Abstract
Background. Temporomandibular disorders (TMD) constitute a heterogeneous group of conditions affecting the temporomandibular joints (TMJs) and the surrounding tissues. The etiology of these anomalies has been extensively discussed due to their multifactorial and diverse nature; however the influence of many factors remains ambiguous. Temporomandibular disorders may be manifested as pain or in a painless form, characterized by acoustic symptoms in TMJs or their dysfunction. The presence of a causal relationship between the use of medications for chronic diseases and TMD symptoms can simplify the diagnostic and therapeutic approach in TMD treatment.
Objectives. The aim of the study was to verify the existence of and assess the correlation between the usage of pharmacotherapy in chronic diseases and the occurrence and severity of TMD symptoms.
Material and methods. This retrospective study was based on the analysis of 252 questionnaires completed by patients who had previously reported to the University Dental Clinic in Krakow, Poland, due to the occurrence of TMD symptoms. The patients were categorized into 4 subgroups, depending on the type of drugs taken: endocrine; cardiological; psychotropic; and other. Data was subjected to statistical analysis.
Results. In the tested group, an association between the usage of endocrine drugs and the risk of headaches was observed. The patients taking cardiological drugs exhibited a reduced likelihood of experiencing difficulties in opening the mouth wide as compared to those under treatment for other reasons. However, no significant impact of the drugs on the intensity of TMD pain symptoms was observed. Furthermore, no correlation was found between the medications taken and the occurrence of clicking in TMJ and behaviors in the form of clenching and/or grinding of the teeth.
Conclusions. The pharmacotherapy of chronic diseases in TMD patients might be associated with an increased risk of headaches. Nonetheless, there were no statistically significant differences between the types of drugs taken with regard to the intensity of TMD pain symptoms, as well as the presence of clicking and behaviors in the form of clenching and/or grinding of the teeth.
Keywords: pharmacotherapy, temporomandibular disorders, TMD, general diseases
Introduction
Temporomandibular disorders (TMD) are a heterogeneous group of disorders concerning temporomandibular joints (TMJs) and the surrounding structures.1 The prevalence of TMD in the general population is estimated at around 40%.1 In young adults, the prevalence of TMD symptoms varies from 43% up to 60%.1 The prevalence of TMD in children and adolescents varies from 16% to 68%.2 They are the 2nd most common cause of craniofacial pain.1, 3, 4, 5, 6 The peak incidence is between the age of 20 and 40. It is more common in women than in men, with a male-to-female ratio ranging from 1:2 in the general population to as high as 1:8 clinically,3, 7, 8 especially in the reproductive age.8, 9 Not only the frequency, but also a higher severity of symptoms is correlated with female gender.10 The prospective OPPERA (Orofacial Pain: Prospective Evaluation and Risk Assessment) study showed that an average of 4 new cases of TMD per 100 people develop each year.11
Due to their multifactorial nature, the etiology of TMD is still not fully elucidated. Modulators can be biological (e.g., internal derangements in TMJ), environmental, social (e.g., a learned response to pain), emotional, and cognitive elements (e.g., depression, anxiety and stress).1, 3, 6 A significant influence of genetic, inflammatory and psychoemotional determinants has been proven.12
The OPPERA study clearly showed that poor general health increases the risk of developing TMD.11 Patients with a subjective sense of impaired health are more likely to suffer from TMD symptoms. Temporomandibular disorders often coexist with other medical conditions. A significant correlation has been shown for headaches, including migraine, and psychoemotional disorders, in particular depression. Rheumatic joint diseases may also affect TMJs or predispose to their dysfunction.10 A cross-sectional study conducted in Korea showed a high level of comorbidity of TMD with asthma, migraine, osteoarthritis, and thyroid disorders.13 The painful form of TMD is often correlated with the presence of the coexisting ache in other areas of the body, such as chronic back pain, abdominal pain, migraine, and others.14
The increase in muscle tone, which is one of the causes of TMD, is influenced, among others, by systemic factors, i.e., hormonal disorders, thyroid diseases, metabolic disorders, migraine, immunological diseases, rheumatoid and degenerative diseases, genetic factors, and many others.3
Symptoms of TMD can be either painful or painless.15 The painful form is characterized by the presence of craniofacial complaints; it affects TMJs, the masticatory muscles, and the head and neck muscles. Typical symptoms include TMJ pain (limited or abnormal abduction of the jaw), and ear, head or face pain. Symptoms can range from mild discomfort to debilitating pain, limiting the jaw function.3 Patients most commonly report feeling tense, spontaneous pain at rest, or muscle ache associated with chewing. The painless symptoms of TMJ dysfunction, occurring locally, are primarily increased masticatory muscle tone, a feeling of muscle stiffness and numbness, the asymmetry of their action, and paresthesia in trigger points. The disorder of the mandible function, especially its limited and incorrect abduction, leads to the unsynchronized work of both TMJs. Reported complaints include clicking in TMJ, which occurs during mandibular abduction and adduction, as well as during protrusion and lateral movements. In advanced stages, some patients complain of the deterioration of their general mental and physical condition as a result of nagging headaches, sleep disorders or poor movement coordination.15
Patients most often seek help because of pain. Excessive muscle tension is predominantly associated with increased stress and subsequent behaviors that allow to relieve emotional tension, such as the clenching and/or grinding of the teeth.12, 16 According to the OPPERA data, the pain intensity level of new TMD cases was rated as mild or very mild.11 The average pain intensity in the TMD population on a scale of 1–10 was 4.6.7 Pain amplification may be modulated by genotypic and phenotypic factors.7, 8 The gender factor is an important determinant – clinical craniofacial pain symptoms are more common in women, they last longer and are more severe.7, 8 Women are also more likely to experience comorbid and multitarget pain.17 It is important to notice that the occurrence of general health conditions also exhibits variability within the population. According to the Migraine in Poland study, headaches tend to occur more often in women (87.1%) than in men.18
Standardizing diagnostic and therapeutic procedures, and creating protocols of medical action are important from the perspective of combating such a complex phenomenon as TMD.19
The existence of numerous presumptions about the impact of general health and the medications taken on the occurrence of TMD was the motivation to conduct our research. The objective of the present study was to verify and assess the correlation between the medications taken for diagnosed chronic diseases and the occurrence and intensity of pain symptoms of TMD among patients admitted to the dental clinic. Establishing specific correlations can be very helpful in clinical practice. Conducting a clinical examination, supported by a thorough medical history could facilitate diagnosis in such cases, and consequently enable the implementation of effective treatment to alleviate the symptoms.19 The presence of a causal relationship between the use of medications for chronic diseases and TMD symptoms can simplify the diagnostic and therapeutic approach in TMD treatment. To the best of our knowledge, no comprehensive study investigating this issue has been conducted so far. Therefore, we aimed to investigate whether the aforementioned correlation exists.
Material and methods
Participants
The study was retrospective and consisted in the verification of the questionnaires completed by patients who had previously reported to the University Dental Clinic in Krakow, Poland, due to alleged TMD in the years 2016–2019.
The study involved individuals who presented at the dental clinic with symptoms related to the stomatognathic system, suggestive of TMD. The data was collected through a proprietary questionnaire, which included questions about general health condition and the most frequent symptoms associated with TMD. Each patient received the questionnaire to complete as part of the initial assessment during their first visit.
After analyzing the completed questionnaire and conducting a clinical examination based on the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD),20, 21 the attending dentist made a diagnosis and established the appropriate treatment. In a situation when a patient reported 2 or more TMD symptoms, each of them was considered separately in relation to the medications taken for comorbid conditions.
The inclusion criteria for the study comprised the presence of at least one TMD symptom. Incomplete questionnaires were excluded from the study.
The analysis included 252 patients; 2 questionnaires were rejected due to incorrect answers to the questions.
Questionnaire
The questionnaire consisted of a part concerning personal data (name, surname, age), a medical history (presence of general diseases, medications taken), and a section on TMD and related symptoms, which included the following issues:
1. What is the reason for reporting to the clinic?
2. What medications are taken regularly?
3. How severe is your current masticatory muscle pain on a scale from 1 to 10?
4. What is the severity of your current TMJ pain on a scale from 1 to 10?
5. What was the average intensity of masticatory muscle pain in the last 8 weeks?
6. What was the average intensity of TMJ pain in the last 8 weeks?
7. Have you ever had a problem with opening your mouth wide?
8. Have you experienced clicks in TMJ? If so, for how long?
9. Are there any other additional symptoms?
10. Do you have headaches; what is their intensity and frequency?
11. Are there tooth clenching and/or grinding behaviors observed?
Statistical analysis
A multivariate analysis of the effect of different types of drugs on the quantitative variable was performed by linear regression. The results are presented as the regression model parameter values with a 95% confidence interval (CI). A multivariate analysis of the effect of different types of drugs on the dichotomous variable was performed by logistic regression. The results are presented as the odds ratio (OR) values with a 95% CI. A significance level of 0.05 was adopted in the analysis, so all p-values below 0.05 were interpreted as statistically significant. The analysis was performed in the R program, v. 4.2.2 (https://www.r-project.org/).
Results
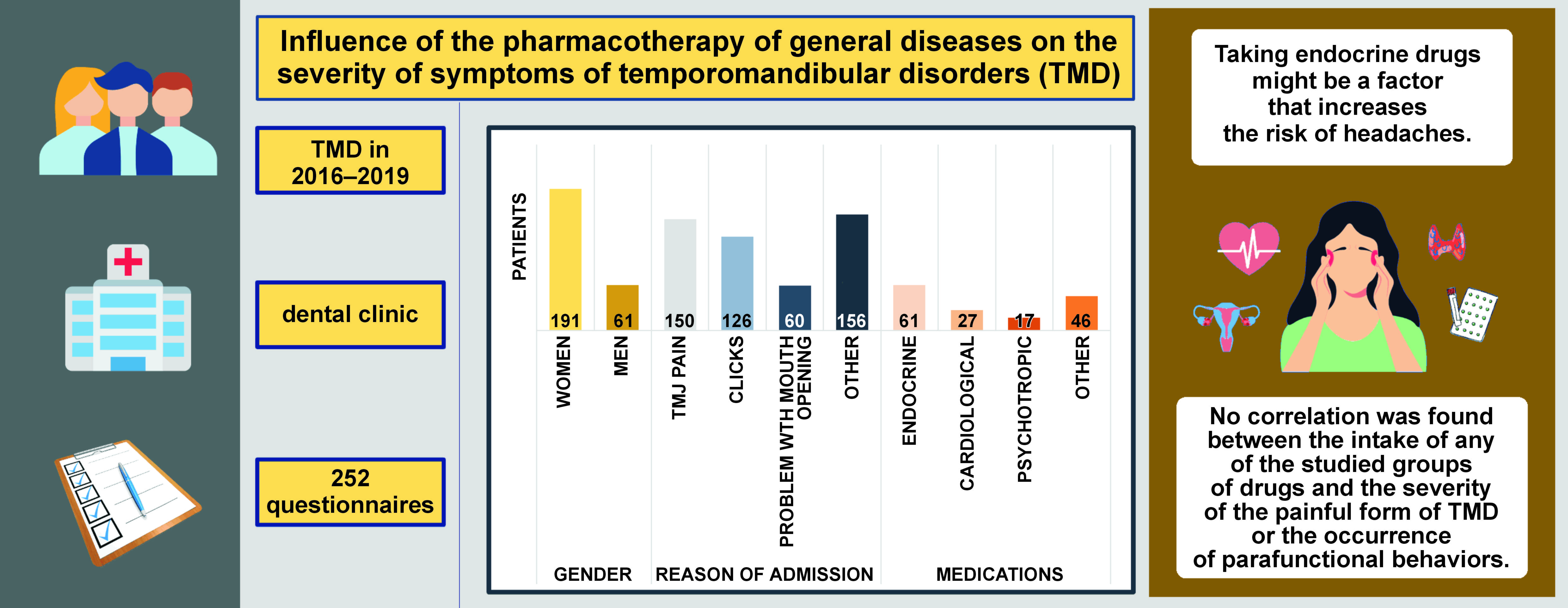
The study included 252 questionnaires. The majority of the respondents were women (76%), while men accounted for 24%. Age ranged from 18 to 42 years. Among the reasons for reporting to the clinic, TMJ pain was the most common cause (n = 150; 60%), followed by clicking in TMJ (n = 126; 50%) and limited mouth opening (n = 60; 24%).
The analysis of the questionnaires made it possible to distinguish 4 groups of drugs taken by the patients: endocrine (24% of patients); cardiological (11% of patients); psychotropic (7% of patients); and other (18% of patients). The emphasis was placed on the medications taken for chronic medical conditions, whereas analgesic medications were classified under the category of ‘other medications’ administered by the patients. The group of endocrine drugs administered by the patients included preparations used for thyroid function disorders (Levothyroxinum natricum) (46%) and contraceptives (23%).
Pain in the masticatory muscles
There was no statistically significant relationship between the drugs taken and the intensity of masticatory muscle pain in the study group (Table 1 and Table 2).
Temporomandibular joint pain
Statistical analysis showed that the intensity of TMJ pain was not affected by taking endocrine, cardiological, psychotropic, or other drugs (Table 3 and Table 4).
Headaches
The results of the analysis show that endocrine drugs are a significant independent predictor of the chance of headache occurrence, and the risk increases by 2.243 times. There was no statistically significant effect of taking cardiological, psychotropic and other drugs on the incidence of headaches (Table 5).
Problem with opening the mouth wide
An important independent predictor of the chance of having a problem with opening the mouth wide is taking cardiological drugs. In this group of patients, an 85.2% reduction in the chance of developing ailments was observed (Table 6).
Clicking
Taking endocrine, cardiological, psychotropic, and other drugs did not affect the occurrence of clicks.
Tooth clenching and/or grinding
There was no correlation between the intake of any of the tested groups of drugs and the occurrence of behaviors in the form of tooth clenching and/or grinding.
Discussion
The aim of the present study was to verify the existence of and assess the correlation between the applied general pharmacotherapy and the occurrence and intensity of subjective symptoms of TMD. Considering the study results, no connection between the use of drugs and the intensification of pain in TMJ can be found. The study showed that taking endocrine drugs increased the likelihood of headaches. No correlation was observed between the intake of any of the tested groups of drugs and the occurrence of clicks and behaviors in the form of clenching and/or grinding of the teeth. The patients taking heart medications had a lower chance of having a problem with opening the mouth wide as compared to those taking other classes of drugs.
Numerous studies have been conducted to determine the impact of various disease states on the occurrence of TMD. Hormones, as active substances, have a great impact on the body. Both thyroid hormones and female sex hormones have been proven to exert a significant influence on the metabolism of the tissues of the musculoskeletal and nervous systems, and pain conduction.
Thyroid hormones
Thyroid diseases are the most common endocrinopathies; they can affect even 2–5% of the population.22 Their incidence increases with age, concerning approx. 15% of the population aged 75 years or older.22 Thyroid diseases are much more common in women than in men.23
Thyroid hormones affect muscle contractility and metabolism. The main target of their signaling is the skeletal muscle, as exemplified by the myopathic symptoms observed in many patients with the thyroid dysfunction.24 Triiodothyronine (T3) is involved in the embryonic and subsequent development of the skeletal muscle by stimulating the growth of fibers. Hypothyroidism is manifested by delayed muscle contraction and relaxation, whereas hyperthyroidism is characterized by excessive muscle contraction.22, 25, 26
The painless forms of TMD include primarily increased masticatory muscle tone, muscle stiffness, the asymmetry of their functioning, and paresthesia. The forms of pain are pressure soreness and the presence of trigger points. The manifestation of thyroid diseases in the musculoskeletal system suggests that they may affect the occurrence and symptoms of the masticatory system dysfunction.22
Studies have reported that there is a clear correlation between the incidence of TMD, especially muscular disorders, and the number of patients with Hashimoto’s thyroiditis.25 The relationship between the occurrence of thyroid diseases and TMJ pain regards about 70% of patients.22
Hypothyroidism is often accompanied by musculoskeletal symptoms ranging from muscle and joint pain to true myopathy and osteoarthritis. It has been speculated that hypothyroidism may manifest itself in the musculoskeletal system in the form of TMD.27
Estrogens
Female sex hormones – estrogens – have a great impact on the functioning of the human body. Numerous studies have been conducted to clearly determine the cause of TMD sex determination, but this dependency is very complex. Since the discovery of estrogen receptors in TMJ in 1986, the potential influence of these hormones on the occurrence and course of TMD has been studied. In addition, they are also present in the nervous system, e.g., in the nucleus of the trigeminal nerve. The impact of estrogens varies greatly, depending on the type of signal and the current conditions, i.e., the presence of inflammation and its type; therefore it is difficult to define it unequivocally.9, 28
Various studies point to several conclusions:
– TMD incidence peaks at childbearing age28;
– the greatest intensity of pain in women of reproductive age occurs in the perimenstrual period, and if they take contraceptives, also during ovulation29;
– the use of contraceptives or hormone replacement therapy (HRT) is positively correlated with the occurrence of TMD, and the severity of symptoms increases with an increasing hormone dose28;
– women with TMD during pregnancy, when there is a significant increase in the estrogen levels, experience a decrease in pain symptoms as compared to non-pregnant women of the same age30, 31, 32;
– estrogen deficiency leads to subsequent structural alterations in the tissues of TMJ, which can lead to degenerative changes.9
Menopausal women are more likely than men of similar age to develop TMJ disease and age-related loss of the alveolar bone.32
Circulatory system
Contrary to the results of the current study, a clinical study conducted among children with cardiovascular diseases (CVD) showed a higher incidence of TMD in these patients than in healthy children, and it concerned acoustic symptoms.33 Notwithstanding, the accompanying pain was milder in the CVD group as compared to controls. The overall occurrence of parafunctional behaviors in both groups did not differ; however, a higher incidence of bruxism was confirmed in children with CVD. The study showed no significant impact of the presence of the main disease on the coexistence of TMD pain or the severity of symptoms.33
Psychoemotional condition
One of the important etiological factors contributing to the development of TMD are psychoemotional disorders, which include depression, dysthymia, panic attacks, anxiety states and neuroses, personality disorders, and sleep disorders.12, 34, 35, 36 Among the global population, 47% of adults suffer from headaches in general, 10% from migraine, 38% from tension-type headaches, and 3% from chronic headaches that last for more than 15 days per month.5 Temporomandibular disorders decrease patients’ quality of life (QoL), potentially limit their daily activities due to pain intensity and pain-related disability, and increase anxiety and depression.6 Chronic stress is a factor that adversely affects the functioning of the stomatognathic system, especially the masticatory muscles.12, 37 An increased level of emotional stress generates behaviors in the form of clenching and/or grinding of the teeth, which are very harmful to the masticatory system.12
Antidepressants administered to patients at risk of depression and/or migraine may cause headaches. Although some patients report clear benefits in terms of headache relief with the use of selective serotonin reuptake inhibitors (SSRIs), there have also been numerous reports of headache or migraine worsening as a result of SSRI use. On the other hand, serotonin-norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs) appear to have the opposite effect, potentially alleviating headaches.38
The potential role of cardiovascular medications in reducing headaches among individuals with migraine is currently under investigation. The first-line medications in this category include beta blockers without intrinsic sympathomimetic activity (such as atenolol, bisoprolol, metoprolol, or propranolol), as well as topiramate and candesartan.39
General medical data on past and ongoing general diseases and pharmacotherapy should be collected from the patient during the first visit, before taking further diagnostic and therapeutic steps. Determining the relationship between the occurrence of specific general disorders or the preparations used and the development and severity of TMD symptoms can significantly facilitate the diagnostic and therapeutic processes with regard to TMD.
There is no clear information in the literature confirming the influence of thyroid, heart and other general diseases on the development of TMD symptoms. It is necessary to conduct in-depth research in this field to determine the relationship between the considered phenomena.
Limitations
The main limitation of this study is its retrospective nature, which does not allow gathering more detailed information from patients; only the data collected in the past can be used. In addition, there was no thorough distinction between the drugs taken and the diagnosed general diseases; their duration and course, and the stage of drug application were not determined as well. The authors did not utilize a validated questionnaire in the study, which represents another limitation. Therefore, to ensure the reproducibility of results, it is recommended to employ a validated questionnaire in future studies. Another constraint is the lack of reference to gender and age in the statistical analysis.
Conclusions
There was no impact of endocrine, cardiological, psychotropic, and other drugs on the intensity of pain symptoms of TMD in the examined population, both currently experienced and their average value from the 2 months preceding the visit.
Taking endocrine drugs is a factor that increases the risk of headaches.
In the patients taking cardiological drugs, a decrease in the chance of having a problem with opening the mouth wide was observed as compared to those taking drugs from other groups.
No correlation was observed between the intake of any of the tested groups of drugs and the occurrence of clicks and behaviors in the form of clenching and/or grinding of the teeth.
Further exploration of these phenomena is needed to establish clear dependencies between systemic diseases and the occurrence of TMD.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.