for the evaluation, prognosis and treatment of OSCC.
Material and methods
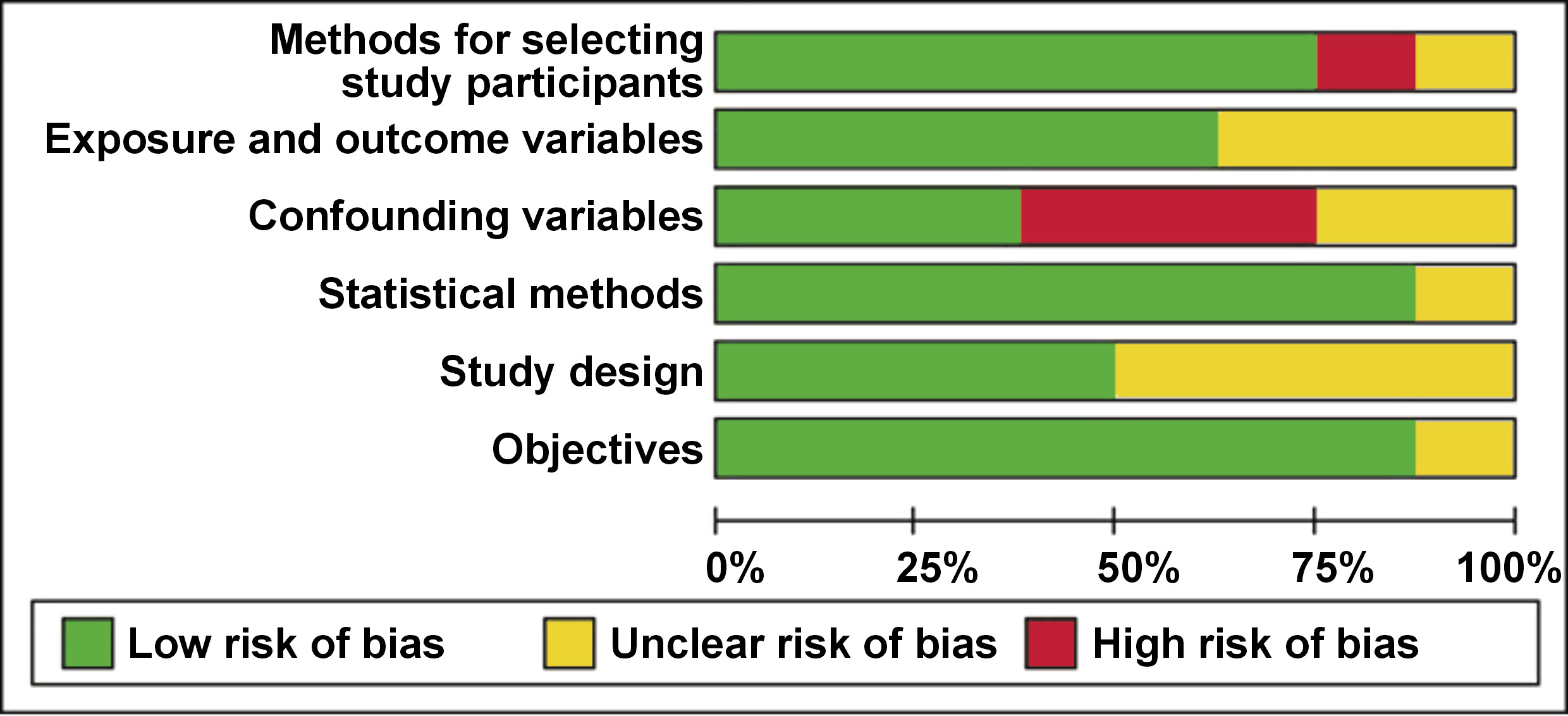
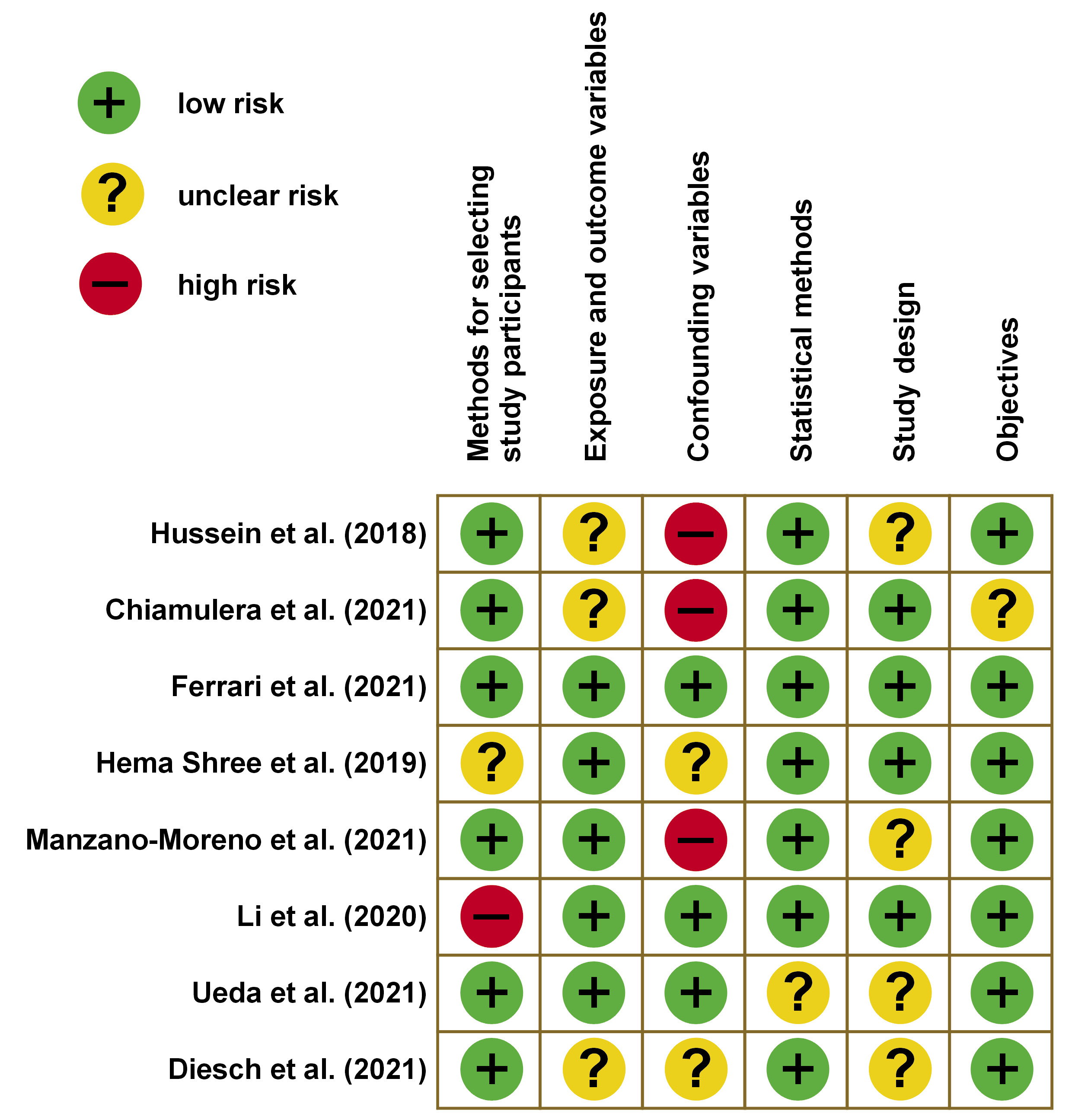
A total of 67 articles published between 2019 and 2024 were identified through manual searches of PubMed®, Scopus, LILACS, Google Scholar, Embase, and Cochrane Library databases. The inclusion criteria for this review were studies published between 2019 and 2024, which utilized salivary cytokines as biomarkers. The case–control studies, longitudinal studies, clinical trials, and reviews were included. Human studies alone were considered for the inclusion, whereas in vitro studies and animal studies were excluded. Two individual reviewers independently evaluated the articles for risk assessment based on the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) criteria. The results are presented in Figure 1 and Figure 2. Following a thorough evaluation of the articles based on the predetermined inclusion criteria and risk assessment, 8 articles were selected for inclusion in this systematic review.
Data extraction
A comprehensive search of PubMed®, Scopus, LILACS, Google Scholar, Embase, and Cochrane Library databases was conducted using the following search terms: alveolar cancer; alveolar cancers; alveolar tumor; alveolar tumors; alveolar carcinoma; alveolar carcinomas; alveolar malignancy; alveolar malignancies; alveolar neoplasm; alveolar neoplasms; gingivobuccal cancer; gingivobuccal cancers; gingivobuccal tumor; gingivobuccal tumors; gingivobuccal carcinoma; gingivobuccal carcinomas; gingivobuccal malignancy; gingivobuccal malignancies; gingivobuccal neoplasm; gingivobuccal neoplasms; cheek cancer; cheek cancers; cheek tumor; cheek tumors; cheek carcinoma; cheek carcinomas; cheek malignancy; cheek malignancies; cheek neoplasm; cheek neoplasms; tongue cancer; tongue cancers; tongue tumor; tongue tumors; tongue carcinoma; tongue carcinomas; tongue neoplasm; tongue neoplasms; tongue malignancy; tongue malignancies; lingual cancer; lingual cancers; lingual tumor; lingual tumors; lingual carcinoma; lingual carcinomas; lingual malignancy; lingual malignancies; lingual neoplasm; lingual neoplasms; buccal cancer; buccal cancers; buccal tumor; buccal tumors; buccal carcinoma; buccal carcinomas; buccal malignancy; buccal malignancies; buccal neoplasm; buccal neoplasms; oral cancer; oral cancers; oral tumor; oral tumors; oral carcinoma; oral carcinomas; oral malignancy; oral malignancies; oral neoplasm; oral neoplasms; mouth cancer; mouth cancers; mouth tumor; mouth tumors; mouth carcinoma; mouth carcinomas; mouth malignancy; mouth malignancies; mouth neoplasm; mouth neoplasms; head and neck tumor; head and neck tumors; head and neck carcinoma; head and neck carcinomas; head and neck malignancy; head and neck malignancies; head and neck neoplasm; head and neck neoplasms.
Results
A comprehensive data search yielded 65 articles, of which 8 were selected for systematic review based on the inclusion and exclusion criteria. Eighty percent of the articles were review articles, encompassing case–control studies and longitudinal studies. Furthermore, 50% of the studies conducted diagnostic meta-analyses. In 80% of the articles, tumor necrosis factor (TNF) (-α, -β, -γ), matrix metalloproteinase-9 (MMP-9), interleukin-1β (IL-1β), IL-6, IL-8, interleukin-1 receptor antagonist (IL-1RA), and interferon-γ (IFN-γ) were discussed as potential markers for OSCC. The sensitivity and specificity of these markers were reported to be 100%.
Consequently, a panel of highly specific markers can be established for the detection of OSCC. These markers could also facilitate the analysis of prognosis and treatment outcomes for patients with OSCC.
Discussion
This systematic review underscores the significant potential of inflammatory cytokines and matrix-degrading enzymes as salivary biomarkers in the detection of OSCC. The consistent appearance of markers such as TNF variants, MMP-9, IL-1β, IL-6, IL-8, IL-1RA, and IFN-γ across the majority of studies highlights a growing consensus in the field. These biomarkers not only reflect underlying pathophysiological processes but also present an opportunity to develop non-invasive, highly accurate diagnostic tools.
IL-6
Interleukin-6 is a highly sensitive and specific marker. Eighty percent of the analyzed studies have used IL-6 as a diagnostic and prognostic biomarker. Interleukin-6 is a multifunctional cytokine that controls inflammatory reactions.10 It aids in the development, migration, invasion, growth, proliferation, apoptosis, progression, angiogenesis, and differentiation of tumor cells. Moreover, IL-6 stimulates the phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways.24 Through the JAK/STAT pathway, IL-6 activates STAT3 by binding to IL-6R, which then forms a complex with gp130. Furthermore, IL-6 can help tumor cells elude immune monitoring, preventing tumor rejection, given its immunosuppressive properties.39
IL-8
In the majority of the included studies, IL-8 has been analyzed along with IL-6 and regarded as the most predictive salivary biomarker. Chiamulera et al. performed a meta-analysis that compared the concentration of salivary cytokines in patients with OSCC to those of healthy controls.37 The results demonstrated a significant increase in the level of IL-8, as indicated by standardized mean difference (SMD) of 1.77 (95% confidence interval (CI): 0.79–1.55).37 The development of OSCC is influenced by the pro-inflammatory and pro-angiogenic IL-1, IL-6, IL-8, and TNF-α, which support cell survival and proliferation.12 They activate pro-cell cycle regulators, including the nuclear factor kappa B (NF-κB), STAT proteins and MAPK/extracellular signal-regulated kinase (ERK) pathways.40
TNF-α
Tumor necrosis factor alpha has been utilized as a salivary biomarker in numerous studies, exhibiting high sensitivity and specificity. A meta-analysis conducted by Chiamulera et al. demonstrated a SMD of 2.04 (95% CI: 0.47–3.61) for TNF-α in patients with OSCC, when compared to healthy individuals.37
Tumor necrosis factor alpha is a multifunctional cytokine that plays a crucial role in various biological processes, including cell survival, proliferation, differentiation, and death.41 Inflammatory cells release TNF-α, a pro-inflammatory cytokine that may contribute to inflammation-related carcinogenesis.42 The cytokine affects various signaling pathways, including NF-κB and c-Jun N-terminal kinase (JNK). NF-κB is a key antiapoptotic cell survival signal, whereas persistent JNK activation promotes cell death. The interaction between NF-κB and JNK exerts an influence on the cellular responses to TNF-α.37
Tumor necrosis factor alpha has a dual role in cancer progression.41 While it can enhance the development, proliferation, invasion, and angiogenesis of cancer cells, presumably acting as an endogenous tumor promoter,43 it also has the potential to eradicate cancer.41 Tumor necrosis factor alpha has been shown to induce cancer cell death, suggesting its potential in cancer treatment. However, significant research is necessary to mitigate the toxicity of TNF-α for routine administration.44 Recent studies have focused on combination therapy, aiming to decrease survival signals such as NF-κB to enhance the susceptibility of cancer cells to TNF-α-induced apoptosis.43
miRNAs
In the nucleus, RNA polymerase II synthesizes long, single-stranded RNA molecules called primary microRNAs (pri-miRNAs).45 These pri-miRNAs undergo sequential processing. Initially, they are processed by the Drosha complex in the nucleus to form precursor miRNAs (pre-miRNAs). Subsequently, the Dicer complex, located in the cytoplasm, produces mature, short, single-stranded microRNAs (miRNAs). The concentration of these mature miRNAs determines their ability to regulate the stability or translation of messenger RNA (mRNA), depending on their sequence complementarity to the target mRNA.46
MicroRNAs can be selectively packed in extracellular vesicles or released in bodily fluids as cell-free miRNAs linked to RNA binding proteins.47 Additionally, miRNAs play a role in the regulation of several biological processes, including cell differentiation, proliferation, apoptosis, and the development of embryos and tissues.48 Numerous miRNAs are involved in the regulation of bone metabolism.49 Their expression is typically tumor-specific and is not altered by bodily fluids or tissues.50 Furthermore, they function as the primary regulators of gene expression, making them crucial for the identification of early stages of malignant transformation.50 As a result, the study of miRNA profiles in cancer patients offers a novel approach to the development of biomarkers for the clinical diagnosis of this illness.51
Additionally, the expression of certain miRNAs, such as miR-24, miR-3P, miR-412-3p, miR-512-3p, miR-302b-3p, miR-517b-3p, miR-134, miR-486-5p, miR-4484, miR-10b-5p, miR-200a, miR-365, miR-21, miR-145, miR-93, miR-184, miR-31, miR-412-3p and miR-34a, has been shown to be either upregulated or downregulated in OSCC patients when compared to healthy controls or patients with oral potentially malignant disorders (OPMDs).
CCL20
The expression of a small cytokine belonging to the CC chemokine family, CCL20, may play a role in the growth and dissemination of OSCC.52 According to a study by Ueda et al., in which 0.069 was used as a cut-off value for the area under the curve (AUC), the researchers found that the specificity and positive predictive value of CCL20 were 0.983 and 0.979, respectively, demonstrating satisfactory accuracy for patients with OSCC as compared to those with OPMD and healthy volunteers.52 According to the microarray analysis, CCL20 mRNA was found to be strongly accumulated in the saliva of OSCC patients. Using quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR), it was ascertained that CCL20 expression was considerably higher in the saliva of patients with OSCC compared to healthy volunteers and patients with OPMD (p < 0.001).52
MMPs
Matrix metalloproteinases are a group of structurally similar zinc-containing endopeptidases.53 A total of 23 MMPs have been documented. Numerous cell types, including immune cells, epithelial cells and fibroblasts, generate MMPs.37 The extracellular matrix (ECM) components that are degraded by MMPs result in altered cell–matrix and cell–cell interactions as well as the activation or inactivation of cytokines, growth factors and cell surface receptors.54
Collagenase-1, also known as MMP-1, is the earliest-identified member of the MMP family and plays a pivotal role in ECM remodeling. It is strongly associated with tumor metastasis, angiogenesis, and inflammation.55 MMP-1 is synthesized as an inactive zymogen (pro-MMP-1) and secreted via a signal peptide-guided pathway. Its activation is triggered by the proteolytic removal of the pro-peptide domain, typically by a serine protease or another MMP, such as MMP-3.56 This activation exposes the catalytic site, enabling MMP-1 to degrade several ECM components, including fibronectin, gelatin and laminin, thereby contributing to ECM remodeling.57
The activity of MMP-1 is controlled by the binding of tissue inhibitors of metalloproteinases (TIMPs) or by autolytic cleavage.53, 58 The equilibrium between the concentration of active metalloproteinases and their inhibitors (TIMPs) may become imbalanced and result in pathological alterations linked to uncontrolled ECM turnover, tissue remodeling, inflammatory response, cell proliferation, and migration.54, 56
Collagen type IV, the predominant glycoprotein component of the basement membrane and a factor in the control of inflammatory and vascular processes, is destroyed by MMP-2 (collagenase-4).58 Numerous elements of ECM, including proteoglycans, fibronectin, laminin, and collagen types III, IV and V, are broken down by stromelysin, also known as MMP-3.59 Type II collagen is best broken down by MMP-13 (collagenase-3).58
Type IV collagen, a significant component of the basal lamina, as well as other types of collagen (V, VII and X), elastin and fibronectin are all degraded by the MMP-9 class of zinc-dependent proteinases,12 which have been linked to a variety of clinical diseases. According to studies,58, 59 stromal cells surrounding the invading front of metastatic tumors exhibit elevated expression of MMP-9. Matrix metalloproteinase-9, along with MMP-2, belongs to the gelatinase subgroup of the MMP family. The overexpression of MMP-9 has been frequently observed in a variety of malignant tumors. Eighty percent of studies included in this review have analyzed MMP-9 as a biomarker, emphasizing its sensitivity and specificity.
Conclusions
This systematic review highlights the growing potential of salivary biomarkers in the early detection and prognostic evaluation of OSCC. The analysis identifies several promising candidates that may serve as non-invasive, reliable tools for clinical application. However, to facilitate their integration into routine clinical practice, future research should focus on large-scale, longitudinal, and multicenter studies aimed at validating these biomarkers and establishing a robust, standardized salivary biomarker panel for OSCC diagnosis and prognosis.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.