Abstract
Background. Chronic systemic diseases and periodontal diseases have an impact on an individual’s quality of life. Both conditions exacerbate an individual’s health status.
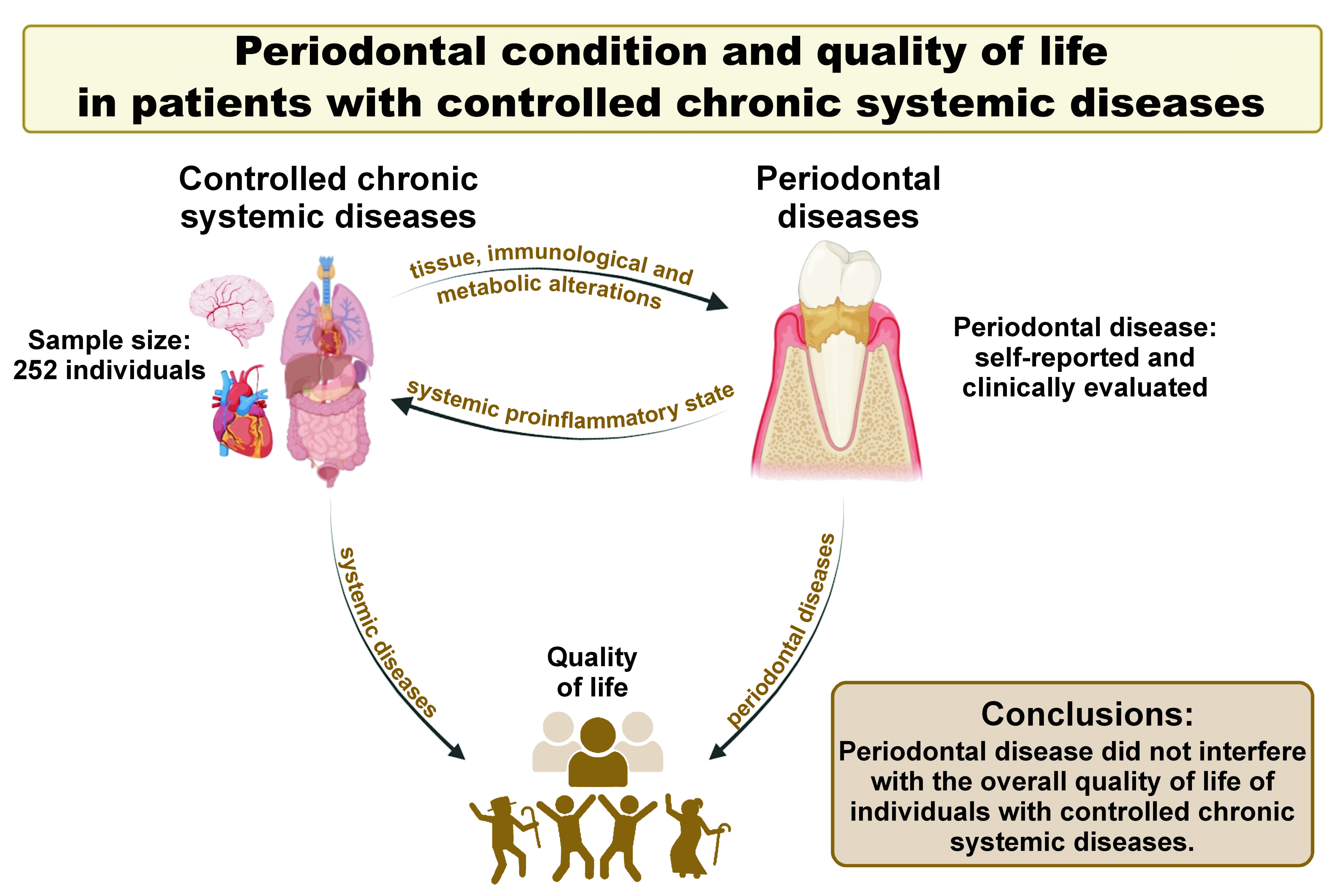
Objectives. The aim of the study was to examine whether periodontal condition could have an impact on the overall quality of life in patients with controlled chronic systemic diseases.
Material and methods. This cross-sectional study included 252 male and female subjects, aged ≥18 years, with a minimum of 6 teeth, and under medical follow-up for chronic systemic diseases. The following exclusion criteria were used: pregnant or lactating women; psychological or neurological limitations; uncontrolled chronic systemic disease; undergoing chemotherapy, radiotherapy, periodontal treatment, or tooth whitening; or use of an orthodontic appliance within the previous 3 months. The Medical Outcomes Study (MOS) 36-Item Short-Form Survey (SF-36) was used to assess the impact of periodontal health on patients’ overall quality of life. To assess self-perception of periodontal condition, a self-reported periodontal disease measurement questionnaire was used. The periodontal assessment was performed by 2 calibrated dentists. Anamnesis forms were completed to collect sociodemographic, behavioral and medical diagnostic data, as well as to identify risk factors.
Results. The majority of the study participants were ≤50 years old (51%), female (65%), had a low education level (≤12 years of study) (60%), and resided in low-income households (93%). The study found no association between periodontal condition and quality of life. The majority of individuals with tooth mobility and 3–10 natural teeth were diagnosed with stage III and stage IV periodontitis. No significant relationship was identified between chronic systemic diseases and periodontitis.
Conclusions. Periodontal disease has been demonstrated to have no effect on the overall quality of life of individuals with controlled chronic systemic diseases. Self-reported cases of periodontal diseases corresponded with the clinical condition. Chronic systemic diseases were not identified as a risk factor for the development of periodontitis.
Keywords: quality of life, periodontitis, chronic disease, self-perception
Introduction
According to the Global Burden of Disease Study 2019 (GBD 2019), approx. 44% of the global population is affected by oral disorders such as dental caries, periodontitis and edentulism.1 These conditions can cause disability, resulting in pain, sepsis, lost school days, decreased work productivity, and overall worse quality of life and well-being.2 Periodontal disease is a multifactorial, chronic inflammatory disease induced by subgingival dental biofilm that causes the loss of tooth-supporting tissues. This can result in a systemic proinflammatory state, which is implicated in the etiology of various chronic diseases, including cardiovascular disease, diabetes, osteoporosis, mental health conditions, and autoimmune diseases.3 Furthermore, the inflammatory mediators present in the gingival and peri-implant sulcus may contribute to the early diagnosis of periodontal and peri-implant diseases.3, 4
The management of health-related habits that contribute to the development of chronic diseases has not improved, and the prevalence of multimorbidity is on the rise, affecting 65% of individuals aged ≥65 years.5 Oral and systemic diseases have a large impact on the quality of life of individuals from all age groups.6 These conditions can influence self-esteem, nutrition, the ability to eat, health, and can cause pain, anxiety and social privation.5, 6, 7
Therefore, patient medical history, in addition to epidemiological indicators, is critical for planning, organizing and monitoring health services.8 The medical history of an individual is further enriched by the integration of their perceptions and social representations of oral and systemic health conditions, as reported by the individual.9 For this purpose, a valid self-reported measurement of disease can serve as a cost-effective method to facilitate epidemiological studies that incorporate population surveillance.10 Self-reporting of diseases consistent with clinical parameters can contribute to the prevention and early diagnosis of periodontal diseases, especially in individuals who require complex clinical care.11, 12 Some studies have demonstrated the effectiveness of self-assessment as a tool for evaluating periodontal status and many other conditions.13 Previous data has shown that self-perception can have acceptable validity, ranging from moderate to high compared to clinical examination. The present study aimed to analyze whether periodontal conditions have an influence on the overall quality of life of patients with controlled chronic systemic diseases. The secondary objectives were to verify the relationship between clinical periodontal diagnosis and self-perception of periodontal condition, and to assess whether chronic systemic diseases are risk factors for periodontal disease.
Material and methods
Study population
This cross-sectional study was designed according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.14 The Institutional Ethics Committee of the State University of Ponta Grossa, Brazil, has approved this study (protocol No. 3028211).
The research was conducted at the Regional University Hospital of Campos Gerais, State University of Ponta Grossa, in the southern region of Brazil, from December 2018 to May 2019. The sample size calculation was based on a previous study that used self-reporting questionnaires regarding periodontal disease.15
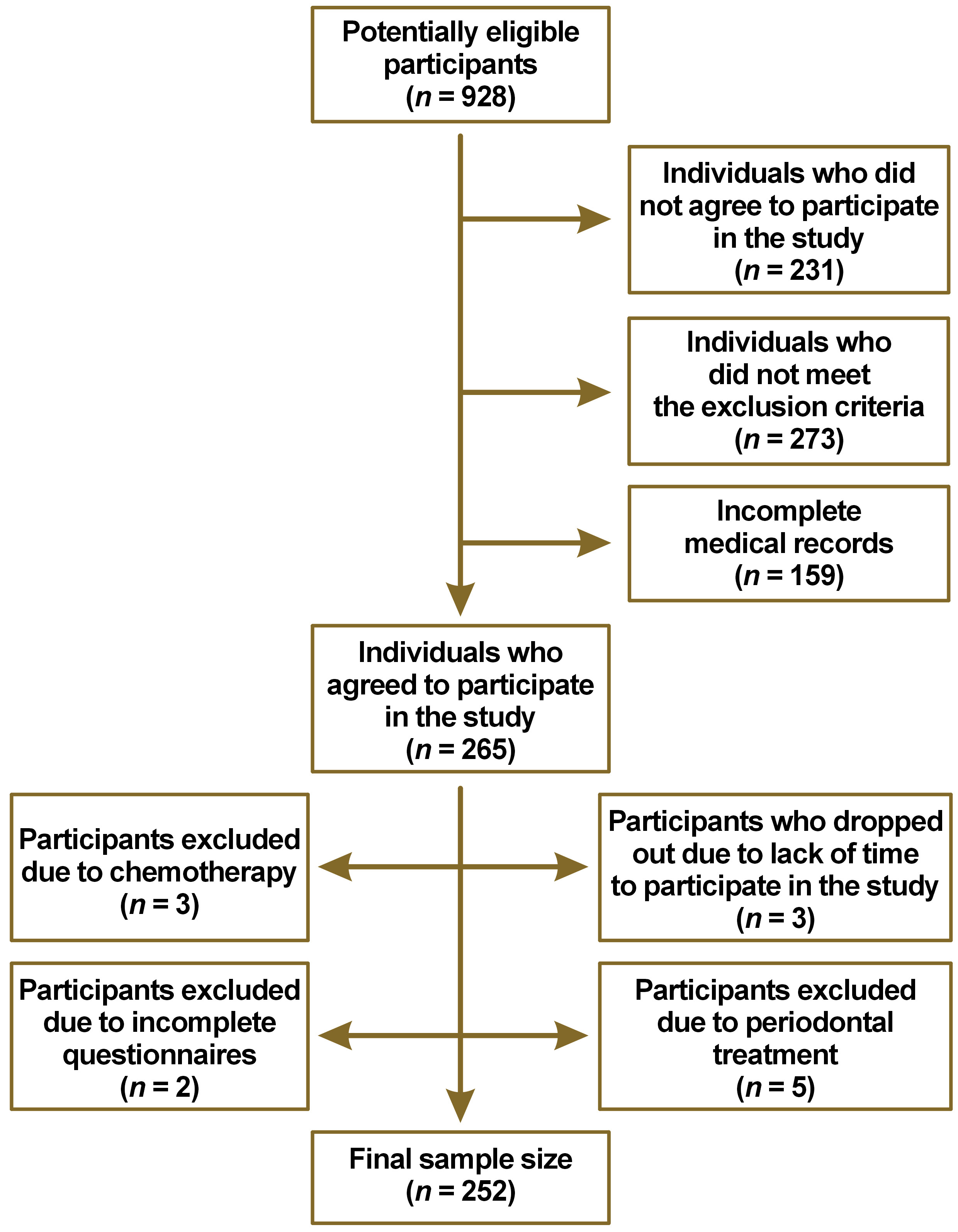
According to a self-report survey, 68% of the population have gingival problems.15 To estimate this proportion with 5% absolute precision and 95% confidence, a minimum sample size of 246 volunteers is required from a potentially eligible population of 928 participants (Figure 1). Based on the sample size calculator (https://www.statulator.com/SampleSize/ss1P.html), if a random sample of 246 is selected and 68% of subjects in a sample exhibit gingival problems, a confidence level of 95% can be ascribed to the hypothesis that between 63% and 73% of the subjects in the population possess the factor of interest.
Male and female subjects aged ≥18 years, with a minimum of 6 natural teeth, under medical follow-up for chronic systemic diseases, and without changes in medication during the previous 2 months were included in the study. The most prevalent systemic diseases were included (circulatory system diseases, nutritional, metabolic or endocrine diseases, respiratory system diseases, and immune system diseases). The following exclusion criteria were used: pregnant or lactating women; psychological or neurological limitations; uncontrolled chronic systemic disease; undergoing chemotherapy, radiotherapy, periodontal treatment, or tooth whitening; or use of an orthodontic appliance within the previous 3 months (Figure 1).
Data collection
An anamnesis form was completed based on patients’ medical records to collect data on sociodemographics, behavioral habits, risk factors for periodontitis, current medication, and medical diagnosis. The patients were categorized into 4 disease groups according to the International Classification of Diseases (ICD-11),16 as follows: group 1 (circulatory system diseases); group 2 (nutritional, metabolic or endocrine diseases); group 3 (respiratory system diseases); and group 4 (immune system diseases). The Medical Outcomes Study (MOS) 36-Item Short-Form Survey (SF-36) and a self-reported periodontal disease measurement questionnaire were subsequently administered to the patients. Then, the patients underwent a comprehensive clinical periodontal examination. Individuals in need of immediate treatment were referred to the university hospital’s dental service.
Assessment of quality of life and self-reported periodontal condition
The instrument used to evaluate the impact of health on patients’ overall quality of life was the SF-36 (Brazilian-Portuguese version). This questionnaire is composed of 36 items grouped into 8 subscales with multidimensional aspects, which evaluate the following: functional capacity; physical aspects; general health status; emotional aspects; social aspects; pain; vitality; and mental health. The SF-36 score ranges from 0 to 100, with 0 representing the worst state of health and 100 representing the best possible state.17
The patients’ self-perception of their periodontal condition was assessed using the self-reported periodontal disease measurement questionnaire (Brazilian-Portuguese version),18 which was based on self-reported questions from previous studies.12, 19 The questions were either objective or had a cognitive basis, prompting the individual to analyze their oral condition. The questionnaire contained 22 items on the following topics: 4 sociodemographic questions; 5 questions related to risk factors (such as smoking, diabetes and pregnancy); 10 self-reported questions concerning oral health and periodontitis; 2 questions on the history of periodontal treatment; and 1 question on the professional report of periodontal disease. The following variables were incorporated into the analysis: gingivitis; tooth migration; tooth mobility; tooth loss; number of natural teeth; oral health classification; scaling and root planing; periodontal surgery; and bone loss. Both questionnaires were previously validated and adapted for the Portuguese language.
Clinical periodontal evaluation
The following dental and periodontal parameters were evaluated: missing teeth; marginal suppuration; dental biofilm; gingival recession; probing depth; bleeding on probing; and clinical attachment loss. Examinations were conducted using a manual Millenium Plus North Carolina CP-15 periodontal probe (Golgran, São Caetano do Sul, Brazil) that included all teeth at 6 sites per tooth, with the exception of third molars. The classification of periodontal disease for subsequent diagnosis was based on the criteria established in the report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.20 We categorized the patients into the following 4 groups: periodontal health or gingivitis (gingivitis was defined as bleeding on probing at ≥10% of the sites); stage I periodontitis (clinical attachment loss of 1–2 mm); stage II periodontitis (clinical attachment loss of 3–4 mm); and stage III and stage IV periodontitis (clinical attachment loss of ≥5 mm).
The periodontal evaluation was conducted by 2 trained and calibrated dentists (LZL and LTN). The training and calibration for assessing clinical parameters (dental biofilm, bleeding on probing and marginal suppuration) were carried out through discussions among the researchers during joint clinical examinations in the preliminary phase of the study. The inter-examiner reliability for gingival recession, probing depth and clinical attachment loss was determined using the weighted Cohen’s kappa. The inter-examiner agreement was substantial, with values of 0.74 for gingival recession, 0.92 for probing depth, and 0.85 for clinical attachment loss.
Statistical analysis
The χ2 test was applied to evaluate the association between general population parameters according to age and sex. The same test was used to verify whether the self-perceived periodontal condition was associated with the periodontal diagnosis. We compared the quantitative periodontal parameters for age and sex using the unpaired t-test. Prior to conducting all analyses, the normality of the data was tested. To ascertain the impact of periodontal condition on patients’ overall quality of life, the analysis of variance (ANOVA) was applied in conjunction with Tukey’s post hoc test. The binary logistic regression model was used to determine whether controlled chronic systemic diseases function as risk factors for periodontal disease. The model incorporated all disease groups as predictor variables. The dichotomous response variable was the presence or absence of periodontitis, and the data was reported as odds ratios (ORs) and 95% confidence intervals (CIs). The results were considered statistically significant for p < 0.05.
Results
Characteristics of the population
The total number of subjects included in the study was 252. The majority of the participants were ≤50 years old (51%), female (65%), had received a maximum of 12 years of education (60%), and resided in low-income households (93% with incomes up to $700.00).
Behavioral habits and oral hygiene
All the variables were analyzed based on sex and age of the participants. The majority of smokers were ≤50 years of age, while ex-smokers were predominantly ≥51 years of age (p = 0.001). In addition, ex-smokers were more frequently male (p = 0.014). With respect to diabetes, no association was identified between age and sex. Patients aged ≥51 years consumed more medications per day (p = 0.002). There was a significant association between the variables of flossing and frequency of flossing in relation to age and sex regarding oral hygiene habits. The use of dental floss was more prevalent among female subjects (p = 0.003) and among individuals aged ≤50 years (p = 0.006). Daily dental flossing, however, was more prevalent among males (p = 0.013) and patients aged ≥51 years (p = 0.016). Women exhibited a higher frequency of toothbrushing than men, with daily toothbrushing significantly associated with female sex (p < 0.0001) (Table 1).
Periodontal conditions
The majority of individuals aged ≤50 years exhibited an average of 20–25 teeth and a lower tooth loss index in comparison to the subjects aged ≥51 years (p < 0.0001). A higher number of sites with biofilm (p = 0.027) and a greater clinical attachment loss of 3–4 mm or ≥5 mm (p < 0.0001) was observed in males aged ≥51 years compared to other groups. The prevalence of probing depth of 1–3 mm was higher among women (p = 0.001), whereas men exhibited a higher number of sites with probing depth of 4–5 mm (p = 0.001) and ≥6 mm (p = 0.005). The group with a mean age of ≤50 years presented a higher percentage of sites with bleeding on probing (p = 0.001) and a lower percentage of sites with gingival recession (p < 0.0001). A lower percentage of women showed sites with gingival recession (1–3 mm: p = 0.002; ≥4 mm: p < 0.0001) (Table 2).
Impact of periodontal condition on quality of life
The results regarding the quality of life subscale of SF-36 were similar among patients with different periodontal diagnoses (p > 0.05) (Table 3). The coefficients of variation between SF-36 subscales according to periodontal condition were low, supporting the similarity between the groups, as follows: functional capacity (3.4%); physical aspects (3.4%); pain (3.3%); general health status (0.4%); vitality (1.5%); social aspects (0.6%); emotional aspects (3.4%); and mental health (1.8%).
Self-reported periodontal disease measurement and clinical periodontal diagnosis
Individuals who reported having tooth mobility, tooth loss, scaling and root planing, and 3–10 natural teeth were mostly associated with stages III and IV periodontitis. A statistically significant association was identified between the stages of periodontitis and these variables (p < 0.05) (Table 4).
Systemic condition and periodontal diagnosis
The predictors were previously defined as follows: circulatory system diseases (group 1); nutritional, metabolic or endocrine diseases (group 2); respiratory system diseases (group 3); and immune system diseases (group 4). When considered as a whole, these factors were not found to be associated with the risk of periodontitis (p = 0.791). After adjustment, the logistic regression analysis did not demonstrate a significant relationship between the risk of periodontitis and the defined predictor variables (Table 5).
Discussion
The findings of this study indicated that the quality of life did not vary according to the periodontal condition of individuals with controlled chronic systemic diseases. The quality of life subscales of the SF-36 demonstrated minimal variation between the patient groups. Although many studies have suggested that periodontal disease has a negative impact on the quality of life of individuals, these studies used specific questionnaires that predominantly addressed oral health aspects rather than generic questionnaires, such as the SF-36, that are widely used in patients with systemic diseases.21, 22, 23 Our observations revealed no impact of periodontal disease on the overall quality of life when using a generic assessment instrument (SF-36). The findings of this study differ from those reported in the literature, possibly due to the fact that the patients included in our research had controlled systemic conditions. Additionally, this discrepancy may be attributed to other criteria used to classify periodontal diseases.17, 23
In the current study, the majority of subjects who completed the self-reported periodontal assessment exhibited advanced stages of periodontal disease and a low family income. These factors could be associated with challenges in accessing healthcare services, which may have contributed to the observed prevalence and severity of periodontal disease.24 The self-reported periodontal condition coincided with the clinical diagnosis.18 Previous studies have attempted to determine the predictive capacity of self-reported periodontal disease measurement variables.13 Although this tool does not estimate the severity of periodontal disease, it has been demonstrated to be effective in the rapid identification of periodontitis due to its non-invasiveness and cost-effectiveness.25 However, the final diagnosis of the severity of periodontal disease must be made through a clinical examination by a qualified professional.
In the present study, chronic systemic diseases were not identified as risk predictors for the development of periodontitis. It should be emphasized that all subjects included in the study were under continuous medical supervision, with their chronic systemic diseases being adequately managed. This factor may explain the absence of associations between variables, a phenomenon that has been previously documented in related studies.26, 27
The present study evaluated a vulnerable population group (systemic involvement, low income and low levels of education). The use of a clinical method for periodontal assessment, in conjunction with a self-reported periodontal disease questionnaire, enabled the identification of subjects requiring both clinical and informational intervention, as many exhibited little knowledge about the disease.11, 12, 13, 18 The individuals adequately self-reported their symptoms yet demonstrated no self-perception of the disease, perhaps due to poor understanding or a focus on medical monitoring for systemic conditions.18, 19, 25
Our study was subject to certain limitations, including the inability to measure incidence or establish causal relationships due to its cross-sectional design, the potential difficulty in interpreting identified associations, and the inability to investigate temporal relations between outcomes and risk factors. Additionally, the unequal distribution of sex among study participants and the use of a generic instrument to assess quality of life may have constrained the generalizability of the findings.28
The patients included in the present study had various systemic diseases, with multiple conditions frequently co-occurring. In addition, they used numerous medications to manage these diseases. Both systemic conditions and medications can cause alterations within the oral cavity, affecting the periodontal tissues and, by extension, the quality of life.7, 29 Therefore, the results of the study may have been influenced by these factors. However, we also identified positive and differentiated aspects, such as the use of 2 methods for evaluating periodontal conditions: the clinical assessment method; and the periodontal disease self-perception questionnaire, an instrument employed in large epidemiological studies. Despite its generic nature, the utilization of the SF-36 to assess quality of life enabled the analysis of functional capacity and subjective well-being in relation to the health status of the study participants.17 Furthermore, to the best of the authors’ knowledge, no previous studies have used the SF-36 to assess the impact of periodontal disease on the overall quality of life of patients with chronic systemic diseases.
The present research offers further insights into the existing literature on this subject, corroborating the idea that self-reported periodontal conditions may serve as indicators of clinical periodontal status.30 Conversely, the findings indicate that periodontal condition does not interfere with the quality of life as measured by a generic assessment instrument (SF-36). This suggests that chronic systemic diseases may have an impact on the physical and mental well-being of individuals. In addition, the outcomes of this study enabled the verification of patients’ levels of knowledge and self-perceptions of periodontal disease. This verified information can facilitate the development of educational policies that encourage the prevention and early diagnosis of this disease.
Conclusions
Based on a sample of patients with systemic diseases who receive ongoing medical care and monitoring at a referral hospital, it can be concluded that periodontal disease did not interfere with the overall quality of life of individuals with controlled chronic systemic diseases. The final diagnosis of the severity of periodontal diseases must be made through a clinical examination by a qualified professional. Controlled chronic systemic diseases were not identified as a risk factor for the development of periodontitis. It is recommended that long-term follow-up studies be conducted to evaluate the impact of periodontitis on quality of life and the association between periodontitis and systemic diseases.
Ethics approval and consent to participate
The Institutional Ethics Committee of the State University of Ponta Grossa, Brazil, has approved this study (protocol No. 3028211).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.