Abstract
Background. Pre- and probiotics may help restore a dysbiotic oral ecosystem. The first years of life provide a window of opportunity to modulate the composition of the oral microbiota and prevent disease.
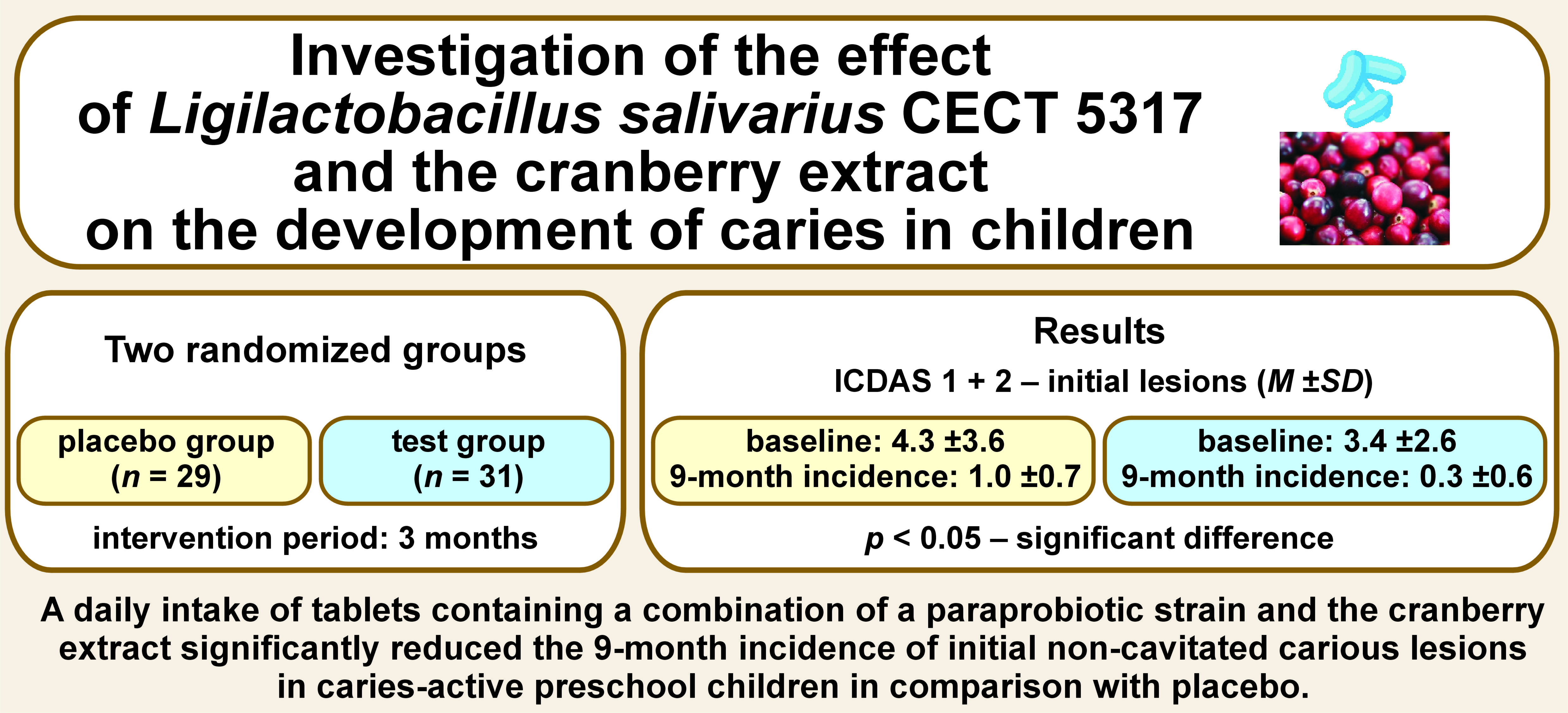
Objectives. The aim of the present study was to investigate the effect of a tablet containing inactivated Ligilactobacillus salivarius CECT 5317 and the cranberry extract on the development of caries in caries-active preschool children.
Material and methods. The study employed a randomized, placebo-controlled, double-blind design. Preschool children (N = 73) with at least one active carious lesion were enrolled and randomly assigned to the test group or the placebo group. The intervention period was 3 months. Caries was assessed according to the International Caries Detection and Assessment System (ICDAS) II criteria at baseline and after 9 months, and oral hygiene was evaluated with the simplified oral hygiene index (OHI-S). The salivary counts of Streptococcus mutans and Lactobacillus spp. were determined at baseline, and then after 3 and 9 months through the conventional cultivation on TYCSB and MRS agar, respectively.
Results. Sixty children completed the trial (a dropout rate of 19%). The baseline caries prevalence was high in both groups (~71%) and there were no major differences between the groups with regard to background variables. The 9-month incidence of initial carious lesions (ICDAS 1+2) was significantly lower in the test group as compared to the placebo group (p < 0.05). The plaque levels, and the salivary counts of S. mutans and Lactobacillus spp. remained unchanged in both groups throughout the study.
Conclusions. A daily intake of a tablet containing a paraprobiotic and the cranberry extract reduced the 9-month incidence of initial non-cavitated carious lesions in caries-active preschool children. The present study is one of the first to show the impact of synbiotics on the development of caries in children.
Keywords: probiotics, prebiotics, Streptococcus, Lactobacillus, Vaccinium macrocarpon
Introduction
Early childhood caries (ECC), defined as the presence of one or more decayed (non-cavitated or cavitated), missing (as a result of caries) or filled tooth surfaces in any primary tooth in a child aged 71 months or younger, is a global health problem associated with impaired quality of life.1 Although ECC is largely preventable, its prevalence remains high in many European countries; for example, in Poland, nearly 80% of 5-year-olds are affected.2 Dental caries is a non-communicable disease (NCD), sharing biological, behavioral and socioeconomic risk factors with other NCDs.3, 4 The main preventive strategies against ECC include regular tooth brushing, restricted intake of free sugars, and daily exposure to fluorides.1 Emerging adjunct technologies may also prove useful. The pivotal role of microbial dysbiosis in the caries process suggests that pre- and probiotics could complement efforts to restore the normal balance of dental biofilm. The early years of life present a window of opportunity for modulating the oral microbiota through such interventions.5 Recent systematic and comprehensive reviews have concluded that lactobacilli-derived probiotics, defined as live bacteria that confer a health benefit to the host, can prevent ECC when administered daily in milk or tablets.6, 7 Paraprobiotics, or inactivated probiotics, are non-viable microbial cells (intact or broken) or crude cell extracts that, when administered orally or topically in adequate amounts, confer benefits to human or animal consumers.8
Research on the impact of paraprobiotics in the oral cavity is limited, but previous findings suggest that inactivated strains of Ligilactobacillus salivarius, isolated from human breast milk, may inhibit biofilm formation.9, 10 Furthermore, a recent short-term trial using chewing tablets containing thermally inactivated L. salivarius HM6 showed a reduced incidence of ECC in comparison with standard treatment.11 Natural polyphenol-containing agents are also interesting biofilm modulators with prebiotic action. For example, natural cranberries may show anti-caries properties by altering the bacterial shape and modifying dental biofilm colonization.12, 13, 14 Interestingly, a combination of paraprobiotics and the cranberry extract may have synergistic effects, warranting further evaluation.15 Therefore, the aim of this study was to investigate the effect of a tablet containing inactivated probiotic lactobacilli and the cranberry extract on the development of caries in preschool children with active caries. The primary outcome was caries incidence over a 9-month period, with secondary endpoints including plaque accumulation and the salivary counts of Streptococcus mutans and Lactobacillus spp. For the primary endpoint, the null hypothesis was that caries incidence would not differ between the active intervention and placebo groups.
Material and methods
Study design
The study used a randomized, placebo-controlled, double-blind design with 2 parallel arms. The intervention lasted for 3 months, and clinical and microbial examinations were conducted at baseline, and after 3 and 9 months. The project received ethical approval from the Bioethics Committee of the Medical University of Warsaw, Poland (No. KB/232/2016), and was registered at ClinicalTrials.gov (NCT 03919838).
The test and placebo tablets were provided free of charge by the manufacturer, and the project was funded by the authors’ institutions.
Participants
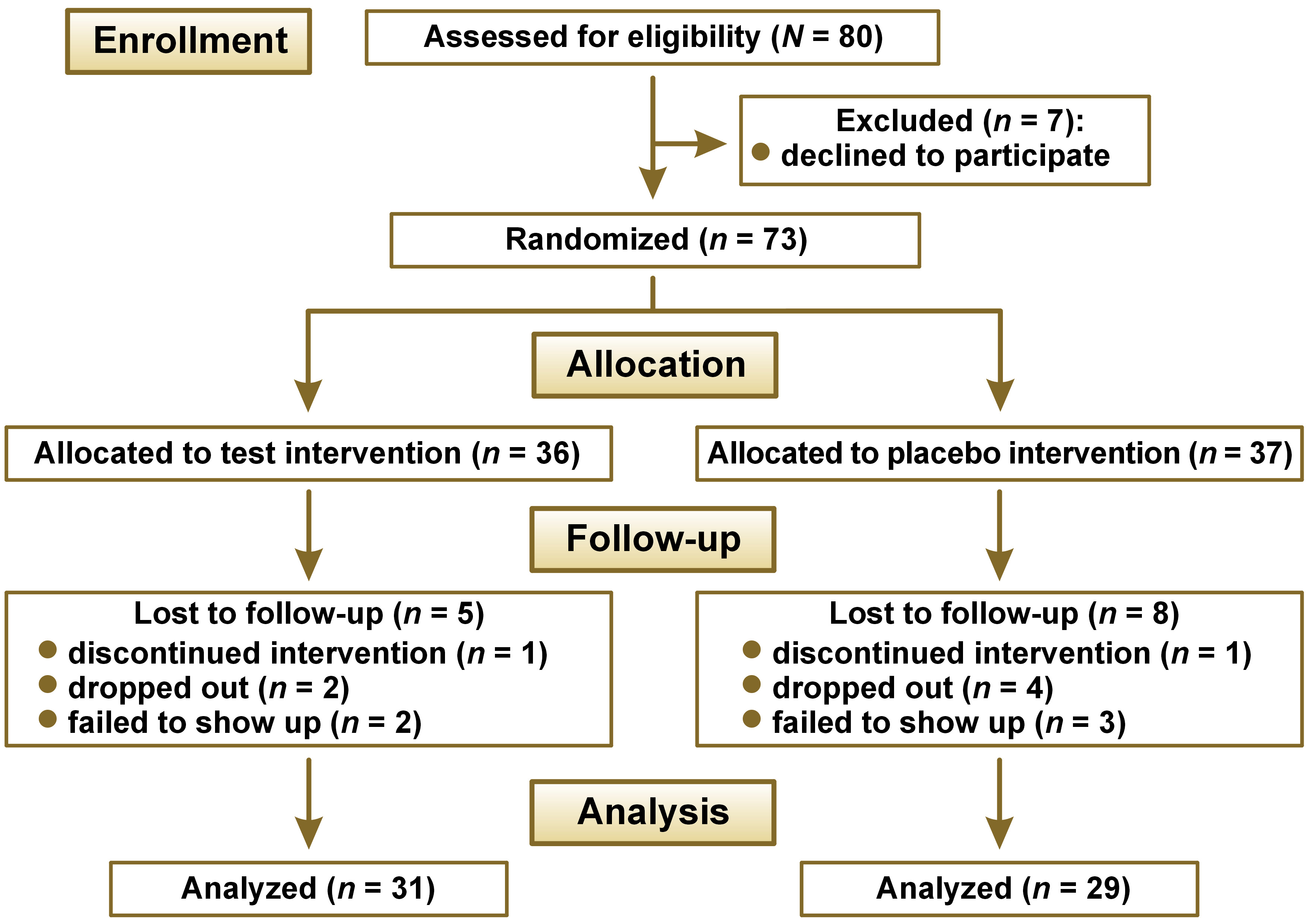
We invited 80 systemically healthy children aged between 3 and 6 years to participate. All participants were outpatients at the Department of Pediatric Dentistry of the Medical University of Warsaw, Poland. The inclusion criteria were as follows: the presence of dental caries (at least one initial or cavitated carious lesion, recent restorations, or missing teeth due to caries (dmft ≥ 1)); the absence of oral inflammatory conditions; and no exposure to antibiotics, probiotics or professional fluoride varnishes within 1 month prior to enrollment. Exclusion criteria were chronic systemic diseases, congenital conditions (e.g., cerebral palsy, clefts, Down syndrome), ongoing medication, family relocation plans, and poor cooperation. Children with hypomineralized second primary molars were also excluded. Written informed consent was obtained from all parents/legal guardians. A review board within the Bioethics Committee of the Medical University of Warsaw monitored the allocation concealment to safeguard the children’s rights throughout the project. We enrolled the children consecutively based on a sample size calculation, in which α (the probability of a type-I error) was set at 0.05 and β (the probability of a type-II error) was 0.20. In order to detect a 50% difference in the development of caries between the groups (the anticipated mean caries incidence 2.0 vs. 1.0), 70 children (35 in each group) were required. Therefore, enrollment was extended to 80 children to allow for potential dropouts. A flowchart is presented in Figure 1.
Randomization
We randomly assigned eligible children into the test group or the placebo group with the aid of software that used permuted blocks of uniform size (three), containing computer-generated numbers. The random number was enclosed in an opaque envelope provided to the examiner before the baseline examination. All people involved (children/parents, clinicians/examiners, research group) remained fully blinded throughout the trial, and an independent monitor guaranteed the allocation concealment.
Intervention
Each child received a jar containing 2 tablets to take daily for 3 months. The active ingredients were the powdered cranberry extract (Vaccinium macrocarpon), standardized to 40% proanthocyanidins, and inactivated L. salivarius CECT 5713 at 10 mg, equivalent to 1 × 109 colony-forming units (CFU). The exact tablet composition is detailed in the supplementary material (available on request from the corresponding author). Placebo tablets were identical in size, color, texture, and sweetness, but contained no active ingredients. The tablets were packaged, coded and supplied by NutroPharma (Lesznowola, Poland). The parents were instructed to administer the tablets in the evening after tooth brushing, and the children were encouraged to let them dissolve slowly in the mouth to maximize tooth contact. Throughout the intervention, the parents were advised against giving their children dairy products containing probiotics, but they could provide traditionally fermented products, such as natural yogurt, kefir or buttermilk. In addition, the parents were asked not to provide any foods or sweets containing xylitol, and to refrain from fluoride exposure other than fluoridated toothpaste (~1,000 ppm, in the mornings and evenings). No specific remineralizing agents were used. During the study, the children received restorative dental treatment for cavitated lesions based on individual clinical needs and ethical considerations. Such treatment impacts the dmft index, but not the International Caries Detection and Assessment System (ICDAS) II score, which exclusively assesses the presence and extent of carious lesions.
Saliva sampling and microbial examinations
We quantified the salivary levels of S. mutans and Lactobacillus spp. through conventional cultivation. Paraffin-stimulated whole saliva was collected over 5 min and the samples were serially diluted in phosphate-buffered saline (PBS). Aliquots of 0.1 mL from serial dilutions were inoculated in duplicate onto selective agar plates from BioMaxima (Lublin, Poland): TYCSB (tryptone yeast extract cystine with sucrose and bacitracin) agar for S. mutans and MRS (de Man– Rogosa–Sharpe) agar for Lactobacillus spp. The agar plates were inoculated using a sensor turntable (Sensorturn pro; WLD-TEC, Arenshausen, Germany) and incubated in an anaerobic environment at 37°C for 5 days. The mean number of CFU for each suspension and dilution was counted, and we expressed the total bacterial load per 1 mL of saliva.
Collection of background data and clinical examinations
At baseline, we collected socioeconomic and medical background data from the parents, as well as information on the child’s oral health behavior and dietary preferences, using predetermined questions. Clinical examinations were conducted in a dental office under optimal illumination by means of a WHO-621 probe. The children refrained from eating and drinking for 2 h before the examination, and did not brush their teeth within 12 h before the appointment. All dental surfaces in subsequent quadrants were examined, and dental caries was scored after cleaning and air-drying according to the ICDAS II criteria at baseline and after 9 months by 3 experienced examiners (A.T.S., P.P.Z., J.G.).
Caries prevalence at baseline and the 9-month incidence were calculated as the mean values of initial (ICDAS 1+2), moderate (ICDAS 3+4) and extensive (ICDAS 5+6) lesions. For the dmft index, ICDAS II scores of 3–6 were considered as “decayed”. To express the baseline caries prevalence, the dmft index captured and described the total burden of the disease (decayed, missed and filled teeth) to indicate the enrollment of a truly caries-active child population. The incidence of new lesions during the study was scored using the ICDAS II index to capture the number of new early (non-cavitated), moderate and extensive lesions, allowing for a detailed description of lesion severity.
Inter- and intra-examiner reliability was assessed by examining 6 randomly selected 5-year-old children with ECC before the study. The results showed very good intra-examiner agreement for the ICDAS II scores (κ = 0.89, 0.79 and 0.91). The interrater reliability was also excellent for the 3 examiners, with kappa values ranging from 0.83 to 0.94.
The oral hygiene levels were determined using the simplified oral hygiene index (OHI-S). The buccal and labial surfaces of 6 teeth were scored after the application of a disclosing solution containing 3% erythrosine. The plaque scores for each individual were summed and divided by the number of the registered surfaces.
Outcome measures
The primary outcome was caries incidence over a 9-month period. Secondary endpoints were the level of oral hygiene and the counts of S. mutans and Lactobacillus spp. in stimulated saliva. For the primary endpoint, the null hypothesis was that caries incidence in the active group would not differ from that in the placebo group.
Assessment of compliance and side effects
The parents were asked to return the tablet packs at the 3-month follow-up to monitor intake. Compliance was categorized as “good” if ≤3 tablets were missed within a week, and “doubtful” if missed more often. The parents were also encouraged to report any perceived adverse or side effects and, in such cases, discontinue tablet use.
Statistical analysis
All data was stored at the Department of Pediatric Dentistry, Medical University of Warsaw. The data was analyzed using the Statistica 13 package (TIBCO Software Inc., Palo Alto, USA). Continuous data was compared between the groups using parametric (t test) and non-parametric (Mann–Whitney U test) tests if variables did not follow a normal distribution. Categorical data and proportions were compared using the χ2 test. Inter- and intra-examiner agreement was assessed with Cohen’s kappa coefficient, and p-values less than 0.05 were considered statistically significant.
Results
Sixty children completed the trial, giving an attrition rate of 19%. The reasons for dropout are shown in Figure 1. There were no significant differences between the groups at baseline regarding the socioeconomic status, oral health behavior or diet, except for a higher proportion of fathers with primary or vocational education in the placebo group (Table 1). The baseline caries prevalence, expressed as dmft, was 71% in the test group and 72% in the placebo group, with the mean values of 7.0 ±4.5 in both groups. The baseline ICDAS II values and the 9-month caries incidence for initial, moderate and severe lesions are presented in Table 2.
A significantly lower incidence of initial lesions (ICDAS 1+2) was observed in the test group as compared to the placebo group after 9 months (p < 0.05). Although the incidence of moderate lesions (ICDAS 3+4) was also lower in the test group, the difference did not reach statistical significance. The intervention had no significant effect on the level of oral hygiene (OHI-S); the baseline value of 0.8 ±0.4 remained unchanged throughout the trial in both groups. Similarly, there were no statistically significant changes in the salivary counts of lactobacilli or S. mutans (Table 3). Compliance with the study protocol was good among all children who completed the trial, with a maximum of 2 missed tablets per week. No side effects or adverse events were reported by the parents or children.
Discussion
This study evaluated the combined effect of a paraprobiotic strain and the cranberry extract on the development of caries and salivary bacterial counts in preschool children over a 9-month period. To the best of our knowledge, this combination has not been investigated so far in a placebo-controlled trial. The use of organic products and natural polymers to prevent disease and maintain health aligns with the concept of green dentistry.16, 17 The rationale behind this combination of natural pre- and probiotic agents was that its synergistic effects could potentially be more powerful than the action of the agents used individually. Cranberry polyphenols (proanthocyanidins) may inhibit the production of organic acids and the formation of dysbiotic biofilm by hindering bacterial adhesion to tooth surfaces. They may also beneficially modulate the microbial ecology of dental plaque in high-caries-risk patients.13, 18, 19, 20, 21 In addition, cranberry polyphenols may affect the production and activity of proteolytic enzymes that contribute to the destruction of the extracellular matrix in dental biofilm.22 We selected a specific high-molecular-weight cranberry extract, previously shown to significantly reduce salivary S. mutans counts in children.23 The ability of probiotic supplements to lower the counts of caries-associated microorganisms in saliva and dental biofilm through the production of bacteriocins, and competition for adhesion and nutrients is well established.24 In this context, paraprobiotic and probiotic strains derived from Lactobacillus spp. appear particularly promising for clinical applications in maintaining oral health.25, 26 The strain used in this trial, L. salivarius CECT 5317, is a non-active strain isolated from human milk.10 The main finding of this study was that the combination of pre- and probiotics significantly reduced the incidence of initial carious lesions after 9 months of a daily intake. Although there were fewer new moderate lesions in the test group, this difference did not reach statistical significance. Consequently, we rejected the null hypothesis.
For all stages of carious lesions, the reduction in new carious lesions was 44%, a magnitude comparable to previous trials using live probiotic bacteria in preschool children.6 This clinical effect is particularly significant given that the participating children were selected for being caries-active. For comparison, Rodríguez et al. reported a 50% reduction in the incidence of cavitated lesions among caries-active preschool children enrolled in a 1-year program of a daily milk intake supplemented with Lactobacillus rhamnosus SP1.27 To our knowledge, only one previous trial has evaluated the effect of thermally inactivated probiotics on the development of caries in preschool children.11 In that study, despite a short intervention period of only 14 days, a reduced incidence of ECC was observed after 12 months as compared to “treatment as usual”.11 Collectively, the findings from our study and the aforementioned research suggest that inactivated L. salivarius strains may have anti-caries properties similar to those of live probiotic bacteria. This information is important for the development, production, storage, and shelf life of future consumer products.
With regard to saliva, numerous studies with probiotic supplements have reported an immediate but short-term decrease in S. mutans, whereas Lactobacillus counts are often slightly increased.28 Surprisingly, in our study, we did not observe any effect on salivary bacterial counts, which contrasts with earlier reports evaluating live or inactivated strains of L. salivarius.10, 29, 30, 31 However, previous studies relied on biofilm models,30, 31 involved short-term protocols with healthy volunteers10 or used simple chair-side tests.29 We found no effect of the intervention on the level of oral hygiene, assessed using OHI-S, which is consistent with many previous reports on probiotic supplements.32 Cranberries, however, contain phenolic compounds that may disrupt biofilm formation, so an effect on biofilm accumulation would not have been surprising. It should, however, be noted that the mean OHI-S score was relatively low (<1.0) among the participating children, which did not necessarily reflect their high cariogenic challenge. This observation underscores the idea that the presence of “virulence hotspots”, rather than the amount of biofilm, is decisive for local caries activity in young children.33
Strengths and limitations
The strengths of this study include its strict randomized, placebo-controlled, double-blind longitudinal cohort design, and high compliance. However, there were limitations, including a relatively high dropout rate, although evenly distributed between the groups. The post hoc power analysis indicated strong statistical power (98.6%). Notably, we presented our data per protocol, and the significant findings persisted in the intention-to-treat analysis. However, we could not investigate the separate contribution of each active ingredient (V. macrocarpon and L. salivarius) with the current study design, as it would have required 4 parallel study arms and a larger sample size. We consider the clinical scoring reliable given the very good inter- and intra-examiner agreement. However, the external validity of our findings may be limited, since the study group represented a selected high-caries population. Therefore, any generalization of these findings to populations with a lower ECC burden should be approached cautiously. Nevertheless, the outcomes of this trial encourage and justify further research into the anti-caries role of this synbiotic mix containing paraprobiotics and natural cranberry polyphenols.
Conclusions
A daily intake of tablets containing a combination of a paraprobiotic strain and the cranberry extract significantly reduced the 9-month incidence of initial non-cavitated carious lesions in caries-active preschool children in comparison with placebo.
Trial registration
The project was registered at ClinicalTrials.gov (NCT 03919838).
Ethics approval and consent to participate
The study was conducted ethically in accordance with the World Medical Association (WMA) Declaration of Helsinki, and approved by the Bioethics Committee of the Medical University of Warsaw, Poland (No. KB/232/2016). Written informed consent for participation was collected from all participants’ parents/legal guardians prior to involvement.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.