Abstract
Background. The formation of biofilm on the tooth surface can lead to the dissolution of the enamel minerals and the onset of tooth decay. Natural compounds may prove to be effective in the prevention of biofilm formation.
Objectives. The present study aimed to evaluate the antibacterial and antibiofilm activity of the Zataria multiflora ethanolic extract in comparison with chlorhexidine, using a novel tooth model.
Material and methods. The study used teeth extracted due to orthodontic treatment or impacted wisdom tooth surgery. Saliva was collected from a volunteer 12 h after tooth brushing, before the test, and it was used freshly. The teeth were placed in 5 test tubes containing a broth medium and serial dilutions of the Z. multiflora extract (50, 25, 12.5, 6.25, and 3.125 mg/mL). A total of 1 mL of the collected saliva was added to each test tube. The growth of microorganisms in the medium was examined visually and the antibiofilm activity of the plant extract was assessed after 72 h, using a spatula. The results were compared with those of positive and negative controls.
Results. Considerable turbidity was observed in the positive control tube containing a tooth, the culture medium and saliva, indicating that the conditions were favorable for the growth of oral flora. No bacterial growth or biofilm formation were observed in the test tubes containing ≥25 mg/mL of the plant extract.
Conclusions. The study results indicated that the Z. multiflora extract had an excellent inhibitory effect against microorganisms and plaque formation in the tooth model, suggesting a suitable substitute for chlorhexidine. However, further studies in this area are recommended.
Keywords: Zataria multiflora, dental plaque, biofilm, tooth model, normal oral flora
Introduction
Dental caries, also known as tooth decay, is caused by cariogenic organisms, including streptococci, lactobacilli, etc., present in the biofilm (dental plaque), which ferment dietary carbohydrates to produce acids, leading in turn to the dissolution of the enamel minerals and the onset of tooth decay.1, 2 Among streptococci, the mutans group, especially Streptococcus mutans, is considered to be the main cause of tooth decay. However, some studies have shown that other bacteria can also be involved in the development of caries.3 On the other hand, dental plaque can be a reservoir of pathogenic strains of Helicobacter pylori and may play an important role in gastrointestinal diseases.4
Commercial mouthwashes act as an adjunct to mechanical methods, such as tooth brushing and flossing; they play an important role in reducing the bacterial load, and thus caries.5 Among antimicrobial agents, chlorhexidine, a biguanide-based chlorophenyl with an extensive antimicrobial activity, has been regarded as the gold standard.6, 7 Unfortunately, the long-term use of chlorhexidine could lead to side effects, such as a decreased salivary flow, the discoloration of the tongue, mouth and teeth, a burning sensation in the mouth, etc.8 Therefore, taking into account the lower resistance of bacteria to plant essential oils, other agents, such as herbal mouthwashes, have been increasingly studied in recent years.9, 10, 11
Zataria multiflora, which grows in different parts of Iran, is one of the dicotyledonous plants belonging to the mint family (Lamiaceae). The plant contains effective antimicrobial compounds, such as carvacrol, thymol and linalool; however, their amount may vary depending on the plant variety and cultivation area.12 As the Z. multiflora extract is natural and inexpensive, apart from other advantages, it could be considered as a substitute for commercial antiplaque agents.13, 14 Although several reports have investigated the antibacterial and antibiofilm activity of different extracts in vitro, using the microtiter method,15 the present study was meant to be novel in terms of using a tooth model with a close simulation of the oral cavity, and it aimed to evaluate the antibacterial properties of Z. multiflora through assessing the inhibition of plaque formation by normal flora in comparison with commercial chlorhexidine.
Material and methods
Collection of teeth and saliva
The study was reviewed and approved by the Medical Ethics Committee of Qom University of Medical Sciences, Iran (code: IR.MUQ.REC.1399.029), and all procedures were carried out in accordance with the relevant guidelines and regulations of the committee. After providing the necessary explanations to the patients referred to a dental clinic in Qom, and receiving informed written consent from all subjects or their legal guardians, teeth that had to be extracted due to orthodontic treatment or impacted wisdom tooth surgery were collected. Therefore, no additional teeth were extracted from the patients except those that were in the treatment process. The collection of saliva from a volunteer was also based on informed written consent.
A total of 27 healthy human teeth, with no caries, cracks or previous restoration, were collected. After extraction, the teeth were placed in 0.9% physiological saline solution until transferred to the laboratory. For disinfection, all the teeth were kept in 10% formalin solution for 7 days. After that, the remaining soft tissue was removed from the tooth surface with a periodontal curette. Then, the teeth were cleaned using a rubber cap and sterilized in an autoclave at 121°C for 15 min.16
Saliva, containing nutrients and a variety of oral microorganisms that could play a significant role in biofilm formation and the development of caries, was collected from a volunteer with no active tooth decay 12 h after tooth brushing, before the test, and used freshly.
Preparation of the plant extract
To prepare the extract, the Z. multiflora plant was purchased from an herbal medicine shop in Qom, Iran. The identification and authentication of the purchased plant were done by the relevant academic staff from the Department of Persian Medicine at Qom University of Medical Sciences. Then, 20 g of the plant powder was dissolved in 100 mL of 96% ethanol solvent (Taghtir Khorasan Co., Mashhad, Iran) and the obtained solution was placed in a dark environment. After 3 days, the solution was filtered, and the resulting clear solution was poured into a glass plate and again placed in a dark environment for 2–3 days so that ethanol could evaporate. The obtained powder was then stored at 4°C until used for further experiments.
Evaluation of antibacterial and antibiofilm effects
For this purpose, the teeth were placed in 5 test tubes containing 3 mL of the tryptic soy broth (TSB) medium (Ibresco, Karaj, Iran). A total of 3 mL of the plant extract stock solution (100 mg/mL) was added to the 1st tube, and then serial dilutions were made to prepare concentrations of 50, 25, 12.5, 6.25, and 3.125 mg/mL. Afterward, 1 mL of the collected saliva was added to each test tube. Four control tubes containing the following materials were also used: a tooth, the culture medium and saliva (positive control PC1); a tooth, the culture medium, saliva, and 0.2% chlorhexidine (positive control PC2); a tooth, the culture medium and 50 mg/mL of the extract (negative control NC1); and a tooth and the culture medium (negative control NC2). The tubes were incubated at 37°C in a shaker incubator and visually evaluated for the growth of microorganisms at 24, 48 and 72 h. The lowest concentration that inhibited the growth of normal flora was identified as the minimum inhibitory concentration (MIC). Finally, the teeth were removed from the culture medium, and the antibiofilm properties of the used materials were evaluated by determining the presence or absence of dental plaque on the tooth surface with a spatula. Toensure the accuracy of the experiment, it was repeated in 3 groups of 9 teeth.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, v. 22.0 (IBM Corp., Armonk, USA), and employing the Kruskal–Wallis test to compare the antibacterial and antibiofilm effects of the Z. multiflora extract with regard to different concentrations. A p-value of less than 0.05 was considered as statistically significant.
Results
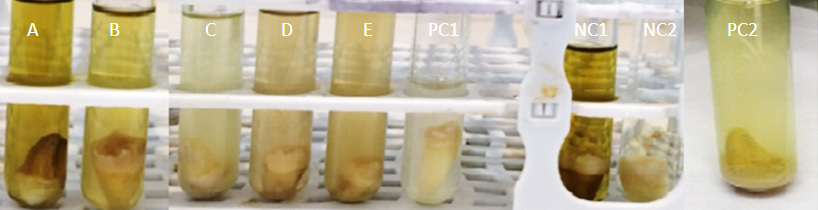
In daily examinations, considerable turbidity was observed in the positive control tube containing a tooth, the culture medium and saliva (PC1), indicating that the conditions were favorable for the growth of normal oral flora. No bacterial growth or turbidity were observed in the tubes containing 25 and 50 mg/mL of the extract (MIC = 25 mg/mL), while these were visible in the tubes containing other concentrations of the extract (p = 0.007). Slight turbidity was also observed in the tube containing chlorhexidine (PC2), indicating a poor growth of microorganisms. No bacterial growth was observed in the negative control tubes (Figure 1).
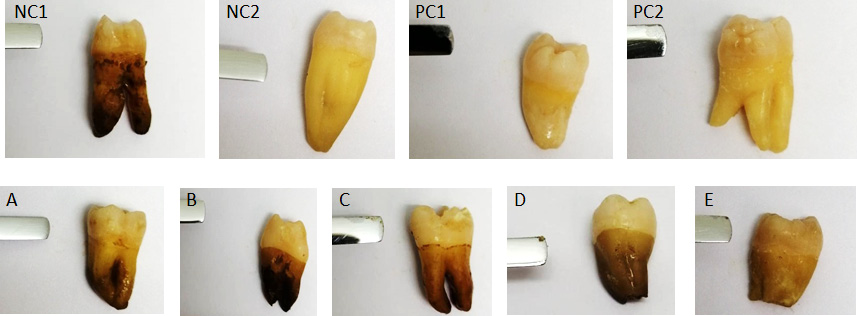
The inhibitory activity of the extract against biofilm formation on the tooth surface was evaluated after 72 h, using a spatula. The results showed that the extract at concentrations of 25 and 50 mg/mL also exhibited a significant inhibitory effect against biofilm formation, and no plaque was formed on the tooth surface, similar to the negative control teeth (p = 0.007). The extract showed no inhibitory effect on biofilm formation at lower concentrations. Therefore, the MIC of the extract against biofilm formation was determined to be 25 mg/mL. Interestingly and unexpectedly, in the positive control tube PC2, commercial chlorhexidine was not able to completely inhibit biofilm formation as compared to the extract at concentrations of 25 and 50 mg/mL (Figure 2). All results were similar in the 3 groups. The findings are summarized in Table 1.
Discussion
To date, tooth decay remains a major challenge in public healthcare systems and one of the most common diseases worldwide, experienced by almost everyone during their lifetime. Microorganisms are the crucial contributors involved in the development of dental caries through the fermentation of carbohydrates and the production of acids.1, 3, 17
Zataria multiflora has been shown to have acceptable inhibitory effects on pathogens, especially antibiotic-resistant bacteria. In a study conducted by Saeidi et al., the antibacterial activity of Mentha longifolia L. (ethyl acetate and aqueous extracts) and Z. multiflora (a hydroalcoholic extract) against some bacterial species was investigated.18 The researchers reported that these extracts were able to inhibit the growth of all the tested species.18 Dadashi et al. showed that Z. multiflora might act in vitro against the multidrug-resistant strains of Klebsiella pneumonia.19 The effects of Z. multiflora against the metallo-beta-lactamase (MBL)-producing Pseudomonas aeruginosa isolates and the antibiotic-resistant Staphylococcus aureus strains isolated from food were also reported by Heidary et al.20 and Soltan Dallal,21 respectively.
As previously mentioned, no study has been conducted so far to investigate the antibacterial and antibiofilm effects of Z. multiflora on a dental model in vitro. In this project, efforts were made to create a close simulation of the oral cavity, which is one of the strongest points of this study. In the present study, it was observed that the Z. multiflora extract at a concentration ≥25 mg/mL significantly inhibited the growth of oral microorganisms.
The antimicrobial activity of Z. multiflora is due to the fact that the plant is a rich source of oxygenated monoterpenes, especially thymol and carvacrol, which have been well established as excellent antimicrobial agents.12, 14 In a study examining the biological importance of the Z. multiflora extract, Saleem et al. showed that thymol was the most important component in the fresh plant, while carvacrol was the most important component in the dried plant.22 It is known that lipophilic compounds in thymol and carvacrol cause damage to the cell membrane through changing its permeability, and ultimately leading to the lysis of bacterial cells.23, 24
In the present study, in the positive control tube (PC2), the commercial chlorhexidine mouthwash could not completely inhibit bacterial growth and plaque formation, indicating that chlorhexidine was less effective than the Z. multiflora extract, which showed significant antibacterial and antibiofilm activity at concentrations of 25 and 50 mg/mL.
Studies have shown that chlorhexidine plays an important role in preventing plaque formation. For example, Haydari et al. investigated the effect of chlorhexidine (0.06%, 0.12% and 0.2%) on plaque formation and bleeding, as well as its side effects on the modified experimental gingivitis model; the results showed that 0.2% chlorhexidine exhibited a significant inhibitory effect on plaque formation as compared to concentrations of 0.06% and 0.12%.25
However, other reports comparing chlorhexidine with herbal mouthwashes, especially Z. multiflora, have indicated that herbal mouthwashes also show good effects in terms of microbial control. Jafari et al. examined the effects of a Z. multiflora essential oil solution and a chlorhexidine mouthwash on the orthodontic elastic rings contaminated with S. mutans in vitro.26 It was found that 0.5 mg/100 cm3 solution had good antibacterial properties, and therefore could be used for controlling microbial plaque in orthodontic rings as a substitute for chlorhexidine.26 In another report by Aghili et al., the antimicrobial effects of the Z. multiflora extract and chlorhexidine on the orthodontic elastomeric ligatures experimentally contaminated with S. mutans, Enterococcus faecalis and Candida albicans were compared.14 Due to statistically significant differences in the count of viable bacteria before and after disinfection with Z. multiflora, they concluded that the plant extract had antibacterial properties and could be used to disinfect orthodontic elastomeric ligatures.14 Moreover, the inhibitory concentrations of both thymol-based and chlorhexidine mouthwashes with regard to the growth of Streptococcus spp. were considered and compared by Khorasani et al.27 They showed that both types of mouthwash were effective in inhibiting the growth of the studied bacteria; however, the chlorhexidine mouthwash was more potent than the thymol-based mouthwash in inhibiting bacterial growth if diluted.27
Limitations
Due to the coronavirus disease 2019 (COVID-19) pandemic, it was not possible to collect saliva from people who had active caries and did not brush their teeth regularly, which may have affected the results. Due to some limitations in our laboratory, certain methods, like atomic force microscopy (AFM) or confocal laser scanning microscopy (CLSM), could not be used to analyze biofilm viability and structure.
Conclusions
This was a preliminary study, designed to use a tooth model. Considering that Z. multiflora is one of the native medicinal plants of the tropical regions of Iran, as well as due to its reasonable price and fewer side effects in comparison with chlorhexidine, it is suggested to use this plant extract as a mouthwash or chewing gum. While confirming the findings of other studies regarding the effectiveness of this plant, the results of the present work with the simulation of the oral cavity environment also showed that Z. multiflora was comparable with chlorhexidine. However, it is recommended that further studies be performed, involving microbiological analysis, the microscopic evaluation of the structure of the biofilm, and finally the use of the extract in vivo.
Ethics approval and consent to participate
The study was reviewed and approved by the Medical Ethics Committee of Qom University of Medical Sciences, Iran (code: IR.MUQ.REC.1399.029), and all procedures were carried out in accordance with the relevant guidelines and regulations of the committee. All subjects or their legal guardians provided informed written consent before the commencement of the study.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.