Abstract
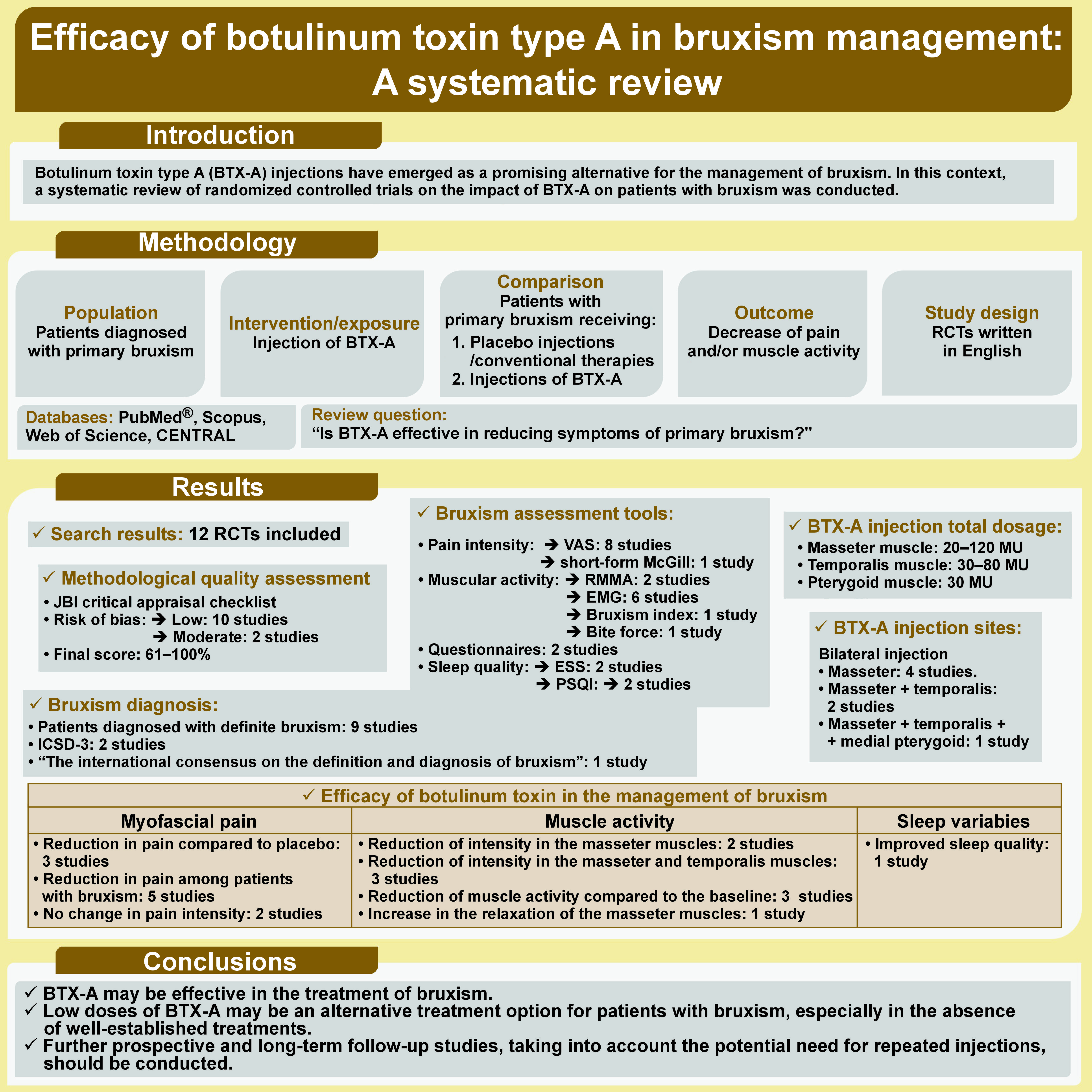
Botulinum toxin type A (BTX-A) injections have emerged as a promising alternative for the management of bruxism. In this context, a systematic review of randomized controlled trials on the impact of BTX-A on patients with bruxism was conducted. A literature search of multiple online electronic databases (PubMed®, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL)) was undertaken from their inception to February 1, 2024. The Medical Subject Headings (MeSH) included “Botulinum Toxins”, “Botulinum Toxins, Type A”, “Bruxism”, and “Sleep Bruxism”, which were combined with the Boolean operators “AND” and “OR”. The methodological quality of each included study was assessed using the Joanna Briggs Institute (JBI) critical appraisal tool. Reducing muscle pain and activity were assessed as primary outcomes, while the quality of sleep was considered as a secondary outcome. Twelve articles met the inclusion criteria. The risk of bias was low in 10 studies and moderate in 2. Bilateral injections of BTX-A into the masseter, temporalis and medial pterygoid muscles were compared to saline injections, the use of occlusal splints and conventional treatment. Of the 12 studies, 6 reported a reduction in muscle activity recorded by rhythmic masticatory muscle activity (RMMA) and electromyography (EMG) after the administration of BTX-A. In addition, 3 studies indicated that the intensity of muscle pain, measured using the visual analogue scale (VAS), decreased significantly in individuals with bruxism who received BTX-A. Finally, 1 study highlighted improved sleep quality in patients with bruxism who were rehabilitated with a single-arch implant overdenture and received either BTX-A or occlusal appliances. Botulinum toxin type A can effectively reduce symptoms of bruxism. However, the included studies exhibited heterogeneity and methodological differences. Long-term follow-up studies with large sample sizes and the incorporation of repeated injections are necessary to further validate the findings.
Keywords: therapeutics, systematic review, botulinum toxin, sleep bruxism
Introduction
Bruxism is a topic of interest in oral medicine.1 Lobbezoo et al. defined bruxism as “a masticatory muscle activity that occurs during sleep (characterized as rhythmic or non-rhythmic) and wakefulness (characterized by repetitive or sustained tooth contact and/or by bracing or thrusting of the mandible)”.2 Research has demonstrated that bruxism can have harmful effects on various structures within the mouth and contribute to tooth wear, damage to periodontal tissue, myofascial pain, headache, and muscular or joint problems.3 Two different categories of bruxism have been identified, namely sleep bruxism and awake bruxism, observed during sleep and wakefulness, respectively.2, 4 Lobbezoo et al. have emphasised the role of the masticatory muscles during sleep and wakefulness in provoking potential clinical consequences.2 Sleep bruxism is no longer considered as a parafunction or a disease but rather as a behavior.5 Classifications and definitions of bruxism have varied widely over the decades, along with the assessment tools and diagnostic criteria.2, 6 Non-instrumental (e.g., questionnaires, oral history, clinical examinations, and diaries), semi-instrumental (e.g., ecological momentary assessment) and instrumental (e.g., electromyography recordings, polysomnography records) approaches have been implemented to diagnose the condition.4 In addition, a grading system has been proposed to determine the degree of validity of these means of assessment in order to facilitate therapeutic approaches.2 Several treatment options are available for the management of bruxism, including occlusal splints, biofeedback, cognitive-behavioral approaches and pharmacological methods.7 Among these, occlusal splints are typically the preferred method for protecting teeth and dental prostheses from damage.7 However, there is insufficient evidence to support the effectiveness of occlusal splints in reducing sleep bruxism.8 Recently, local injections of botulinum toxin (BTX) have been increasingly used in the treatment of movement disorders and have received the attention for its efficacy in treating bruxism.
Botulinum toxin, also known by the brand name Botox, is an anaerobic bacterial endotoxin produced by the Clostridium botulinum bacterium.8 It has been the subject of research since the late 1970s regarding its therapeutic potential in the management of various neuromuscular disorders.9 Botulinum toxin blocks the action of neuromuscular transmission, which leads to muscle relaxation and decreased muscle contractions. A recent systematic review has shown the efficiency of BTX in treating refractory myofascial pain associated with temporomandibular disorders (TMD) by alleviating pain and increasing the pressure pain threshold.10 Since bruxism is a behavior characterized by repetitive masticatory muscle activity that may lead to TMD, researchers have proposed the administration of BTX injections to mitigate bruxism by reducing the contractions of the masseter muscles.9 Despite the widespread use of BTX in clinical practice, the efficacy and safety of this approach in the treatment of bruxism have not been fully established. Previous systematic reviews on the subject have included articles with subjective outcome values, such as reported pain reduction, instead of objective reduction of muscular forces and/or episodes of bruxism.7, 11, 12, 13, 14, 15 As a result, the conclusions that could be drawn from these reviews were limited to more subjective interpretations. It is therefore important to conduct a novel review in order to obtain more reliable and consensus-based results. This objective can be achieved by incorporating newly published articles that have not been systematically reviewed in a scientifically rigorous manner. Hence, this systematic review was carried out as an attempt to evaluate the efficacy of botulinum toxin type A (BTX-A) in the treatment of bruxism.
Material and methods
Protocol and eligibility criteria
The present study adhered to the guidelines established by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.16 The review protocol was registered with PROSPERO (identification No. CRD42023472755).
The inclusion criteria were established in accordance with the PICOS criteria, as follows: P (population) = patients diagnosed with primary bruxism; I (intervention/exposure) = injection of BTX-A; C (comparison): patients with primary bruxism receiving placebo injections or treated by conventional therapies or with a lower dose of BTX-A; O (outcome): decrease of pain and/or muscle activity; S (study design): randomized clinical trials (RCTs) written in English. The review question posed was: “Is BTX-A effective in reducing symptoms of primary bruxism?”.
The present study was conducted with no restrictions applied concerning setting, country, or period of the study. Studies that did not share the purpose of this systematic review, as well as studies reported in proceedings, books, dissertations, theses, and monographs, were excluded from consideration. Studies not fully published and those with data associated with other health problems and with secondary bruxism caused by psychological or neurological disorders were also excluded from the analysis.
Search strategy
A comprehensive electronic search of the PubMed®, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL) databases was conducted by 2 independent reviewers (GO and MK) to identify studies assessing the efficacy of BTX-A in the management of bruxism. The following Medical Subject Headings (MeSH) were used: “Botulinum Toxins”, “Botulinum Toxins, Type A”, “Bruxism”, and “Sleep Bruxism” combined with the Boolean operators “AND” and “OR”, as follows: (“Bruxism”[MeSH] OR “Sleep Bruxism”[MeSH]) AND (“Botulinum Toxins”[MeSH] OR “Botulinum Toxins, Type A”[MeSH]).
Disagreements among reviewers regarding the final inclusion of articles were resolved by consensus. The search was limited to articles published in English language before February 1, 2024.
Study selection
Two investigators (SY and GO) used the EndNote™ software, v. 9.0 (Clarivate™, London, UK) to eliminate duplicates and perform the initial screening of the articles based on their titles and abstracts. Subsequently, the full texts of eligible articles were assessed according to the established inclusion criteria. Any discrepancies between the 2 reviewers were resolved by the third reviewer (MK).
Data extraction
The data from the included studies was extracted in a specified format, including the population, the parameters being investigated, the periods of parameter collection, and the significant findings. The extracted data was then reviewed and analyzed by 2 authors (SY and GO). Subsequently, it was verified by the third author (MK). Any divergence in data collection was resolved through consensus.
Methodological quality assessment
The quality of the included studies was assessed according to the Joanna Briggs Institute (JBI) critical appraisal tool for the assessment of risk of bias for randomized controlled trials.17 The tool encompasses 13 items: randomization component; allocation concealment; similarity of treatment groups at baseline; blinding of participants; blinding of personnel; blinding of outcome assessors; groups treated identically other than the intervention of interest; follow-up; intention to treat; similar way of outcome measurement; reliable way of outcome measurement; statistical analysis; and trial design.
The methodological quality and risk of bias of each included study were analyzed independently by 2 authors (SY and OG). The reliability was scored as “yes”, “no”, “unclear”, or “not applicable”. In case of discrepancy, the third author (MK) reviewed the studies to reach a consensus.
The risk of bias in the studies was categorized as low (“yes” scores ≥70%), moderate (“yes” scores between 50% and 69%) or high (“yes” scores ≤49%).18
Results
Search results
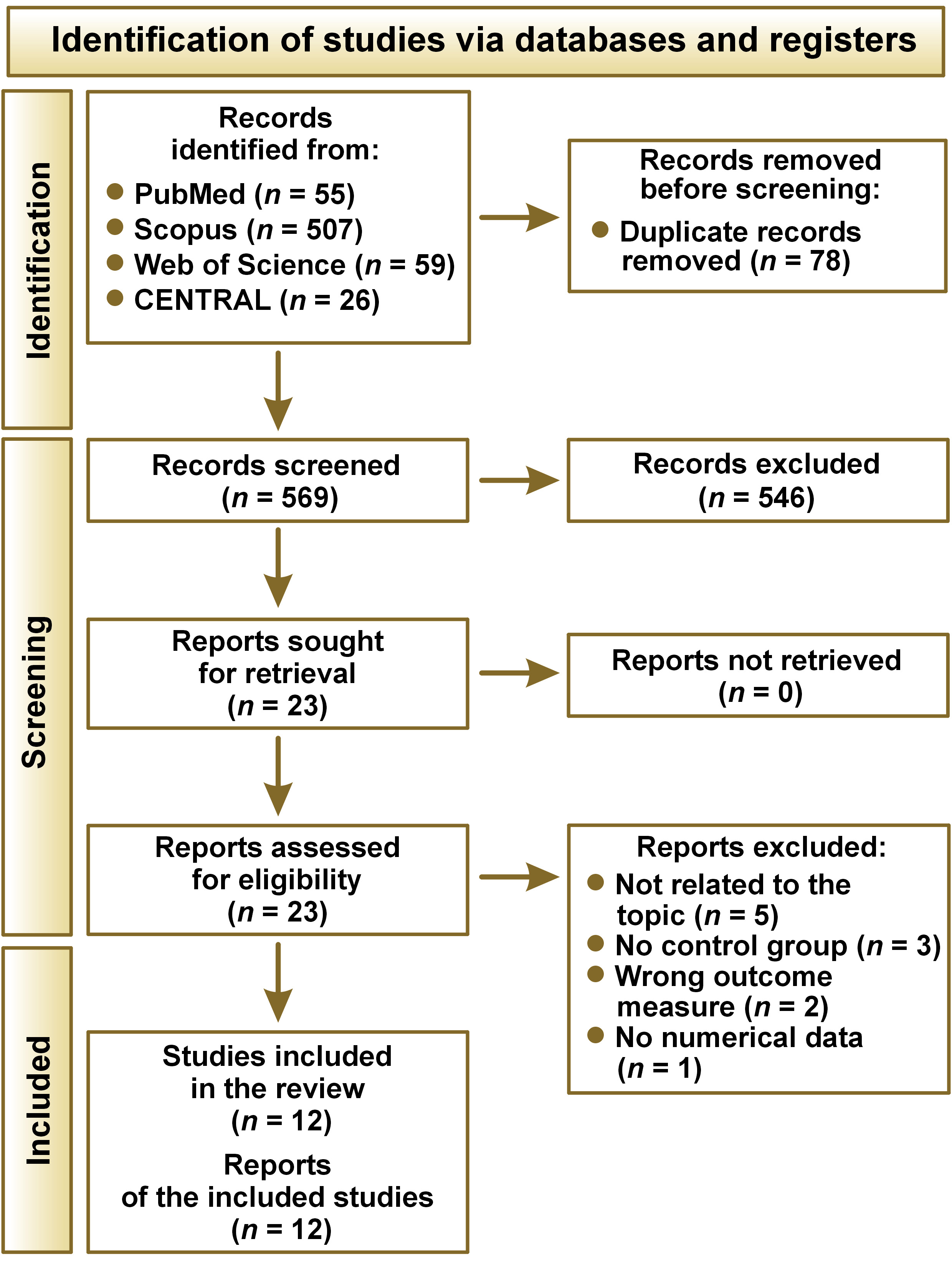
The search of the electronic databases yielded a total of 647 articles published between 2008 and 2024. After the elimination of duplicates, 569 papers remained for further consideration. In the first phase of the study, the titles and abstracts of these articles were reviewed, resulting in the selection of 23 studies.3, 8, 9, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 No additional paper was added after screening the reference lists of the 23 articles. In phase 2, the texts of the articles were read in full, with 11 papers excluded.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Ultimately, 12 studies3, 8, 9, 19, 20, 21, 22, 23, 24, 25, 26, 27 were included in the systematic review (Figure 1).
Methodological quality assessment results
The methodological quality of the included studies was assessed using the JBI critical appraisal checklist, with 10 articles classified as low risk of bias and the remaining 2 papers classified as moderate risk of bias. The final score ranged from 61% to 100% (Table 1). All studies reported data on the following items: 1 (i.e., randomization component); 7 (i.e., groups treated identically other than the intervention of interest); 8 (i.e., follow-up); 9 (i.e., intention to treat); 10 (i.e., similar way of outcome measurement); 11 (i.e., reliable way of outcome measurement); and 13 (i.e., trial design).
Study characteristics
After the selection of 12 articles,3, 8, 9, 19, 20, 21, 22, 23, 24, 25, 26, 27 the following information was extracted: first author; year of publication; study period; study design; follow-up duration; diagnosis of bruxism; inclusion and exclusion criteria; sample size; age and sex of participants; collected data and how they were assessed; and characteristics of BTX-A injection (Table 2). The included studies were published between 200826 and 2024.19 They were conducted in Saudi Arabia,19, 20 Egypt,25 Turkey,23 Australia,21 the United States,27 South Korea,3, 8, 24 Italy,26 India,9 and Syria.22 All studies were RCTs. The sample size ranged from 1224 to 5020 subjects, with 3 studies reporting a sample size calculation.19, 22, 25 Ten studies3, 8, 19, 21, 22, 23, 24, 25, 26, 27 included both males and females and 1 study included females only.20 Additionally, 1 study did not report any information related to sex.9 The mean age of the study participants ranged from 253, 24 to 5825 years. The duration of follow-ups varied from 4 weeks3 to 12 months.20, 25
Diagnosis of bruxism
The included studies showed different approaches to bruxism diagnosis through a combination of both subjective and objective criteria. These methods included the evaluation of clinical signs and symptoms (such as muscle pain, tooth grinding, attrition in occlusal surfaces of posterior teeth), medical questionnaire and electromyography (EMG). Patients were diagnosed with definite bruxism in 9 studies.3, 9, 19, 20, 21, 23, 25, 26, 27 Two studies21, 27 applied the International Classification of Sleep Disorders – Third Edition (ICSD-3), whereas “The international consensus on the definition and the diagnosis of bruxism” by Lobbezoo et al. was followed in 1 study.19
Outcome assessment
Different bruxism characteristics were evaluated, including muscle pain19, 20, 21, 22, 23, 26, 27 and muscular activity.22 Pain intensity was measured using the visual analogue scale (VAS)9, 19, 20, 21, 22, 23, 26, 27 and the short-form McGill Pain Questionnaire.21 Muscular activity was recorded through rhythmic masticatory muscle activity (RMMA),3, 8 EMG,3, 8, 21, 22, 24, 27 bruxism index (BI),21 and bite force.23 Bruxism symptoms were also investigated using various questionnaires.21
The secondary outcome was the participants’ quality of sleep. The Epworth Sleepiness Scale (ESS)21, 27 and the Pittsburgh sleep quality index (PSQI)25, 27 were used to evaluate this outcome.
BTX-A injection
Botulinum toxin type A was injected in all subjects in the included studies. Among the 12 RCTs, in 48, 19, 22, 24 and 2 trials,26, 27 respectively, bilateral injections were administered into the masseter muscles and both the masseter and temporalis muscles. Controls received placebo injections of isotonic saline. Cruse et al. extended their comparisons to include bilateral injections into the masseter, temporalis and medial pterygoid muscles, with a control group receiving saline injections.21 On the other hand, bilateral injections of BTX-A into the masseter muscles were compared to occlusal splints23 and conventional treatments for bruxism, which included behavioral strategies, occlusal splints and pharmacological treatment.20 Only 1 investigation compared bilateral BTX-A injection in the masseter muscles alone with combined injections in the masseter and temporalis muscles.3 Jadhao et al. included 3 groups and compared bilateral BTX-A injections into the masseter and temporalis muscles to a control group.9 The control group received saline injections, and a second control group was not subjected to any intervention.9 Finally, a study by Ali et al. used bilateral injections of BTX-A into the masseter and temporalis muscles and compared it to conventional occlusal stents and a second control group that did not receive any intervention (patients were instructed to only remove the overdenture at night).25
The total dosage of BTX-A injections administered to the masseter muscles ranged from 20 mouse units (MU)22 to 120 MU.27 In the temporalis muscles, the dosage ranged from 30 MU21 to 80 MU.27 Cruse et al. injected 15 MU into each medial pterygoid muscle (Table 2).21
Efficacy of botulinum toxin in the management of bruxism
The main results of the published studies assessing the impact of BTX-A on bruxism are presented in Table 3.
Myofascial pain or jaw stiffness
Three studies20, 22, 27 indicated a significant reduction in pain scores in the BTX-A group compared to the placebo group, while 1 investigation20 reported the same reduction in the treatment group compared to conventional treatments. On the other hand, 5 studies demonstrated the efficacy of BTX-A in addressing bruxism-related myofascial pain symptoms.19, 20, 22, 23, 26 Specifically, Guarda-Nardini et al. observed a significant pain reduction during chewing after 6 months in individuals with bruxism who received BTX-A, as compared to those who received a placebo.26 In addition, Kaya and Ataoglu reported that both occlusal splints and BTX-A were effective in alleviating pain associated with bruxism.23 However, BTX-A was found to be slightly less effective in pain reduction compared to occlusal splints. Notably, BTX-A injections still provided a significant reduction in pain symptoms, making them a viable and alternative treatment option.23 Two other studies21, 27 reported contradictory results in terms of pain reduction following the injection of BTX-A. No evidence was found for change in pain intensity as measured by the short-form McGill Pain Questionnaire21 or the VAS21, 27 compared to the control group and before injection.
Muscle activity
Three studies3, 9, 22 demonstrated the efficacy of BTX-A injections for sleep bruxism, as evidenced by the reduction in the intensity of both the masseter and temporalis muscles in comparison to the control groups. However, this reduction was observed only in the masseters in 2 other studies.8, 24 Three studies reported that the administration of BTX-A into the masseter muscle8, 24 and into both the temporalis and masseter muscles3 reduced muscle activity compared to the baseline. On the other hand, Kaya and Ataoglu reported that BTX-A administration effectively increased the relaxation of the masseter muscles.23 The study demonstrated that BTX-A injections offer a promising approach to alleviate muscle-related pain and discomfort in patients with bruxism, providing a potential alternative for those who may not be able to use traditional occlusal splints or who have contraindications for other treatments.23 Cruse et al. demonstrated a decrease in BI in the masticatory muscles evaluated by EMG between the experimental and control groups.21
Sleep variables
Ali et al. reported that BTX-A and occlusal appliances effectively improve sleep quality in patients with bruxism who have been rehabilitated with a single-arch implant overdenture, as compared to the control group and the values registered prior to the injection.25
Discussion
The present systematic review included 12 RCTs3, 8, 9, 19, 20, 21, 22, 23, 24, 25, 26, 27 that reported the effects of BTX-A on primary bruxism. Although BTX-A doses and injection sites varied widely between the studies, a promising conclusion was retained: BTX-A injection can be an effective management strategy for bruxism.
Scope of the study
Bruxism is defined as repetitive masticatory muscle activity, which refers to the involuntary and non-functional grinding or clenching of teeth, occurring during sleep or while awake.2, 32 Bruxism is a behavior that may have several etiologies.5, 30 Central nervous system involvement in the pathophysiology of bruxism has been demonstrated, with a role of brain neurotransmitters,39 including the serotoninergic pathway.40 Bruxism affects the quality of sleep and muscle activity, causes pain in the teeth, temporomandibular joints and mastication muscles, as well as leads to tooth decay.3 Despite the existence of various therapeutic modalities, including pharmacological and non-pharmacological approaches, none of these techniques has been fully effective for bruxism management.21, 36 Firstly, behavioral approaches necessitate that the patient be conscientious and observant. The latter is imperative for massage sessions.14 Secondly, pharmacological treatment involves the administration of various drugs, such as clonazepam, clonidine, buspirone, clozapine, gabapentin, and amitriptyline.41 Although these molecules are effective in reducing bruxism pain, many drug interactions in patients with other diseases contraindicate their use.42 Particular care should be taken when prescribing these medications due to their potential adverse effects.41 Moreover, de Baat et al. concluded that “there are insufficient evidence-based data to draw definite conclusions concerning medications attenuating sleep bruxism and/or awake bruxism”.41 Additionally, given that bruxism is a condition that causes phasic or tonic masticatory muscle activity, the use of splints has been explored as a means of mitigating muscle contractions.29 A recent systematic review has concluded that there is an absence of sufficient evidence to prove the effectiveness of occlusal splints in the treatment of sleep bruxism, thus recommending a multidisciplinary approach encompassing the use of occlusal splints in conjunction with complementary therapies, such as massage therapy.43 Over the last 2 decades, an increasing number of studies have explored the efficacy of BTX-A in mitigating nocturnal bruxism, with encouraging outcomes reported.22 In addition, patients may exhibit a higher motivation for BTX-A injections compared to the nocturnal use of splints or repeated psychotherapy sessions.14
Efficacy of BTX-A injection
The present review provides a summary of the current evidence regarding the efficacy of BTX-A in the targeted management of bruxism. The studies included in this systematic review reported a short-term assessment that consolidated the analgesic effect of BTX-A; only 2 studies reported a follow-up of more than 6 months after the end of the intervention.20, 25 However, of the 12 studies, only 6 reported a reduction of muscle activity after administering BTX-A.3, 8, 9, 21, 22, 24 Nevertheless, this reduction was observed in the masseter muscles, and not in the temporalis muscles, in 1 study.24 Shim et al. found that the injection of BTX-A to the muscles reduced their activity in the temporalis muscles, but not in the masseters.3 In addition, 3 studies19, 22, 27 indicated that the intensity of muscle pain significantly decreased in patients with bruxism who received BTX-A. Finally, 1 study highlighted that patients with bruxism experienced enhanced sleep quality following the administration of BTX-A.25
The heterogeneity of the results may be attributed to methodological differences among the included studies, which precluded the possibility of conducting a meta-analysis to synthesize the data. Three remarks related to the differences regarding the BTX-A injection should be highlighted. First, concerns have been raised about the usefulness of injecting areas other than the masseter muscles. The masseters are the principal muscles responsible for both grinding and clenching movements observed during bruxism.44 Secondly, the variability concerned doses which ranged from 20 MU22 to 120 MU27 and from 30 MU21 to 80 MU27 for masseter and temporalis muscles, respectively. In addition, the dosage of BTX-A products is influenced by their formulation.45 For example, 1 MU of Botox (Allergan Aesthetics, Irvine, USA) is equivalent to 3–5 MU of Dysport® (Ipsen, London, UK).46 Thirdly, the varied frequency of injections could possibly lead to different results. Ali et al. opted for repetitive injections every 3 months,25 despite the fact that recent studies have demonstrated the longevity of toxin action for months after its single use.47 In fact, the attenuation of symptoms was still significant 12 months later despite the unique intervention in 1 study.20
In this context, future studies should ascertain the optimum characteristics of BTX-A injections for individuals with bruxism. The authors of the present study recommend that injections be administered exclusively into the masseter muscles, with ultrasound assistance. The principal advantage of this technique is the quantification of muscle thickness.30 In addition, musculoskeletal ultrasound is a useful guidance for interventional procedures, has high spatial resolution, allows for serial evaluations, and is widely available.48 A maximum dose of 100 MU of BTX-A is proposed per dental session.49 Larger doses can cause side effects such as dysphagia, dysphonia, dry mouth, headache, and nervous atrophy.50 In addition, sensitivity and mild cutaneous reactions at injection site are frequently observed.50 Animal studies reported the possible systemic adverse effects after the injection of BTX-A, which include transient weakness, fatigue, nausea, and pruritus.51 These effects are presumed to result from BTX-A diffusion into the bloodstream.51 However, the studies included in this systematic review did not report any side effects. Therefore, the injection of BTX-A may be considered safe for the management of pain in patients with bruxism.20, 21
Biological mechanisms underlying the effects of BTX-A injection
Botulinum toxin, recognized as the most potent neurotoxin, is produced by C. botulinum.20 The present systematic review revealed that BTX-A may be efficacious in managing symptoms of bruxism. Botulinum toxin acts as a muscle relaxant and provides an analgesic effect in neuromuscular disorders.47 Additionally, it reduces bruxism pain through 2 mechanisms.52, 53 The first mechanism involves the antinociceptive effects of BTX-A. Studies have highlighted that BTX-A impedes the release of substance P and calcitonin gene-related peptide (CGRP).52, 53 Moreover, based on the examination of mechanical sensitivity of dural afferents, BTX-A has been shown to reduce the activity of mechanosensitive receptors and the transient receptor potential ankyrin 1 (TRPA1) channel. These processes elucidate the antinociceptive activity of BTX-A.47 The second mechanism involves the masseter muscle, which is induced to a state of rest by BTX-A injection. The final mechanism involves the reduction of exocytotic release of acetylcholine among the motor nerve terminals by inhibiting the fusion of synaptic vesicles with the pre-synaptic membrane at the neuromuscular junction.54 This mechanism stems from hydrolysis of synaptosomal-associated protein 25 (SNAP-25), a vital component within the vesicle docking system responsible for exocytosis.54 It is an integral part of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex, which plays a crucial role in facilitating the docking and fusion of synaptic vesicles.55 Consequently, the source of muscle contraction is blocked.47 Based on these 2 theories, the efficacy of BTX-A was tested in several pathologies that cause increased painful muscle tone, and it might be useful to manage bruxism and myofascial pain.47, 52 In addition, some studies indicated that the BTX-A effect persists for months after its single use.47 The principal contributing factor is the BTX-A protease, which has the capacity to escape from cellular degradation mechanisms and survive in the cell cytoplasm.47
Despite the recent biological findings, no study has yet reported on the efficacy of BTX-A injections for reducing pain with a constant and long-lasting effect following a single injection. Patients were followed up for short periods, whereas bruxism is a chronic condition. Longer follow-up observations are necessary to determine the long-term effectiveness of BTX-A in treating bruxism. Therefore, upcoming controlled clinical studies should extend their observation periods to more than 4 months.
Discussion of the methodology
The evaluation of treatment efficacy or preventive interventions is more rigorous through RCTs.56 In effect, a low score of risk of bias was attributed to 83% of the included studies. As systematic reviews have the risk of being affected by bias at the level of individual studies, the validity of these studies is necessary to estimate when conducting this type of review.57, 58 Indeed, the true intervention effect may be over- or underestimated.57
The included studies employed a variety of tools and methods for bruxism diagnosis, a factor that can introduce discrepancies. None of the included studies mentioned the depth of the BTX-A injection.3, 19, 20, 22, 27 The primary outcome assessment of patients in the included studies focused on bruxism characteristics, which were evaluated using various assessment tools. Initially, muscle pain was assessed and analyzed. To this end, 8 studies employed VAS, a unidimensional pain rating scale,9, 19, 20, 21, 22, 23, 26, 27 while only 1 study used the short-form McGill Pain Questionnaire,21 a multidimensional questionnaire that provides a description of pain aspects in adults with chronic pain.21, 59
In order to assess the impact of BTX-A injection on patients’ quality of life, 3 studies considered sleep quality as a secondary outcome and used ESS and/or PSQI for evaluation.21, 25, 27 Although ESS is a valid tool commonly used to measure sleepiness, it is important to consider its limitations in order to avoid bias.31 Recent studies have demonstrated the clinical application of PSQI and its efficacy in sleep measurement.60
Limitations
The current systematic review is subject to 4 limitations. The first limitation concerns the number of patients included in the studies, which varied between 12 and 50. In fact, only 3 studies reported on the method of sample size calculation.19, 22, 25 While the results of studies are promising, the quality level of the evidence is not high enough to provide explicit guidelines for bruxism. Consequently, upcoming studies should include a sufficient sample size to ensure the representativeness of the studied population and to minimize the risk of bias.61 The second limitation is related to the assessment of bruxism characteristics. Only 3 studies evaluated the quality of sleep,21, 25, 27 while the muscle activity was assessed in 6 studies.3, 8, 9, 22, 23, 24 Future works should formulate common criteria for the assessment of bruxism to ensure the attainment of conclusive results. Thirdly, the included RCTs explored events related to pain and bruxism only. Subsequent studies should also evaluate the alleviation of bruxism complications after BTX-A injections, such as tooth wear.7 Fourthly, concerning control groups, it would be preferable to opt for traditional therapies rather than placebo injections. In fact, this comparison allows for widening the gap between the 2 therapeutic approaches and, thus, emphasizes the effect of BTX-A injections. From an ethical standpoint, even if control patients are engaged in research activities, it is advisable to recommend a standard therapy to them in order to alleviate their pain, even if only minimally.
Conclusions
This study investigated the impact of BTX-A injections on patients diagnosed with bruxism. A comprehensive review of the relevant literature revealed that BTX-A may be effective in the treatment of bruxism. Therefore, low doses of BTX-A may be an alternative treatment option for patients with bruxism, especially in the absence of well-established treatments. Further prospective and long-term follow-up studies, taking into account the potential need for repeated injections, should be conducted.
Trial registration
The protocol of the review was registered with PROSPERO (identification No. CRD42023472755).
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.