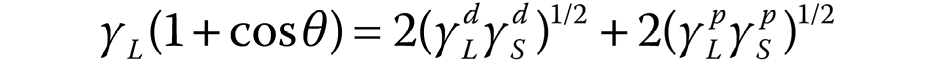Abstract
Background. Biomodifiers can reinforce the collagen matrix, improving the biomechanical and biochemical properties of dentin.
Objectives. The present study aimed to evaluate how 2.5% phosphorylated chitosan (P-Chi) and 0.5 mol/L carbodiimide (EDC) affect the surface wettability, surface free energy and surface morphology of the eroded dentin.
Material and methods. Bovine tooth specimens (N = 144) were randomly divided into 6 groups according to the dentin substrate (sound or eroded) and surface treatment (2.5% P-Chi, 0.5 mol/L EDC or no biomodification (control)). Half of the specimens (n = 72) were submitted to erosive challenge by immersion in 0.3% citric acid (pH 3.2) for 2 h. For the surface wettability analysis (n = 12), the contact angles between the dentin surface and the 3M™ Scotchbond™ Universal adhesive were measured with a goniometer. For the surface free energy analysis (n = 3), the contact angles between the dentin surface and 3 organic solvents of distinct polarities (water, formamide and diiodomethane) were recorded. Surface morphology (n = 3) was analyzed with the use of scanning electron microscopy (SEM). The data was statistically analyzed using the two-way analysis of variance (ANOVA) (α = 0.05).
Results. Neither 2.5% P-Chi nor 0.5 mol/L EDC influenced the dentin surface wettability (p > 0.05). Surface free energy decreased in the eroded substrate after biomodification with EDC (p < 0.05). Biomodification with P-Chi demineralized the dentin surface and increased the dentin tubule embouchure.
Conclusions. It can be concluded that 2.5% P-Chi and 0.5 mol/L EDC did not impact the surface wettability of the eroded dentin. However, EDC promoted lower surface free energy, while P-Chi altered surface morphology, causing demineralization and the opening of dentin tubules.
Keywords: chitosan, carbodiimides, tooth erosion
Introduction
Dental erosion can result from the prolonged contact of dental tissues with the acidic substances originating from intrinsic factors, extrinsic factors, or a combination of both.1, 2, 3 It is characterized by a chemical process that does not involve bacterial action. From a clinical point of view, this process is defined by the progressive and irreversible loss of the tooth structure through mineral dissolution, culminating in tissue demineralization.4 Depending on the severity of the lesion, erosion may lead to dental hypersensitivity (DH),5, 6 as well as functional and esthetic damage.
The state of insecurity experienced during the coronavirus disease 2019 (COVID-19) pandemic, mainly due to stress, caused muscular hyperactivity, and the exacerbation of bruxism and temporomandibular disorders (TMD),7 increasing their prevalence not only in adults, but also in children and adolescents.8 Bruxism causes a type of pathological wear of the tooth structure due to the clenching and/or grinding of the teeth, and it is another condition that can aggravate the effects of dental erosion. Oral health conditions, such as TMD, bruxism and/or dental erosion, can significantly affect an individual’s oral health-related quality of life (OHRQoL). This may have a negative impact on the physical, social and psychological aspects of a person’s overall well-being.9
What also affects OHRQoL is DH, which is often caused by the exposure of dentin due to erosion, especially in younger patients.10 It is recommended to adopt healthier dietary practices, including reducing or avoiding acidic foods and beverages, as well as destructive parafunctional behaviors, such as tooth clenching and/or grinding, which can lead to the erosive loss of the tooth structure, and should be discouraged in order to prevent or relieve DH.11
Dental erosion causes damage to the tooth structure, leading not only to mineral dissolution, but also to the enzymatic degradation of collagen. This can affect the quality of the collagen network of the hybrid layer, which is a major challenge to overcome in adhesive dentistry,12, 13 as it impacts the long-term integrity of the dentin-bonded interfaces.14 It directly determines the success of composite restorations, which are the materials of choice in dental practice, being based on minimally invasive approaches,15, 16, 17 and being resistant to erosion from intrinsic and extrinsic acids.18
To improve the quality and longevity of adhesive restorations, various studies have evaluated the use of biopolymers, such as chitosan and carbodiimide, to increase the number of cross-links between collagen fibers and neutralize matrix metalloproteinases (MMPs),19, 20, 21 thereby increasing the resistance of the water-rich collagen matrix to enzymatic degradation and preserving the integrity of the hybrid layer.22, 23
Cross-linking with the use of biomedical agents is one of the strategies used in dentin biomodification.24, 25, 26 Chitosan is a biopolymer obtained by the alkaline deacetylation of chitin; it can be used as a biomodifier increasing the mechanical strength of collagen, which is the main component of dentin, and the resistance of collagen fibrils, which are used as a support to form adhesive interfaces, to hydrolytic and enzymatic degradation.22, 23, 27, 28, 29 Chitosan is biocompatible, with its low tissue toxicity, potent antimicrobial activity and chelating capacity.30, 31, 32 Dentin treatment with chitosan increases immediate bond strength and improves adhesive infiltration in healthy dentin.20, 33, 34
Chitosan is an important natural polymer used not only in medicine, but also in dentistry, having a wide range of applications.35 It is currently employed for tissue engineering scaffolds to regenerate the dentin–pulp complex,36 and bone regeneration due to its biocompatibility, biodegradability, osteoconductivity, and affinity for biomolecules.37 It can also be added to dental materials, such as glass ionomer cements, which may increase cell viability,38 chitosan-based drugs in the endodontic treatment of root canals due to its anti-inflammatory, antifungal and antiseptic properties,39 and the experimental solutions of mouthwashes, which could reduce dentin erosion.40, 41
The incorporation of new chitosan derivatives by using chemical products, known as biomodification, has been investigated. Biomodification does not alter the functional backbone of chitosan, and retains the original physicochemical and biochemical properties of the biomodifier while providing a matrix with new properties with respect to the added products.42 Phosphorylated chitosan (P-Chi) derivatives are promising inducers of accelerated resistance,43 as they induce calcium phosphate mineral deposition on the partially demineralized dentin surfaces, resulting in low interfacial energy and facilitating dentin surface remineralization.44 In addition to their proven ability to remineralize the dentin surface, P-Chi derivatives have a high antimicrobial potential.45
Carbodiimide (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, or EDC) is an isomeric cyanamide that can aggregate amino acids into peptides without incorporating other groups during the binding process. Carbodiimide has low cytotoxicity, and its mechanism of action involves the formation of covalent peptide bonds between proteins to activate the carboxy-free groups in glutamic acid and aspartic acid present in protein molecules.46, 47, 48 These covalent peptide bonds generate O-acylisourea, which reacts with the epsilon amino group of lysine or hydroxylysine present in the nearest polypeptide chain to form covalent amide bonds,49, 50 resulting in urea as the only residual product, which is easily disposed of with water. These chemical interactions provide EDC with increased collagen strength and assist in the inhibition of protease activity.33, 44
Dentin treatment with 0.5 mol/L EDC can prevent the degradation of the resin–dentin adhesive interface for up to 12 months, especially when the biomodifier is applied for 60 s, which prevents the degradation of collagen by MMPs.49 Cadenaro et al. demonstrated that EDC-treated dentin collagen denatured at higher temperatures than the untreated control at the concentrations tested (0.5 M and 1.0 M) during immersion for 10 min or longer.51 This indirectly indicates a more resistant and highly cross-linked collagen network. Another advantage of EDC is that this non-specific agent acts on a broad spectrum of collagenase-like enzymes (MMPs, cathepsins and others), thus eliminating the need to use different types of agents for each of these enzymes.12, 23, 25
Given the properties of the P-Chi and EDC solutions, they can be used to improve the biomechanical and biochemical properties of dentin, thereby reducing the biodegradation of this tissue and maintaining bond strength over time.20, 22, 26, 42, 52 The aim of the present study was to evaluate the effect of 2.5% P-Chi and 0.5 mol/L EDC on the surface wettability, surface free energy and surface morphology of the eroded dentin.
The null hypothesis tested was that the biomodification of the eroded dentin with 2.5% P-Chi or 0.5 mol/L EDC would not affect the surface wettability, surface free energy or surface morphology of the eroded dentin.
Material and methods
Experimental design
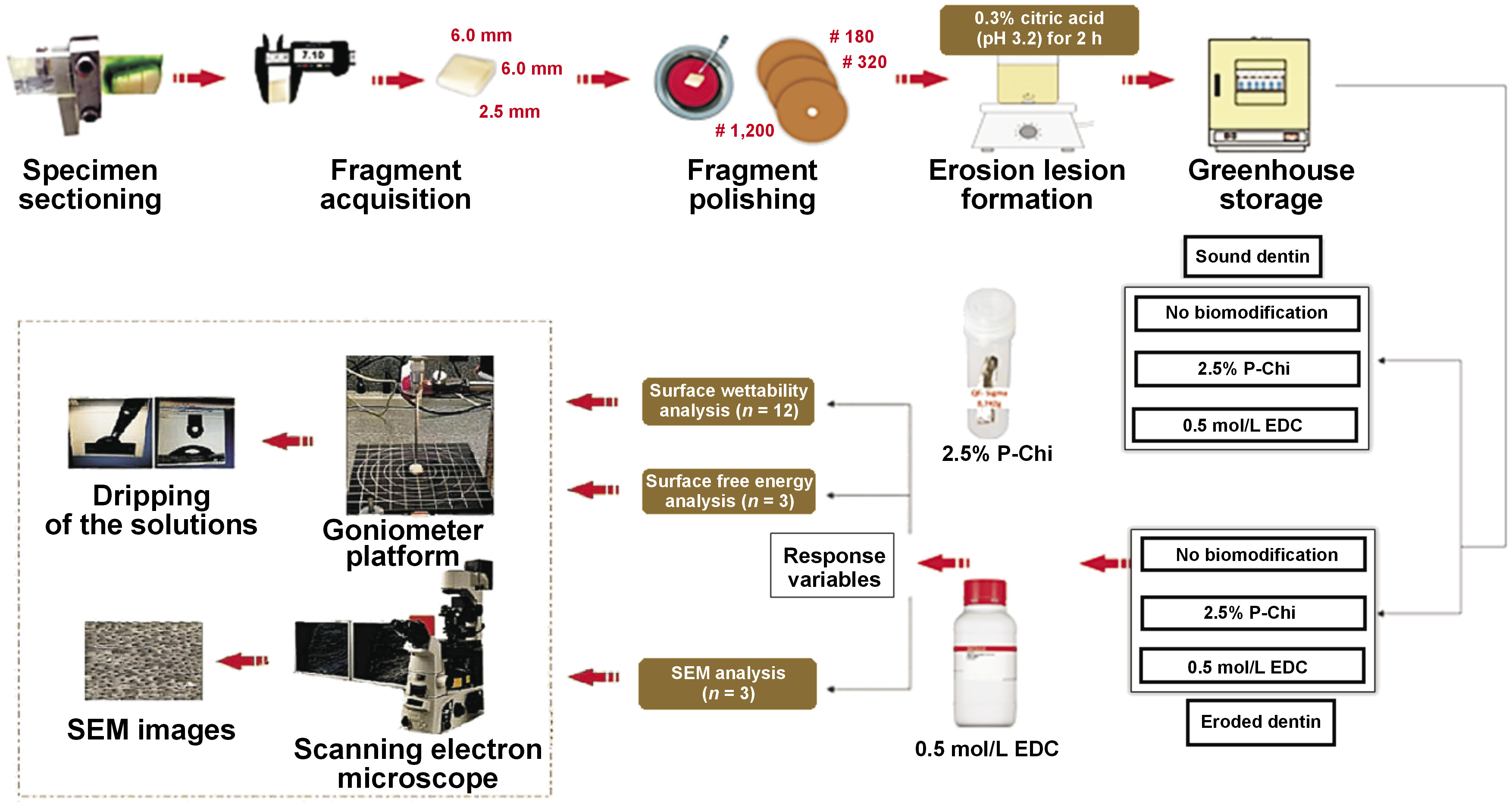
The factors studied were the dentin substrate at 2 levels (sound or eroded) and surface treatment at 3 levels (2.5% P-Chi, 0.5 mol/L EDC or no biomodification (control)). The experimental sample consisted of 144 bovine incisors from the meat-processing industry that would otherwise be discarded. The response variables were as follows: surface wettability, measured as the contact angle between the adhesive system and the dentin surface (n = 12) – quantitative variable; surface free energy, measured as the contact angle between the dentin surface and 3 organic solvents of different polarities (n = 3) – quantitative variable; and surface morphology, analyzed by means of scanning electron microscopy (SEM) (n = 3) – qualitative variable. A flowchart of the study is presented in Figure 1.
Tooth selection
Bovine incisors, previously preserved in distilled water, were examined microscopically under a magnifying glass (Leica Microsystems, Wetzlar, Germany) at ×20 magnification. Incisors without fracture lines or crown-deep cracks were selected for the study (N = 144).
Sample preparation
The teeth were sectioned transversely at the cementoenamel junction (CEJ) to separate the crowns from the roots using a double-faced diamond disk (15HC 11-4244; Buehler, Lake Bluff, USA) mounted on a low-speed handpiece (IsoMet® 1000; Buehler). The crowns were then sectioned in the mesial-distal direction, providing 2 hemi-sections of each crown (buccal and palatal). A dentin fragment with dimensions of 6.0 mm × 6.0 mm × 2.5 mm was obtained from the vestibular hemi-section for all analyses.
The dentin fragments were fixed in Teflon® matrices, using fused wax (Kota, São Paulo, Brazil), with the enamel surface downward. The dentin surface was then polished with Arotec APL-4 polyester (Arotec, Cotia, Brazil), water-cooled and polished with #180–320-grit sandpaper (Hermes Abrasives, Virginia Beach, USA) to adjust the fragment size. Each dentin specimen was subsequently polished with #1,200 grit sandpaper (Hermes Abrasives) for 10 s to flatten the dentin surface. Finally, the specimens were polished with 0.3-micrometer alumina paste (Arotec) on the polishing felt (ATM, Altenkirchen, Germany) for 5 s.
Erosion lesion formation
A protocol established by Vanuspong et al. was used to induce the formation of erosion lesions.53 Each dentin specimen from a group of 72 (half of the specimens) was immersed in 20 mL of 0.3% citric acid (pH 3.2) and placed on a shaker table (CT-155; CIENTEC Equipamentos Científicos, Belo Horizonte, Brazil), where it remained under constant stirring at 50 rpm for 2 h. Afterward, the specimens were washed with distilled water, stored individually in Eppendorf tubes containing artificial saliva and kept in an oven at 37°C for 24 h. Artificial saliva consisted of methylparaben (2.0 g), sodium carboxymethylcellulose (10.0 g), KCl (0.625 g), MgCl2•6H2O (0.059 g), CaCl2•2H2O (0.166 g), K2HPO4 (0.804 g), and KH2PO4 (0.326 g) in 1,000 mL of distilled water, according to the protocol described by McKnight-Hanes and Whitford,54 and modified by Amaechi et al.55
Experimental groups
A total of 144 dentin specimens were randomly divided into 6 groups (n = 12), with 24 specimens per group: sound dentin – no biomodification; sound dentin – P-Chi; sound dentin – EDC; eroded dentin – no biomodification; eroded dentin – P-Chi; and eroded dentin – EDC.
Dentin surface treatment with P-Chi
Dentin surface biomodification with P-Chi was carried out according to the procedure described by Wang and Liu.43 First, the P-Chi solution was prepared by adding 2.5 g of low-molecular-weight chitosan (Sigma-Aldrich, Saint Louis, USA) (75–85% deacetylation), 5 g of urea and 10 mL of phosphoric acid to 40 mL of dimethylformamide (DMF). The mixture was stirred at 150°C for 1 h and filtered. The precipitate was thoroughly washed with distilled water and anhydrous ethanol, and dried under vacuum. The mixture was slowly added to 100 mL of a 1% acetic acid solution under magnetic stirring (Marconi Equipment Laboratories, Piracicaba, Brazil) for 1 h, which was sufficient to solubilize the polysaccharide. Next, 20 μL of 2.5% P-Chi was applied to the dentin surface with a micropipette for 60 s. The surface was then dried with absorbent paper.
Dentin surface treatment with the EDC-HCl solution
The EDC-HCl solution (Sigma-Aldrich) was prepared immediately before use. To obtain an EDC concentration of 0.5 mol/L, 2.3 mg of EDC-HCl was diluted in 230 μL of Milli-Q® water to give a solution with pH 7.34. Then, 20 μL of 0.5 mol/L EDC was applied to the dentin surface for 60 s. The surface was then washed with distilled water for 15 s and dried with absorbent paper.
Surface wettability analysis
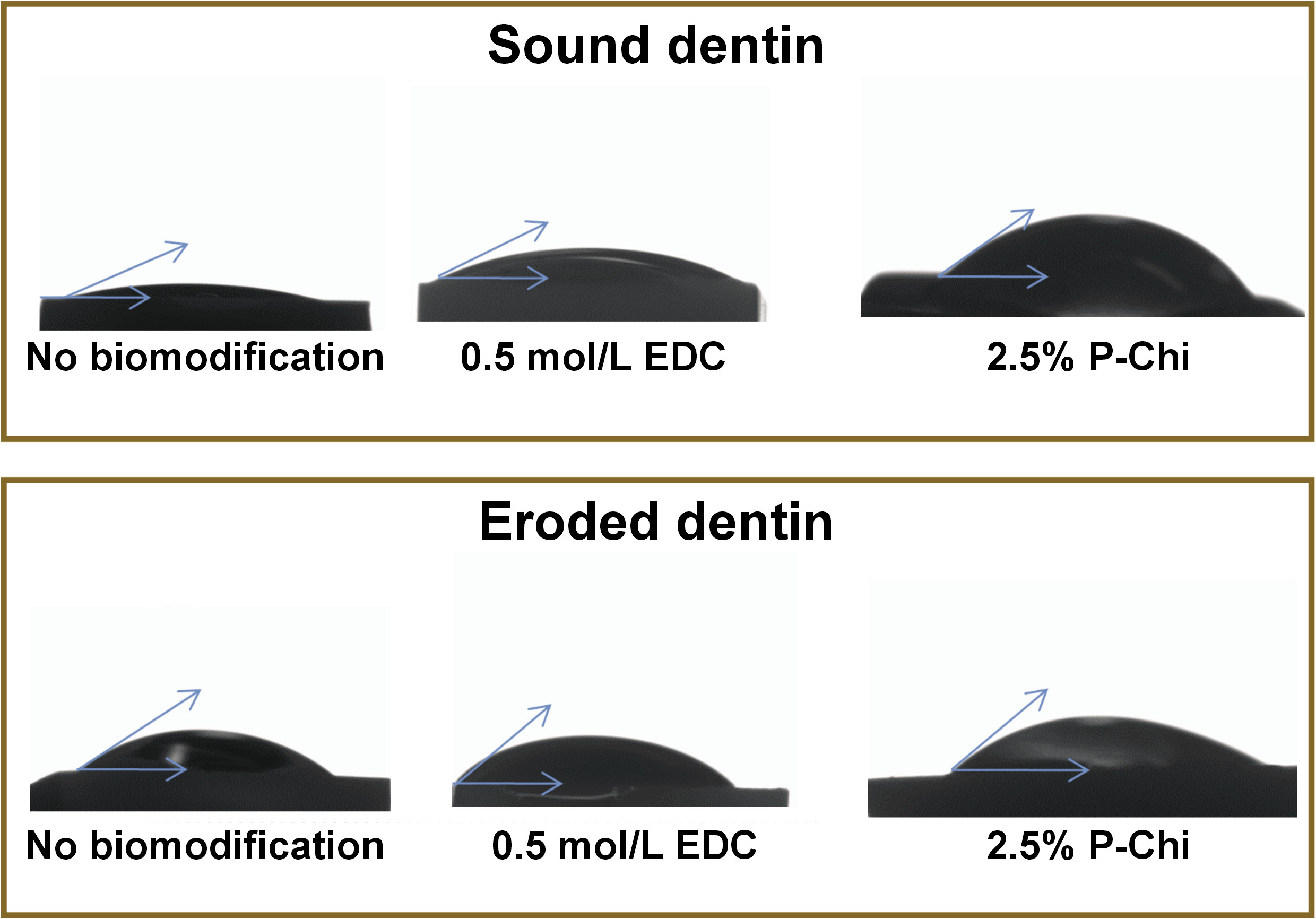
Dentin wettability was determined by measuring the contact angle (Θ), using the sessile drop method with a goniometer (OCA 20; DataPhysics Instruments, Filderstadt, Germany). Each specimen was positioned on a movable platform with leveling screws. A 20-microliter drop of an adhesive (Scotchbond™ Universal adhesive; 3M Germany, Neuss, Germany) was then placed onto the specimen and the drop image of the dentin surface was captured for 2 min at intervals of 1 ms, using a lighting system equipped with a tungsten lamp and a charge-coupled device (CCD) camera. A microcomputer processed the image, and the tangent formed between the drop and the surface was adjusted to determine the Θ values by using the SCA20 software (DataPhysics Instruments). The Θ values were analyzed using the SCA20 program. All procedures involving the Θ measurements were carried out indoors at a controlled ambient temperature of 25 ±1°C.
Surface free energy analysis
Three liquids of different polarities were used to analyze surface free energy: water (a polar universal solvent); formamide (of intermediate polarity); and diiodomethane (highly apolar).
The values for formamide and diiodomethane were used to calculate surface free energy and its components. However, only the water contact angle values were required to explain the surface state.
The contact angle was measured with a goniometer (OCA 20; DataPhysics Instruments), using the sessile drop method. Each sample was positioned on a movable platform with leveling screws. A 20-microliter drop of each liquid (water, formamide or diiodomethane) was then dropped onto the specimen. In the case of formamide and diiodomethane, a micropipette was used to deliver the drop onto the dentin surface; in the case of water, the drop was delivered directly from the goniometer needle. Surface free energy (γS) was calculated from the Θ values regarding the contact angles between the liquids of different polarities and the solid surface. The Owens–Wendt 1969 formula was used to separate the total surface energy into its disperse (d) and polar (p) components (Equation 1):
For each liquid with γL, and its dispersive (γLd) and polar (γLp) components, Θ was measured between the liquid itself and the sample. The γS value is obtained as the sum of its dispersive (γSd) and polar (γSp) components.
Specimen preparation for SEM
The previously prepared specimens (n = 3) were placed in Eppendorf tubes containing distilled water, and prepared according to the following protocol: ultrasonic cleaning (Ultrasonic Cleaner T-1449-D; Odontobrás, Ribeirão Preto, Brazil) for 10 min; drying with absorbent paper; immersion in a 2.5% glutaraldehyde solution buffered with a 0.1 M sodium cacodylate solution, pH 7.4 (Merck, Darmstadt, Germany), at 4°C for 12 h; washing with distilled water for 3 min, followed by immersion in distilled water for 1 h, changing the water every 20 min; and dehydration in the ascending gradations of ethanol (Labsynth Products Laboratories, Diadema, Brazil) – 25% (20 min), 50% (20 min), 75% (20 min), 95% (30 min), and 100% (60 min). After dehydration, the specimens were immersed in the hexamethyldisilazane (HMDS) solution (Merck) for 10 min for chemical drying. All procedures were performed under a fume hood. After drying, the specimens were fixed in carbon double-sided tape stubs and coated with gold in a vacuum metallization apparatus (SDC 050; Bal-Tec, Balzers, Liechtenstein). The specimens were examined under a scanning electron microscope (XL30 FEG; Philips, Eindhoven, the Netherlands) at the Chemistry Laboratory of the Faculty of Philosophy, Sciences and Letters, University of São Paulo, Ribeirão Preto, Brazil.
The entire surface of the specimens was scanned, and the most representative area of each group was photographed at a single magnification of ×1,500.
Statistical analysis
The Θ values obtained during the surface wettability analysis were subjected to the Kolmogorov–Smirnov normality test, and the data was shown to be normally distributed. The data was then subjected to the analysis of variance (ANOVA) for 2 factors – surface treatment and the dentin surface, with a significance level of 5%. Statistical analysis was performed using the SPSS for Windows, v. 12.0 (SPSS Inc., Chicago, USA).
Results
Table 1 shows the lowest contact angle values obtained based on the surface wettability analysis. ANOVA did not reveal any statistically significant differences between various kinds of treatment (p > 0.05). However, biomodification with 0.5 mol/L EDC reduced surface free energy as compared to the control group (p < 0.05). The mean surface free energy values ranged from 39.01 mJ/m2 for the EDC-modified group to 49.23 mJ/m2 for the control group (Table 2).
Figure 2 shows a schematic representation of the contact angle measurements.
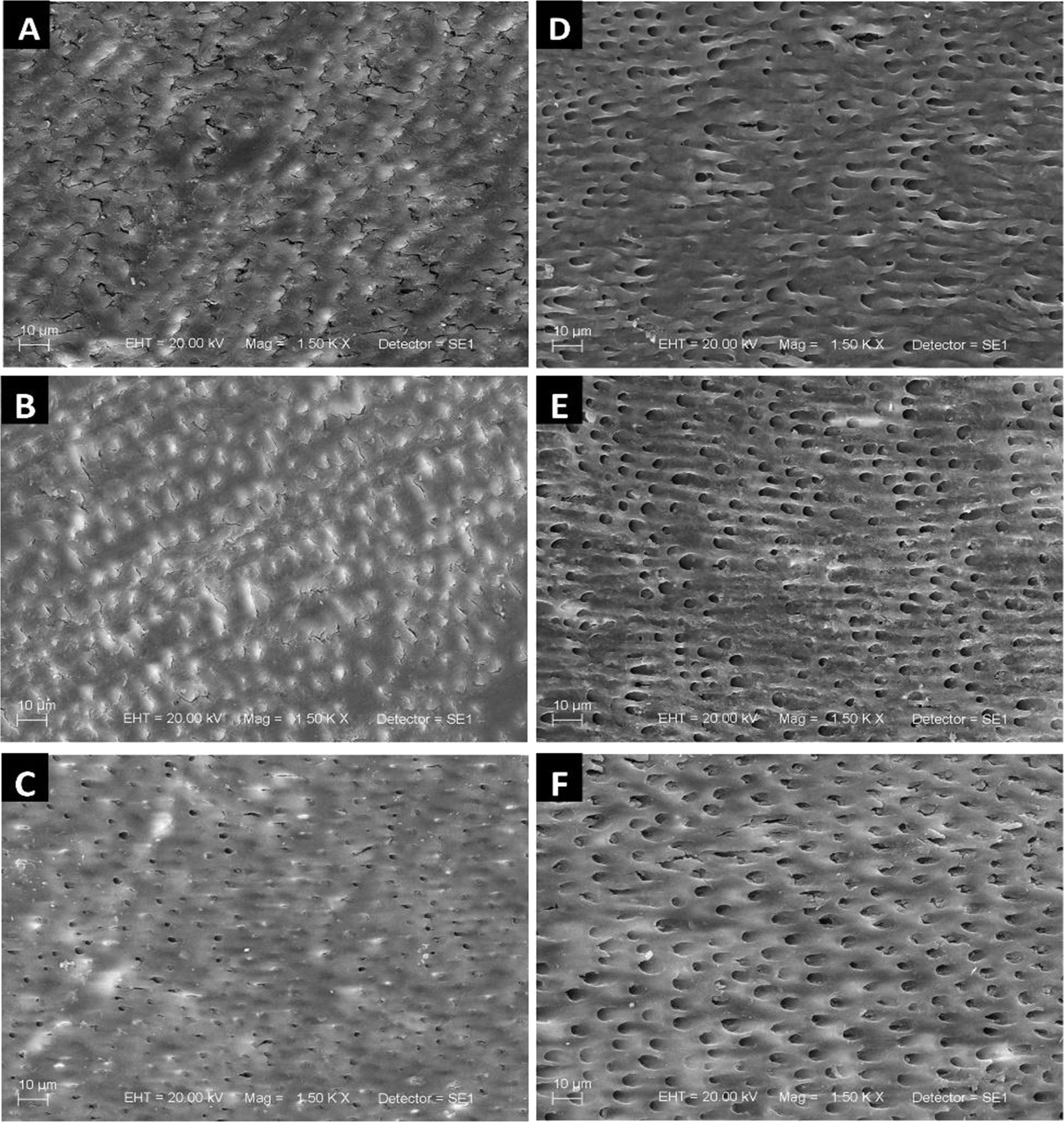
Figure 3 shows the SEM images of the sound and eroded dentin after biomodification, and with no biomodification (control). The sound dentin showed a smear layer and the dentin tubules occluded by peritubular dentin deposits, as well as irregular mineral deposition in intertubular dentin (sclerotic molds) (Figure 3). After biomodification with EDC, dentin tubules remained obliterated (Figure 3). Biomodification with P-Chi resulted in partially visible dentin tubules (Figure 3). The eroded dentin showed a demineralized organic matrix surface, with no smear layer and open dentin tubules (Figure 3). Surface treatment with EDC promoted a demineralization pattern similar to that of the non-biomodified group (control), but dentin tubule embedding was slightly increased (Figure 3). Surface treatment with P-Chi induced surface demineralization and slightly increased dentin tubule embedding, possibly due to greater peritubular dentin removal (Figure 3).
Discussion
The use of the chitosan solution has shown favorable results in remineralizing the exposed structure of the demineralized dentin30 and appears to be effective due to its ability to cross-link with dentin type I collagen, which provides protection against enzymatic degradation.31 Similarly, biomodification with EDC improves the collagen structure and adhesion to it by inactivating dentin MMPs.25
Chitosan and EDC are biopolymers widely studied in dentistry, but there are also other natural agents with applications in this field, namely, the propolis extract with cancer-selective toxicity and an anti-inflammatory effect on tongue cancer cells,56 or as a promising substitute for synthetic remineralizing and antibacterial agents, acting on deep carious dentin,57 as well as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) as scaffolds in the process of tissue regeneration, the treatment of intrabony defects and regenerative endodontic treatment.58 In addition, in organic toothpaste formulations, it is possible to use hectorite (natural clay) as an emulsifier because of its excellent absorption capacity, the Commiphora myrrha resin extract that functions as a flavoring agent and a natural antiseptic, among many other natural ingredients, such as Stevia rebaudiana, erythritol or xylitol, which have no associated adverse events.59
The biomodification of the dentin matrix improves its biochemical and biomechanical properties, with benefits for preventive and restorative treatment.26, 46 In the present study, the analyzed biomodifiers did not affect the surface wettability of the eroded dentin. This result is in agreement with a study by Ururahy et al., in which no statistically significant differences in wettability were observed after biomodification with chitosan at different concentrations,60 but partially different from the observations of Curylofo-Zotti et al.61 In the latter study, when chitosan was used, there was a change in the wettability of the remaining dentin after the selective removal of carious lesions with an erbium-doped yttrium aluminum garnet (Er:YAG) laser, whereas with EDC, there was no change in the dentin wettability.61 Rehumidification without affecting the wettability of the dentin surface is favorable, since adhesion depends on the flow of the adhesive system on the surface. In this way, we would achieve the positive effects of biomodification (antimicrobial and chelating properties) without losing adhesion.
In terms of surface free energy, it was demonstrated that surface biomodification with 0.5 mol/L EDC promoted a decrease in free energy as compared to the unmodified surface. The total surface free energy is the sum of its disperse and polar components. Changes in these components result in different interactions between the surface and the liquid (biomodifier). The dispersive component favors London-type interactions (dipole-induced or Van der Walls forces) between non-polar molecules, whereas the polar component favors electrostatic, metallic and dipole–dipole interactions between polar molecules.62 Therefore, these intermolecular interactions, in addition to anion co-adsorption and hydrogen bonding between the surface and the protein, are important factors contributing to the overall interaction.62, 63 The higher the surface free energy, the higher the surface propensity to chemical reactions. In terms of biological environment, high surface free energy values imply a high surface propensity to bind biomolecules.
Surface free energy is related to the chemical groups present on the surface of the sample and, consequently, to the nature of the surface molecular interactions. The polar and non-polar groups of proteins are expected to interact selectively with different surfaces (sound and eroded, modified or not). In the present study, the decrease in surface free energy promoted by biomodification with 0.5 mol/L EDC was due to a decrease in the polar component and a slight increase in the disperse component, indicating an increase in the surface affinity, which was caused by non-polar substances after biomodification with EDC. From a chemical point of view, the fact that EDC showed a higher affinity in the eroded substrate than in the sound substrate could be related to the hydrogen bonding interactions between EDC and the eroded substrate. The treatment of the dentin substrate with diluted acid (in this case, citric acid) promotes the acidic hydrolysis of collagen molecules into amino acid residues, generating amino (-NH2) and carboxyl (-COOH) groups that are hydrogen bond donors to the nitrogen atom of the tertiary amine of the EDC structure. Consequently, the ‘non-polar’ (less polar) moiety of EDC would be exposed to the substrate surface, thus justifying the decrease in surface free energy by decreasing its polar component and increasing it disperse component. Therefore, the mechanism of action of EDC is expected to involve collagen and occur via intermolecular interactions.
Regarding surface biomodification with 2.5% P-Chi, there is a strong ion–ion interaction between the hydroxyapatite phosphate anions and the cationic moiety of P-Chi (NH2+ group), which is protonated when chitosan is solubilized in acetic acid. Consequently, the polar moiety of the P-Chi structure would remain on the substrate surface, which from a chemical point of view would justify the lack of the influence of 2.5% P-Chi on surface free energy. Therefore, the mechanism of action of P-Chi is via ionic interactions and must involve hydroxyapatite. Based on this explanation, the 2.5% P-Chi treatment was more efficient than the 0.5 mol/L EDC treatment in terms of both surface wettability and surface free energy.
The SEM images showed that the 2.5% P-Chi treatment was more effective in removing the smear layer and increasing the diameter of dentin tubules. This superficial modification is favorable, since chitosan has antimicrobial activity and could contribute to tubule antisepsis in addition to facilitating the flow of the adhesive system.
Limitations
Although the texture and fractal dimension analyses (TA and FD, respectively) have been used in dentistry to evaluate the properties of the surrounding tissues, as well as the incorporated materials,64, 65 the authors of the present study did not have the possibility to use these and other methods of analysis besides SEM images. Another limitation is that, even though the surface wettability, surface free energy and surface morphology analyses were conducted, a long-term bond strength test was not performed. Therefore, it is suggested that further studies be conducted using adhesive bond tests, along with TA and FD, with a long-term follow-up to better evaluate the bond strength with these biomodifiers.
Conclusions
It can be concluded that 2.5% P-Chi and 0.5 mol/L EDC did not impact the surface wettability of the eroded dentin. However, EDC promoted lower surface free energy, while P-Chi altered surface morphology, causing demineralization and the opening of dentin tubules.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.