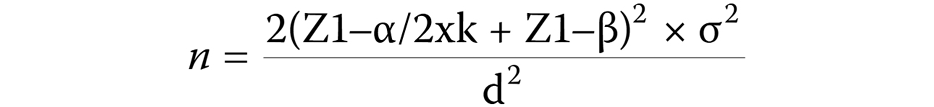Abstract
Background. Periodontal disease is the most prevalent chronic inflammatory condition that can cause the destruction of supporting periodontal tissues. It has been hypothesized that while the synthesis of pro-inflammatory cytokines causes tissue destruction and disease progression, anti-inflammatory cytokine production can result in protective immunity. The balance of inflammatory cytokines is central to the immunoregulation of the disease.
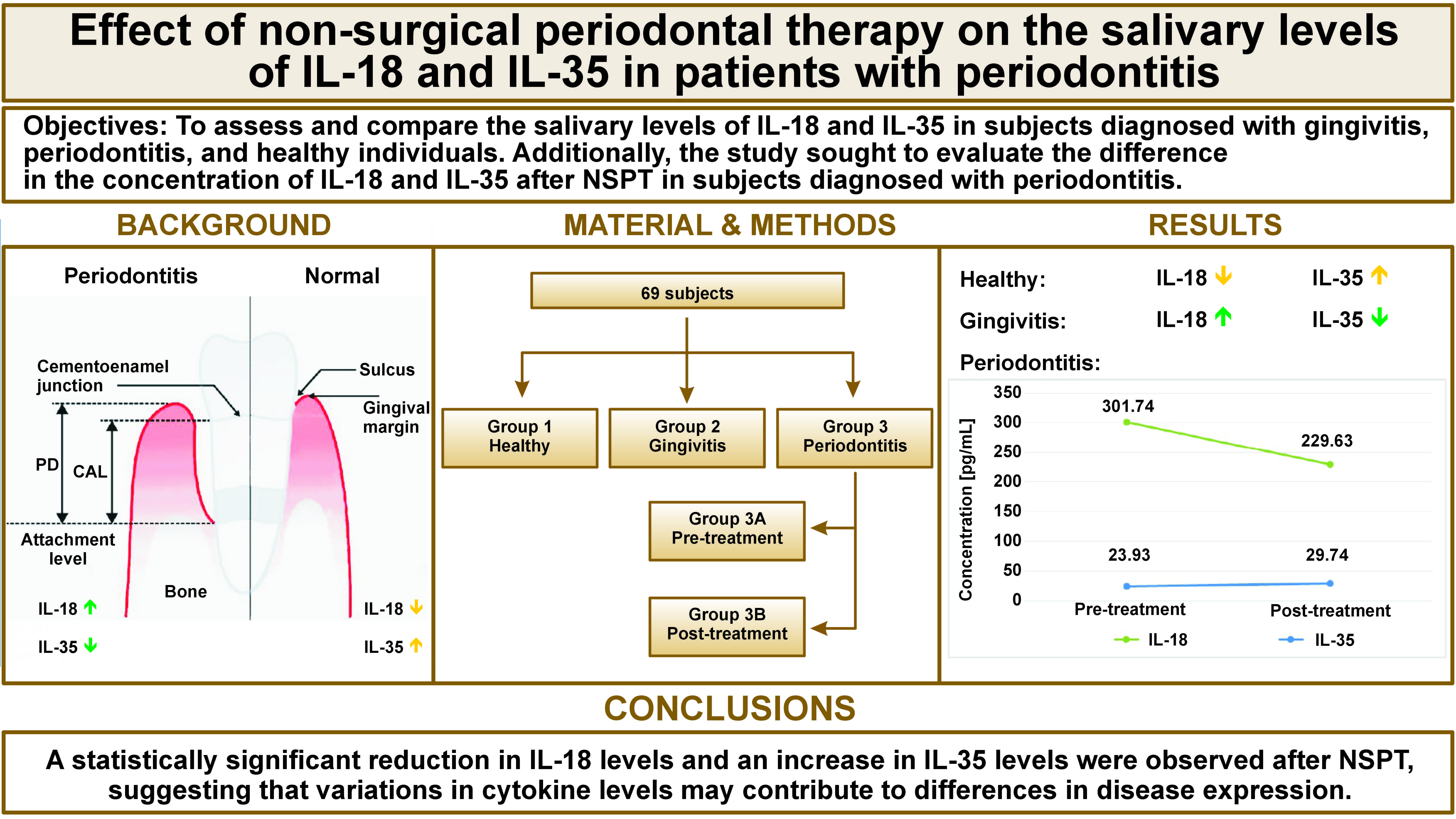
Objectives. The aim of the study was to assess and compare the salivary levels of interleukin (IL)-18 and IL-35 in subjects diagnosed with gingivitis, periodontitis, and healthy individuals. Additionally, the study sought to evaluate the difference in the concentration of IL-18 and IL-35 after non-surgical periodontal therapy (NSPT) in subjects diagnosed with periodontal disease.
Material and methods. A total of 69 individuals were divided into 3 groups: healthy (group 1; n = 23); gingivitis (group 2; n = 23); and stage II periodontitis (group 3A; n = 23). Saliva samples were obtained from each participant at baseline and, in the periodontitis group, at baseline and 12 weeks after NSPT (group 3B; n = 23). Probing pocket depth (PD), bleeding on probing (BoP), gingival index (GI), and clinical attachment level (CAL) were recorded and IL-18 and IL-35 levels were analyzed using an enzyme-linked immunosorbent assay (ELISA).
Results. The mean salivary level of IL-18 was significantly higher in the gingivitis group compared to the other groups (p < 0.05), whereas the mean IL-35 level was significantly higher in the healthy group compared to the other groups (p < 0.05). Twelve weeks after NSPT, the periodontitis group demonstrated a statistically significant difference in cytokine levels, characterized by a decline in the IL-18 concentration (229.63 ±49.35 pg/mL) and an increase in the concentration of IL-35 (29.47 ±17.88 pg/mL).
Conclusions. In the present study, a significant difference in the salivary levels of IL-18 and IL-35 before and after NSPT was observed. Therefore, these cytokines could serve as potential inflammatory biomarkers.
Keywords: cytokines, interleukin-35, periodontitis, interleukin-18, human enzyme-linked immunosorbent assay
Introduction
Periodontal disease is the most prevalent inflammatory condition that can cause the destruction of supporting periodontal tissues.1 Gingivitis can revert to its original state of health; however, a patient diagnosed with periodontitis remains with the condition even after successful treatment and requires supportive periodontal care.2 Periodontitis is clinically diagnosed by measuring probing pocket depth (PD), clinical attachment level (CAL), bleeding on probing (BoP), and radiographic evaluation, often after the destruction of connective tissue and bone.3 Biomarkers can quantify and analyze accurately the signs of normal biological or pathogenic processes or identify objectively physiological responses to a therapeutic intervention.4 Circulating molecules are putative disease biomarkers that are present in whole saliva and gingival crevicular fluid (GCF) of patients diagnosed with periodontal disease at elevated concentrations.5
Every aspect of immunity and inflammation, including antigen presentation, bone marrow differentiation, cellular attraction and activation, transcription of adhesion molecules, and acute phase reactions, involves cytokines.6 The synthesis of pro-inflammatory cytokines has been observed to be a primary driver of tissue destruction and disease progression. However, the production of anti-inflammatory cytokines can result in the establishment of protective immunity.7, 8 Some cytokines function primarily to induce inflammation and are referred to as pro-inflammatory cytokines. Conversely, anti-inflammatory cytokines play a role in the suppression of their action.9 In the context of periodontal pathogenesis, the inflammatory activity is regulated due to the coexistence of pro- and anti-inflammatory cytokines, suggesting its destructive and protective immune mechanisms.10
Interleukin (IL)-18 is a pro-inflammatory cytokine that belongs to the IL-1 superfamily due to its expression in chronic inflammation and autoimmune diseases. It is recognized as the regulator of immune responses.11, 12 Interleukin-18 levels are elevated in GCF, saliva, serum, and gingival tissue samples from individuals with periodontal disease. The cytokine has been demonstrated to modulate inflammation by stimulating Th1 and Th2 cell responses.13, 14, 15 Studies have reported increased concentrations of IL-18 in GCF of individuals with periodontitis compared to healthy individuals, and its decrease in concentration after initial periodontal therapy.16 Thus, the expression of IL-18 in saliva can relatively signify its importance in the regulation of immune response in gingival inflammation.
Interleukin-35 is a novel anti-inflammatory signal molecule that mediates immunosuppression.17 Some researchers have demonstrated the immunomodulatory response of IL-35 in various conditions.18 Hence, an increase in the IL-35 levels plays a crucial role in the suppression of periodontal inflammation and is indicative of its potential as a biomarker in maintaining periodontal health.
A paucity of research has been conducted on the salivary levels of IL-18 and IL-35 in healthy patients, as well as in individuals with gingivitis and periodontitis.16, 18 A limited number of studies have investigated the difference in cytokine levels in periodontitis patients before and after non-surgical periodontal therapy (NSPT).19 This study aims to assess and compare the salivary levels of IL-18 and IL-35 among individuals with gingivitis, periodontitis, and in healthy patients. Additionally, the study will evaluate the effect of NSPT on IL-18 and IL-35 levels in patients diagnosed with periodontitis.
Material and methods
Data collection
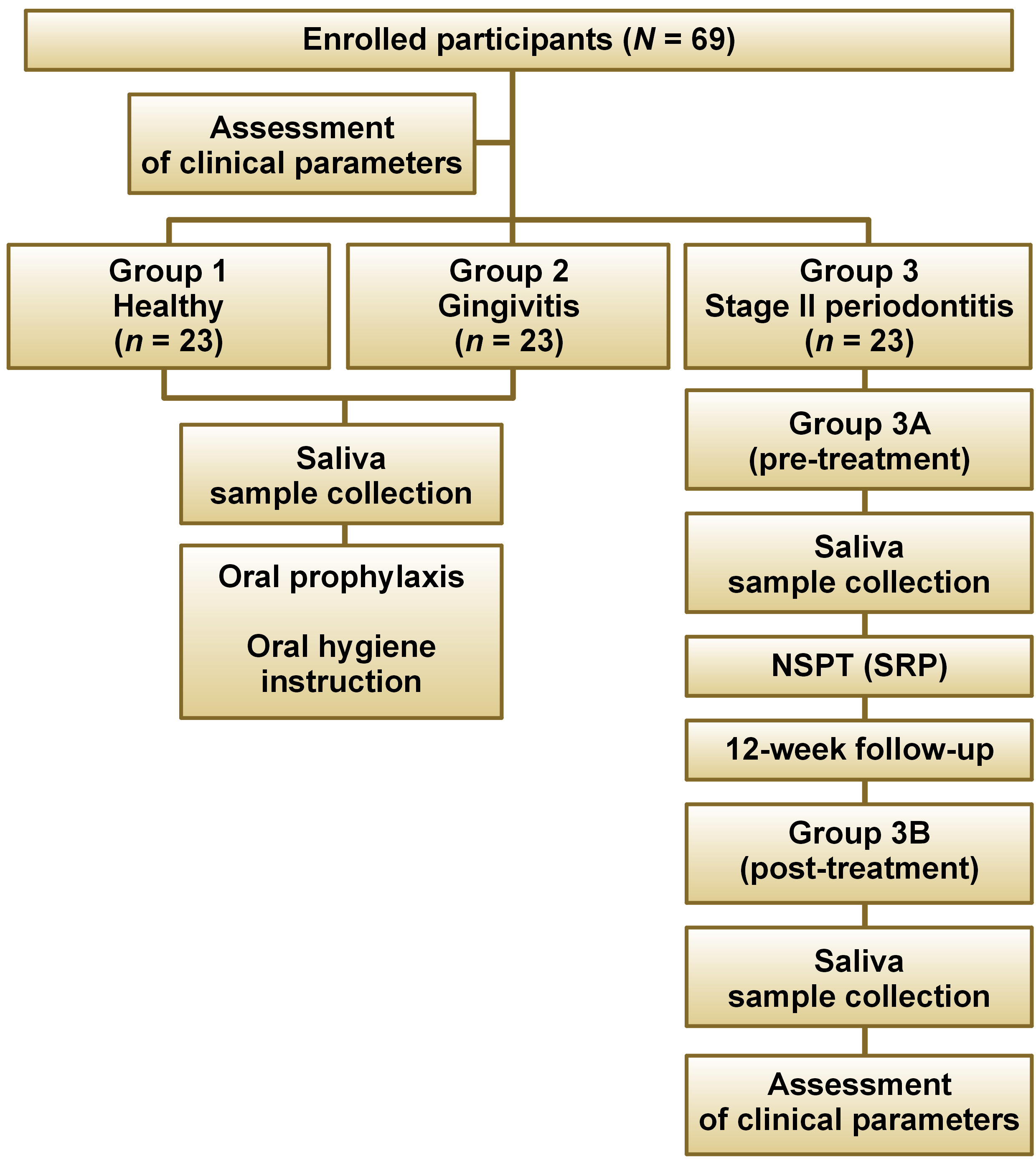
Each patient underwent a comprehensive oral examination. Socioeconomic demographic data, sex, age, and medical and dental history were recorded using structured proformas. The study participants were recruited from the Department of Periodontology at A B Shetty Memorial Institute of Dental Sciences, NITTE (Deemed to be University), Mangaluru, India. Prior to the initiation of the study, ethical approval was obtained from the Central Ethics Committee (NITTE) (Cert. No. ABSM/EC/114/2021). The study was registered with the Clinical Trials Registry – India (CTRI) under the identification No. CTRI/2021/12/038961. Written informed consent was obtained from all study participants. A total of 69 subjects were recruited for the study and subsequently categorized into 3 distinct groups: group 1 (healthy; n = 23); group 2 (gingivitis; n = 23); and group 3A (stage II periodontitis; n = 23). Saliva samples of 5 mL were collected from all study participants, and routine scaling procedures were performed on each subject. In group 3A, NSPT was performed, and the results were recalled after 12 weeks (group 3B (post-treatment)) to evaluate clinical parameters. Five milliliters of saliva sample were collected for the estimation of cytokine levels.
Clinical parameters
The UNC 15 probe was used to assess various periodontal health parameters at all sites, namely PD, CAL, gingival index (GI) (Löe and Silness, 1963),20 and BoP. The inclusion criteria for the study encompassed systemically healthy male and female patients aged 20–45 years with a minimum of 20 teeth present and who were non-smokers. The study excluded patients with less than 20 teeth, pregnant or lactating women, and menopausal women. The subjects who had undergone periodontal therapy within the preceding 6 months, patients with diabetes, hypertension, or other systemic diseases, and patients who were taking any medications were also excluded from the study.
Criteria for diagnosis
The flowchart of the study is presented in Figure 1. The participants were divided into 3 groups according to the American Academy of Periodontology (AAP) 2017 Classification of Periodontal and Peri-Implant Diseases and Conditions.21 Subjects with GI ≤ 1, PD ≤ 3 mm and BoP ≤ 10% were categorized as periodontally healthy (group 1). Patients with GI > 1, PD ≥ 3 mm and BoP ≥ 10% were diagnosed with gingivitis (group 2). Individuals with GI > 1, CAL ≥ 3 mm and PD ≤ 5 mm were classified as having stage II periodontitis (group 3). Group 3 was further subdivided into group 3A (before NSPT) and group 3B (after NSPT), as subjects with stage II periodontitis underwent NSPT i.e., scaling and root planing (SRP) (Figure 2), and were recalled after 12 weeks for the assessment of periodontal health parameters and saliva sample collection.
Saliva sample collection
The study participants were instructed to abstain from drinking and eating for 1 h prior to the collection of saliva samples. Participants were directed to sit comfortably in a quiet, enclosed space and thoroughly rinse their mouths with distilled water to ensure the removal of any food particles. They were then advised to adopt an upright posture with their heads in a lowered position, allowing the saliva to drip passively from the lower lip. Unstimulated whole saliva (5 mL) was collected into a graduated sterile container.
Analysis of IL-18 and IL-35 levels
Salivary levels of IL-18 and IL-35 were analyzed using a commercially available human enzyme-linked immunosorbent assay (ELISA) (Krishgen Biosystems, Cerritos, USA) at Central Research Lab of K.S. Hegde Medical Academy (NITTE). The centrifugation process was executed at a speed of 3,000 rpm for 10 min to remove cells and debris. The supernatant obtained was then transferred into a microcentrifuge tube in 0.5-mL aliquots. The samples were stored at −80°C until further analysis. The salivary concentrations of IL-18 and IL-35 were measured in each subject using human ELISA kits (Krishgen Biosystems) specific for each analyte, according to the manufacturer’s instructions.
Sample size calculation
In this interventional study, the calculation of sample size for one-way analysis of variance (ANOVA) was performed using the following formula (Equation 1):
where:
Z – confidence interval;
x – number of subjects;
k – number of groups;
σ – standard deviation (SD) = 0.925;
d – margin of error = 1; and
α, β – probabilities used to evaluate the results of the hypothesis.
The groups were compared at a 5% level of significance, with Z1−α/2xk = 2.39, and Z1–β = 1.28 (at 90% power).
The sample size required for the study was 23 subjects per group, resulting in a total of 69 subjects from groups 1, 2 and 3 being evaluated.
Statistical analysis
The descriptive data was expressed as mean (M) ±SD. A p-value of <0.05 was considered statistically significant. The χ2 test was performed to analyze subjects based on age, sex, occupation, and address. To compare IL-35 and IL-18 concentrations across different groups, the Kruskal–Wallis ANOVA test was carried out. Additionally, the Mann–Whitney U test was conducted for intergroup analysis. The Wilcoxon signed-rank test was used to compare the cytokine concentrations in saliva among groups 3A and 3B (non-normal distribution). The data was analyzed using nMaster Software, v. 2 (https://nmaster.software.informer.com/2.0/).
Results
There was no statistically significant association among the 3 groups based on age, sex, occupation, and type of settlement (Table 1). A comparison of IL-18 and IL-35 concentrations between healthy individuals, patients with gingivitis and stage II periodontitis (pre-treatment) exhibited the highest mean IL-18 levels in the gingivitis group (307.21 ±80.96 pg/mL) and the highest mean IL-35 levels in the healthy group (139.96 ±91.29 pg/mL) (Table 2). The mean differences (MDs) between groups 1 and 2 and between groups 1 and 3A were found to be −126.80 and −121.34, respectively. A statistically significant MD of 5.47 was reported between groups 2 and 3A (Table 3). A comparison of IL-18 and IL-35 levels between the pre- and post-treatment groups revealed a reduction in IL-18 levels, accompanied by an increase in mean IL-35 levels post-treatment. These values were statistically significant (Table 4). The observed and expected probing PD, GI, BoP, and CAL between the 3 groups were analyzed using the Kruskal–Wallis test (Table 5). Marked differences in clinical parameters were identified in GI, PD and BoP in group 1 when compared with groups 2 (−1.03; −0.58; −10.00), 3A (−1.10; −1.09; −9.69) and 3B (−0.02; −0.30; 1.69). The MDs observed between group 2 and group 3A as well as group 3B were statistically significant (GI: −0.08; PD: −0.51; BoP: 0.30 and GI: 1.01; PD: 0.28; BoP: 11.69, respectively) (Table 6).
Discussion
Periodontitis is a chronic, microbes-associated, host-mediated inflammatory condition that results in the loss of supporting periodontal tissues.22 Although the microorganisms present in the dental plaque biofilm are the cause of gingivitis and periodontitis, the etiology and concurrent tissue loss are the result of a chronic, inflammatory host immune response.23, 24 Cytokines are the signaling molecules that play an important role in inflammation, functioning as either pro- or anti-inflammatory agents in the immune system.25
In the present study, a significant difference and change in the level of IL-18 was observed between the healthy patients and individuals with gingivitis and stage II periodontitis. The gingivitis groups exhibited the highest levels of IL-18. The observed variations in IL-18 levels between the study groups were found to be statistically significant. The mean difference in the concentration between the stage II periodontitis (post-treatment) and gingivitis groups was found to be lower, whereas the difference in the concentration between the healthy and gingivitis groups was comparatively higher. The variation in IL-18 levels can be attributed to the progression of the disease, with the similar values in the gingivitis and stage II periodontitis groups potentially indicative of a nearly clinically unnoticeable transition in the gingivitis group to periodontitis. Prior studies have reported elevated concentrations of pro-inflammatory cytokines in periodontitis; however, few studies have evaluated the alterations in the expression levels of certain pro-inflammatory cytokines during the progression of periodontal disease.26, 27 The results of the present study are consistent with those of the study by Figueredo et al., who analyzed IL-18 levels in pooled GCF obtained from individuals with gingivitis and sites with periodontitis and gingivitis in patients with chronic periodontitis.16 According to the study, gingivitis sites in patients with periodontitis exhibited greater GCF IL-18 levels.16 Similar results were reported in a study by Pradeep et al.,11 which reported changes in the IL-18 levels during the progression of the disease, with only a limited number of samples from the gingivitis group exhibiting values that approximated those observed in the periodontitis group.27
Interleukin-35 is a novel member of the IL-12 family and an anti-inflammatory cytokine that possesses effective inhibitory properties by regulating Treg cells. It has been demonstrated that the strong inhibitory effect of T cell proliferation can be exerted by preventing mitosis without inducing apoptosis.28 The present study reported that the difference in the salivary concentration of IL-35 between the groups is statistically significant. The healthy group exhibited a significantly higher concentration compared to the gingivitis and stage II periodontitis groups. These findings align with those reported by Köseoğlu et al., who sought to determine the expression of IL-35 in GCF, saliva and plasma of participants with gingivitis, chronic periodontitis, and healthy individuals.29 In comparison with the gingivitis and chronic periodontitis groups, the healthy patients’ saliva showed a significantly higher concentration of IL-35.29
It has been suggested that the cellular response of pro-inflammatory cytokines is subject to negative regulation by anti-inflammatory cytokines, thereby establishing a balance between inflammatory cytokines and serving as a pivotal factor in determining the immune pathology of periodontal disease.30 In the present study, the levels of IL-18 and IL-35 were assessed at baseline and 12 weeks after NSPT in patients with stage II periodontitis. The mean IL-35 level was observed to be increased post-treatment compared to baseline, and this difference was statistically significant. A marked improvement was observed across all clinical parameters from baseline to 12 weeks following NSPT. The results demonstrated a decrease in the mean GI, BoP, PD, and an improvement in CAL 12 weeks after NSPT in patients with stage II periodontitis (p < 0.05). The obtained results are analogous to those of the study by Goswamy et al., who assessed and compared the levels of IL-35 in GCF of patients with moderate to severe periodontitis before and after NSPT.31 The results showed that, from the 1st to the 3rd week of post-periodontal treatment, there was an increase in IL-35 concentration in GCF along with a notable improvement in clinical indicators.31
In the present study, a marked reduction in the level of IL-18 was observed from baseline to 12 weeks after NSPT. These findings are consistent with the results obtained by Oliveira de Campos et al., who evaluated the effect of NSPT on the concentration of IL-18.32 The patients were categorized into 2 groups (gingivitis and periodontitis). Gingival crevicular fluid samples were obtained at baseline and 1 month after SRP. A significant difference in the plaque index (PI), GI, PD, CAL, and IL-18 was observed between baseline and 1 month post-NSPT.32
The primary goals of periodontal treatment are to eliminate inflammation, halt the progression of the disease, restore aesthetics, and establish an environment conducive to maintaining health.33 The first method recommended for treating periodontal infections is SRP, which is widely acknowledged as the gold standard.34 Scaling and root planing resulted in a significant enhancement in PD reduction, and an improvement in CAL and BoP values 4 weeks after its implementation compared to baseline. In addition, NSPT has significantly reduced the salivary concentration of IL-18, a key player in establishing a link between acquired and innate immune responses involving both amateur and expert immune cell lineages.35 Coversely, an increase in IL-35 levels post-treatment was also observed. The results reported in the previous studies are similar to these obtained in the present study.35
Van der Weijden and Timmerman evaluated the effect of subgingival debridement on clinical parameters (BoP, PD and CAL) in chronic periodontitis patients.36 Badersten et al. assessed the impact of NSPT on plaque scores, BoP, PD, and CAL at baseline and 5 months after NSPT, and observed marked improvement in clinical parameters.37 The results of the current study align with those of the abovementioned studies, as it reported improvements in clinical parameters 12 weeks after NSPT, with a reduction in PD, GI, BoP, and an improvement in CAL. These findings support the effectiveness of NSPT in patients with stage II periodontitis.
The balance between cytokines determines the degree of the host response to antigenic stimulation during the course of chronic inflammatory processes. In this study, changes in the IL-18 and IL-35 levels after SRP, along with the enhancement in the clinical parameters, are indicative of a balance between pro- and anti-inflammatory cytokines. This balance plays a pivotal role in the immunoregulation of periodontal disease. Therefore, this study supports the notion of counter-regulation of cytokines as a strategy to avert subsequent tissue destruction. There was no statistically significant difference between the 3 groups regarding the demographic data. This finding aligns with the longitudinal research conducted by Faddy et al., who reported no effect of age on periodontitis except for the regression in the healing process.38
In general, NSPT is an effective treatment for the suppression of periodontal disease progression. The observed improvement in CAL, BoP, PD and GI, with a lower salivary IL-18 concentration and an increase in the concentration of IL-35 suggest that IL-18 may comprise the subclinical activation of periodontal inflammation, while IL-35 may have a suppressive effect on its activity. The findings of this study reported a significant difference in IL-18 and IL-35 levels among healthy individuals and those with gingivitis and stage II periodontitis. This observation suggests that both interleukins could serve as possible diagnostic biomarkers for periodontal disease due to their distinct levels after NSPT.
Conclusions
The present study reported a decrease in the salivary levels of IL-18 and an increase in IL-35 levels between baseline and 12 weeks after NSPT in subjects with stage II periodontitis, suggesting that NSPT may be an effective mode of treatment in periodontitis. However, the study is not without its limitations, including a relatively small number of follow-up patients and a short follow-up period. Further investigations with prolonged follow-ups are warranted to provide a comprehensive understanding of the correlation between IL-18 and IL-35 and the impact of NSPT on periodontal health.
Ethics approval and consent to participate
The study was approved by the Central Ethics Committee (NITTE) (Cert. No. ABSM/EC/114/2021). Written informed consent was obtained from all study participants.
Trial registration
The study was registered with the Clinical Trials Registry – India (CTRI) under the identification No. CTRI/2021/12/038961.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.