Abstract
The presence of toxic metals in the human environment can have detrimental effects on people’s well-being. This literature review examines the ways in which various environmental and non-environmental factors can contribute to the accumulation of heavy metals in hard dental tissues. It is of the utmost importance to ensure the safety of the environment by restricting the presence of toxic metals originating from both industrial and non-industrial sources. The aim of this study is to analyze current research and identify the primary sources of heavy metal exposure and the mechanisms by which these metals are deposited in dental tissues. Moreover, the objective of this review is to synthesize data from various studies to determine the main environmental and non-environmental sources of toxic metal exposure that contribute to their presence in dental tissues, as well as the biological and chemical processes that are responsible for the deposition of heavy metals in hard dental tissues. Additionally, the review aims to assess the impact of heavy metal accumulation on dental health and its potential systemic effects on overall well-being. The accumulation of heavy metals in the teeth is influenced by a number of factors, such as age, systemic conditions, the nutritional status, and dental caries. The presence of supernumerary teeth results in altered levels of microelements, including an increase in cadmium (Cd) and copper (Cu). Additionally, smoking exacerbates toxic metal accumulation, especially Cd and lead (Pb), and disrupts the balance of essential minerals within the teeth. These findings underscore the impact of environmental pollution on dental health and highlight the potential of teeth as biomarkers of environmental exposure, emphasizing the need for continued research to address the health risks associated with environmental toxins.
Keywords: teeth, biomonitoring, toxic metals, bioelements
Introduction
The environment in which humans reside and work is characterized by a notable and pervasive presence of heavy metals. This category encompasses a variety of elements, such as zinc (Zn), copper (Cu) and iron (Fe), that play crucial roles as micronutrients in the human body. It also includes metals that are not essential for life processes, such as cadmium (Cd), mercury (Hg) and lead (Pb). It is vital to carefully manage the levels of these metals as trace elements, since both deficiencies and excesses can have harmful effects on human health.1, 2, 3
The role of Zn in the body is crucial for the production of insulin, as well as the synthesis of proteins and nucleic acids. Insufficient levels of Zn within the body may contribute to the development of obesity and diabetes. The uneven distribution of Zn may impede the onset of metabolic disorders and diabetes by regulating several biological processes. The influence of Zn on a range of hormones, including testosterone, growth hormone and gonadotropins, is widely recognized. Zinc also takes part in the synthesis, storage and secretion of insulin. Additionally, it is a crucial component of thymulin, which is responsible for T cell maturation and differentiation. Recent studies have indicated that Zn is crucial in the development and maintenance of bone tissue homeostasis. The element plays an essential role in bone tissue, not only serving as a constituent but also facilitating the formation of the collagen matrix, the process of mineralization, and the turnover of bone. Zinc deficiency can lead to a gradual reduction in bone density. Bones that lack or have a severe deficiency of Zn are known to be thin, fragile and exhibit increased resorption.4, 5
Copper is an essential element in numerous physiological processes. It plays a key role in the synthesis of hemoglobin, the formation of bones, the deposition of calcium (Ca) and phosphorus (P) in bones, the absorption of Fe, and the hardening of collagen. Additionally, Cu exhibits antiviral properties and aids in detoxification processes.6, 7 It functions as a coenzyme in a number of enzymes, such as cytochrome c oxidase, representing a crucial component in the process of cellular respiration. Furthermore, Cu participates in the process of keratinization of hair and coat. A deficiency in Cu results in the inhibition of melanin synthesis, a reduction in the immune response, an increased likelihood of infections, an elevated risk of cardiovascular disorders, and impaired cholesterol metabolism.6, 8
Lipid metabolism is a complex process that involves various factors, one of which is Fe. Iron plays a crucial role in oxidation processes and serves as a constituent of important molecules such as cytochromes, hemoglobin and myoglobin.9, 10 One of its primary functions is to facilitate the transportation of oxygen, storage of transitional tissues and cellular utilization of oxygen. Additionally, it is equally significant in the cytochromes that are housed within the mitochondria. Iron is involved in the transfer of electrons in the electron transport chain.6 It is stored in the liver, spleen and bone marrow through proteins called ferritin and hemosiderin, both of which have a strong affinity for the element. Additionally, Fe plays a role in erythropoiesis, the formation of leukocytes and immune reactions, impacting both cellular and humoral immunity. Furthermore, the presence of Fe is of the utmost importance for the optimal performance of osteoblasts, which are the cells responsible for bone development.11, 12
Nevertheless, it is important to acknowledge the presence of heavy metals under specific circumstances. The elements that occur naturally within the Earth’s crust play a role in various natural phenomena, including the erosion of rocks, oceanic evaporation, the formation of soil, and volcanic eruptions.13 The human body can be exposed to heavy metals through various means, including air, water and soil. Soil contamination can occur due to the presence of fertilizers, plant protection products, industrial dust, and sewage. The absorption of water by plants from contaminated soil results in the uptake of toxic metals, which subsequently accumulate in their tissues. However, it is worth noting that the inhalation route provides the most accessible means of absorption. A number of industrial sectors serve as the primary sources of environmental contamination. These include metallurgy, electrotechnology and the chemical industry, as well as the production of fertilizers, paints and solvents. Metals are also heavily present in car exhaust emissions. Additionally, cigarette smoking is a significant non-industrial factor that exposes individuals to high levels of heavy metals. It is crucial to take into account the presence of toxic metals in amalgam fillings and the alloys utilized in prosthetics and orthodontics. Furthermore, the presence of heavy metals has been identified in a range of cosmetics and dietary supplements.1, 2, 3
Metal toxicity operates through various mechanisms, which can be classified into 3 primary categories. These classifications encompass the obstruction of vital functional groups within proteins, the displacement of metal ions that act as cofactors for enzymes and other functional proteins, and the modification of the spatial arrangement of proteins.14 A wide array of illnesses can be attributed to the presence of heavy metals. The manifestation of the harmful impact of toxic metals on the human body is contingent upon a number of factors, including the specific type of metal, the dosage, the method of exposure, the duration of exposure, and the individual’s personal susceptibility. Heavy metals have an impact on various cellular organelles and components within biological systems. These include the cell membrane, mitochondria, lysosomes, endoplasmic reticulum, nuclei, and certain enzymes that are critical for metabolic processes, detoxification and the repair of damage.15 Toxic metals, in addition to their toxic properties, possess the capability to accumulate within human parenchymal tissues. Once they enter the body, these metals tend to accumulate in the kidneys, liver and pancreas.16 Additionally, heavy metals accumulate in the hard tissues of teeth.1, 2, 3 The gradual deterioration of physical, muscular and neurological capabilities, which resembles the symptoms of diseases such as multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, and muscular dystrophy, can be attributed to extended contact with specific metals and their compounds. Some metals possess properties that have been linked to the development of birth defects and cancer. The progression of cancers can be influenced by certain heavy metals, which stimulate cancer cells through various pathogenic connections and may also decrease their receptiveness to treatment.1, 2, 17
The significance of biomonitoring in the context of health and the environment is gradually increasing. As a component of environmental dentistry, biomonitoring encompasses a range of actions aimed at evaluating the condition of the environment through the use of biomonitors. These biomonitors, which are utilized to assess the exposure to toxic metals in humans, are known as non-invasive matrices. Examples of such matrices include hair, nails, urine, saliva, and teeth. Among these, deciduous teeth are particularly convenient for obtaining biological material, resulting in a wealth of studies that analyze the levels of toxic metals present in their structure.1, 3 The teeth of an individual are an enduring testimony to their lifestyle within a specific environment and an indicator of the influence of environmental pollutants. During the process of mineralization, noxious metal cations become incorporated into the crystalline framework of hydroxyapatites. These detrimental substances gain access to the tooth tissues via the bloodstream.18, 19
It is important to acknowledge that chelation therapy has been the main approach to the management of cases of heavy metal poisoning. This method employs the use of chelating agents to form chelates, which are complex ring-like structures that trap metal ions and facilitate their removal from the body. However, the use of metal chelators has its limitations. Metal chelators have the potential to facilitate the migration of heavy metals from other regions of the body to the brain, which can escalate the neurotoxicity of the individual in question. Moreover, the use of these chelators may lead to the depletion of important metals, including Zn and Cu, resulting in serious side effects, such as liver damage.20, 21
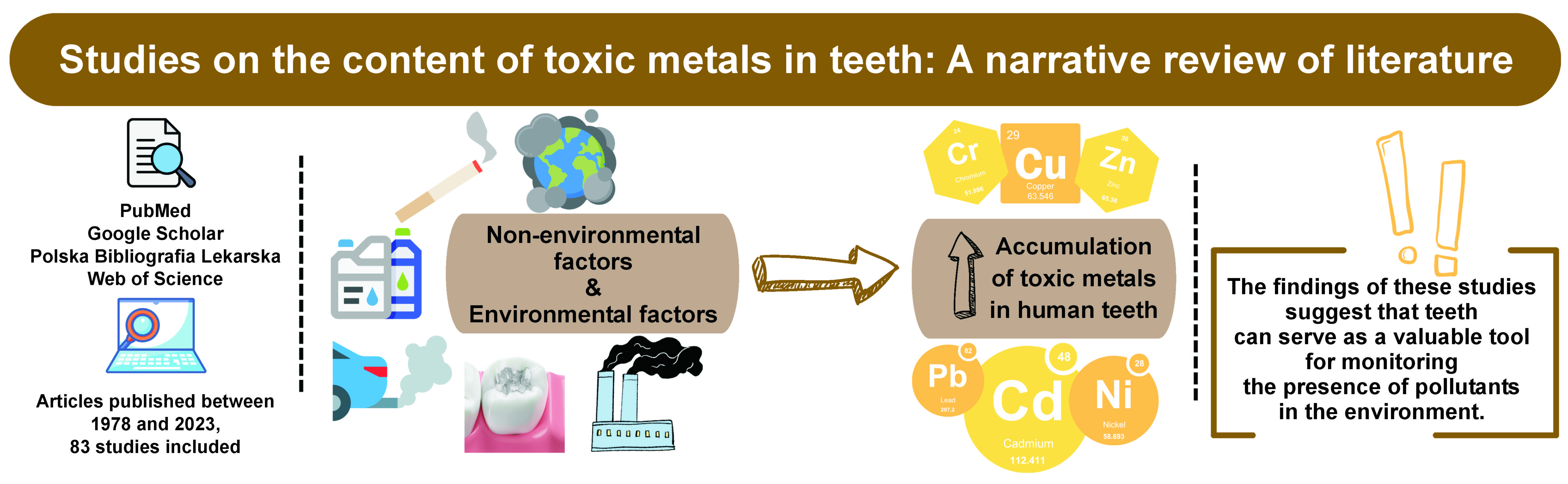
The content of toxic metals in dental tissues is influenced by both industrial and non-industrial factors. The following paragraphs will present a comprehensive analysis of this dichotomy, offering a more exhaustive investigation and inquiry. Within the discourse, certain publications that explore the composition of heavy metals within teeth and the various factors that contribute to their presence will be discussed.
This review offers a novel perspective by examining the interplay between environmental and non-environmental factors in the accumulation of heavy metals in dental tissues. It integrates recent research to identify key sources of toxic metal exposure, elucidate the biological mechanisms of metal deposition in teeth, and assess the broader health implications of this accumulation. The aim of this review is to enhance understanding of the potential of teeth as biomarkers for environmental exposure, to assess the impact of environmental factors on both dental and systemic health, and to underscore the importance of developing diagnostic and preventive strategies. Furthermore, the study emphasizes the need for improved environmental safety measures.
Material and methods
Literature search strategy
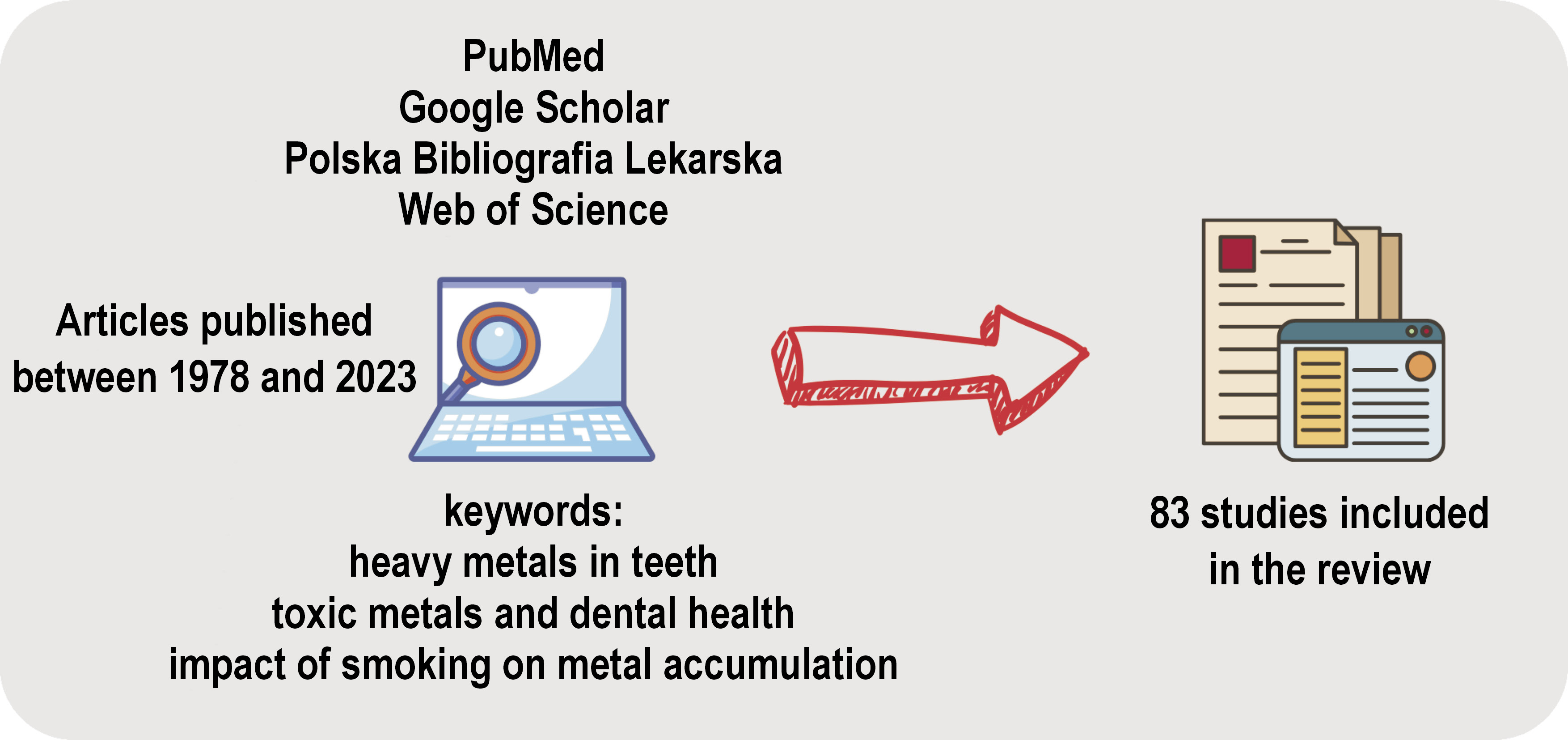
A comprehensive literature search was conducted using the following databases: PubMed; Google Scholar; Polska Bibliografia Lekarska; and Web of Science. The search was limited to articles published between 1978 and 2023. The keywords and phrases used in the search included “heavy metals in teeth,” “toxic metals and dental health” and “smoking impact on metal accumulation.” Boolean operators AND and OR were employed to refine the search results. The search was limited to articles published in English and Polish.
Inclusion and exclusion criteria
The articles included in the review examined the impact of heavy metals on dental health or associated environmental factors, were peer-reviewed and focused on human subjects. Non-peer-reviewed articles, articles not available in full text, and studies with an unrelated focus were excluded from consideration.
Selection process
The articles were subjected to a screening process based on their titles and abstracts. Full-text articles were reviewed for relevance, resulting in the inclusion of 83 studies. Duplicate articles were excluded during the screening process. The methodology is presented in Figure 1.
Data extraction and analysis
Data extraction involved reviewing abstracts and full-text articles to identify relevant studies. The extracted information included study design, sample size, metal types examined, and key findings related to heavy metal impact on dental health. We employed a narrative synthesis approach to integrate and summarize findings across studies. This involved categorizing results according to themes such as types of metals, mechanisms of impact, and health outcomes.
Quality assessment
The quality of the included studies was evaluated based on the study design, the sample size and the methodological rigor. No specific assessment tools were employed; rather, a critical appraisal was conducted to evaluate the reliability and relevance of each study.
Results
Age and sex
The levels of toxic metals found within human teeth can be influenced by age. Fischer and Wiechuła conducted a study with the objective of analyzing the accumulation of Pb in the calcified tissue of permanent teeth.22 Atomic absorption spectroscopy (AAS) was employed to measure the concentration of Pb in teeth samples from a particular group of Polish individuals. The research aimed to determine the accumulation of Pb in the human body based on the changes in Pb concentration in teeth from individuals aged 13–84 years. The results of the study showed that the concentration of Pb increased with age in calcified tooth tissues, and this was a statistically significant process. The research also revealed that subjects over the age of 60, born in the 1930s, had a lower concentration of Pb compared to those born in the 1950s. The concentration of Pb in the teeth of younger individuals (<60 years) was observed to increase. The analysis of changes in Pb levels revealed that even low exposure can result in a relatively high accumulation of Pb concentration in calcified tooth tissues. Fischer et al. have analyzed the changes in the concentrations of various elements, including manganese (Mn), Fe, magnesium (Mg), Cu, potassium (K), chromium (Cr), Pb, Cd, and Ca, in deciduous teeth.23 The study aimed to investigate whether metal concentration changes with age and to describe changes in mineral composition. The researchers obtained deciduous teeth samples from children aged 5–14 years living in southern Poland through non-invasive physiological replacement. Metal concentration was determined via AAS. The results demonstrated a significant decrease in the concentration of the analyzed elements in the deciduous teeth of older children, when compared to those of younger children. However, no significant correlation was observed between the total metal concentration and the Ca content in relation to age.23
Chromium is a vital element for the human body. Although it has a significant physiological function, it can also be harmful, with potential carcinogenic, mutagenic, embryotoxic, and teratogenic effects, which depend on its valence state. Malara et al. conducted research into the various factors that influence the presence of Cr in teeth.24 The objective of the research was to evaluate the influence of tooth type, age and sex on the level of Cr present in the tooth structure. The study sample consisted of permanent teeth sourced from individuals aged between 20 and 68 years residing in Ruda Śląska, Poland. Atomic absorption spectrometry was employed to measure the concentration of Cr in the teeth. The type of tooth and the sex of the donor had no significant influence on the level of Cr in the tooth tissue. Furthermore, there was no meaningful correlation between the Cr contents in teeth and the age of the donors.
Weight
Eating disorders represent a prevalent social phenomenon that may manifest in weight loss and weight gain. Malnutrition, whether due to an excess or deficiency of nutrients, can result in severe health consequences. The study conducted by Fischer and Wiechuła aimed to determine the levels of certain elements, including Cr, Ca, Cu, Fe, and Mn, in the deciduous teeth of children in relation to their body weight.25 Although there were no significant differences in the concentration of the metals between children with normal and abnormal body weight, the correlation between the metals in teeth varied according to the children’s weight.25 This observation may indicate fluctuations in the mineral composition of tissues that are associated with metabolic disorders.
Systemic diseases, neurodevelopmental disorders and food allergies
The assessment of element levels in teeth, like in other bodily tissues, has the potential to be valuable in the diagnosis of diseases. The concentrations of heavy metals in both the tissue and serum have been linked to a wide range of illnesses, making them potentially valuable biomarkers for early disease detection.
Orzechowska-Wylęgała et al. investigated the presence of Cd and Pb in the teeth of children from the Upper Silesia region of Poland suffering from celiac disease or food allergies.26 This particular region is known for its prevalent heavy industries, including coal mining, which have led to considerable environmental contamination. As all the children who participated in the study resided in the same location, their exposure to environmental pollution was consistent across the studied groups. While several factors can impact the levels of heavy metals in dental tissues, the focus of their research was on systemic illnesses that necessitate long-term adherence to restricted diets.26 The most commonly observed illnesses in pediatric patients include celiac disease and food allergies. The prolonged implementation of restricted diets has been associated with mineral deficiencies in children, as well as quantitative and qualitative changes in the mineral composition of bones and dental tissues. Such alterations may lead to an increased accumulation of toxic metals within the body, such as Pb, Cd, strontium (Sr), Hg, and arsenic (As). The study revealed that children with celiac disease and food allergies have a higher tendency for the accumulation of certain metals in their deciduous teeth, in comparison to healthy individuals. Moreover, the Pb to Ca and Cd to Ca ratios were observed to be elevated in children with celiac disease and food allergies, providing additional evidence of the presence of these harmful metals in the body.26 The results of this study suggest that children who undergo restrictive diets may require adjustments to their dietary intake, with a particular focus on increasing the consumption of proteins and sulfur amino acids.
In a study conducted by Yalçin et al., the teeth and blood samples of both healthy children and those with congenital heart disease (CHD) were examined.27 The study included 39 children with CHD and 42 healthy children. The researchers used inductively coupled plasma mass spectrometry to evaluate the levels of 13 different elements, namely Mg, P, Ca, Cr, Mn, Fe, Cu, Zn, Sr, Cd, Pb, Hg, and molybdenum (Mo). After adjusting for potential confounding variables, it was observed that children with cyanotic and acyanotic CHD exhibited significantly lower levels of tooth Ca and a lower Ca:P ratio in comparison to the control group. Furthermore, children with acyanotic CHD exhibited markedly elevated levels of Cu in their teeth, along with increased levels of Mo in their blood and reduced levels of Mg in their blood, when compared to the control group, which consisted of healthy children.27
The research conducted by Sitarik et al. investigated the correlation between in utero and postnatal levels of Pb, which were measured using deciduous baby teeth, and the bacterial and fungal gut microbiota of infants in their first year of life.28 The discovered associations between Pb exposure and gut microbiota could potentially affect the development of a child.28 However, since there is a lack of research that investigates these correlations in humans, particularly with regards to fungal microbiota, further examination is necessary.
The study conducted by Abdullah et al. aimed to investigate the potential correlation between the presence of heavy metals in children’s tooth enamel and the occurrence of autism and disruptive behaviors.29 The researchers analyzed the concentrations of Pb, Hg and Mn in both prenatal and postnatal enamel regions of deciduous teeth from children diagnosed with Autism Spectrum Disorder (ASD), those exhibiting high levels of disruptive behavior, and typically developing children. The usage of laser ablation inductively coupled plasma mass spectrometry revealed no statistically significant disparities in the levels of these neurotoxicants between children with ASD and typically developing children.29
The variations in sex regarding ASD diagnosis and the mutagenic influence of toxic exposures suggest that these factors might have a significant impact on the causal relationships observed in any potential associations.30
In a study conducted by Adams et al., the levels of Hg, Pb and Zn in the baby teeth of children with autism were compared to those of a control group.31 The results showed that children with autism exhibited significantly elevated levels of Hg, while the levels of Pb and Zn were comparable to those observed in the control group. This study indicates that children with autism may have had a greater accumulation of Hg in their bodies during fetal and infant development. The researchers also suggest that the increased use of oral antibiotics in children with autism could have hindered their ability to eliminate Hg, thereby contributing to the higher levels of the element observed in their baby teeth.31
The study conducted by Figueroa-Romero et al. aimed to investigate the potential dysregulation of metal uptake during childhood in individuals who were later diagnosed with amyotrophic lateral sclerosis.32 By examining the co-exposure to different elements, the researchers found a strong association between childhood metal dysregulation and the development of amyotrophic lateral sclerosis.32
The impact of childhood exposure to low levels of Pb was assessed by Needleman et al.33 The study revealed a strong correlation between elevated Pb levels during childhood and lower social standing during high school, increased rates of absenteeism, diminished language skills and logical thinking abilities, decreased hand-eye coordination, delayed reaction times, and reduced finger tapping speed.33
The study by Haavikko et al. investigated the concentrations of Zn and Cu in the deciduous teeth of Finnish children and adolescents.34 The study focused on ways in which these mineral levels could serve as indicators of potential atherosclerosis precursors and systemic diseases. By analyzing the mineral content in dental tissues, the study aimed to understand the relationship between these trace elements and the risk of developing atherosclerosis and other systemic health issues.34
Although Mn is a necessary component for growth and development, elevated Mn levels have been associated with neurobehavioral impairment in children. The aim of the study conducted by Bauer et al. was to determine whether there is a correlation between visuospatial learning and memory test results and prenatal or postnatal Mn levels, as measured in deciduous teeth.35 The deciduous teeth were collected from 142 participants who resided in areas with varying ferromanganese industries in Italy. The prenatal and postnatal tooth regions were analyzed for Mn concentrations using laser ablation inductively coupled plasma mass spectrometry. The results of the study indicate that the prenatal period could be a crucial timeframe for the influence of environmental Mn on executive function and visuospatial ability, particularly in females.35
In a study conducted by Gunier et al., the relationship between Mn levels in teeth and neurodevelopment was analyzed in young Mexican-American children.36 The researchers used laser ablation inductively coupled plasma mass spectroscopy to measure Mn levels in both prenatal and postnatal dentin from children’s shed teeth. The children’s scores on the Mental Development Index (MDI) and Psychomotor Development Index (PDI) on the Bayley Scales of Infant Development at 6, 12 and 24 months were examined and compared to Mn levels. A unique biomarker was used to measure prenatal and early postnatal Mn levels in tooth dentin. The findings revealed a negative, temporary correlation between postnatal Mn levels and early neurodevelopment, with sex-specific modifications and prenatal hemoglobin interactions.36
Horton et al. investigated the relationships between dentin biomarkers of Pb, Zn and Mn and behaviors observed in later childhood.37 The behaviors exhibited during childhood could reveal postnatal periods of vulnerability to both individual and combined metal levels found in deciduous teeth. While Mn present in prenatal dentin may offer protective effects, elevated levels of Mn during early postnatal development may increase the risk of negative behaviors. Furthermore, the simultaneous presence of higher concentrations of Mn, Zn and Pb may adversely affect behavior, with Pb specifically associated with an increase in anxiety symptoms.37
Mora et al. analyzed the levels of Mn in the dentin of shed teeth during both prenatal and early postnatal stages.38 The teeth were collected from children residing near agricultural fields that had been treated with Mn-containing fungicides in California, USA. The study aimed to examine the relationship between Mn levels and various aspects of behavior, cognition, memory, and motor functioning in children. The results revealed a significant association between higher levels of prenatal and early postnatal Mn in deciduous teeth and adverse behavioral outcomes, including internalizing, externalizing and hyperactivity problems, in both boys and girls.38
By utilizing tooth-matrix biomarkers and examining detailed temporal patterns of exposure, scientists have identified specific periods of development during which Mn is linked to visual-spatial skills. The findings indicate that the associations between Mn and cognitive abilities are significantly influenced by the timing of exposure, with positive effects observed for prenatal levels and detrimental effects observed for postnatal levels.39
Environmental pollution
It is crucial to underscore the significant impact of environmental pollution, such as that of the air, soil and water, on the accumulation of toxic metals in the human body, including the teeth. There are a number of methods that can be used to evaluate the extent of heavy metal exposure in the environment. One of these methods is the analysis of various biological samples. Specifically, human teeth are a particularly suitable option because they possess a stable elemental composition. This stability allows for the comparison of the effects of long-term exposure to heavy metals among individuals inhabiting regions with varying degrees of pollution. It should be noted that the placenta is a crucial organ that receives considerable attention during pregnancy. Unfortunately, this organ provides an inadequate barrier against the transfer of dangerous heavy metals, especially Pb, to the developing fetus. The presence of Pb in this organ poses a serious environmental threat to the well-being of future generations. Hormonal fluctuations during pregnancy result in the release of Pb from long-term deposits in bones and teeth into the mother’s bloodstream, which can have harmful effects due to exposure to a contaminated environment.40
In order to assess early-life metal exposure in a community that had expressed concerns about previous exposures, Friedman et al. conducted a study using deciduous teeth.41 The teeth were collected from children who had lived in Holliston, Massachusetts (USA) from the time of conception. By analyzing naturally shed teeth, the researchers were able to obtain detailed information about the timing and dosage of metal exposure during early life. This study effectively demonstrates the usefulness of deciduous teeth in community-based research, particularly in cases with a history of water contamination.41
Anttila and Anttila investigated the absolute concentrations of Mn, Fe, Ni, Cu, Zn, Sr, and Pb in whole enamel, as well as in the labial and lingual surface enamel of deciduous incisors, using proton-induced X-ray emission.42 The mean concentration values derived from the 19 samples collected in the urban area did not exhibit significant differences when compared to the 9 samples obtained from the rural region of Finland.42
The study by Anttila et al. focused on the Pb content in the enamel of deciduous teeth from an area with high radon (Rn) levels.43 The study aimed to assess the concentration of Pb in the enamel of teeth from children living in a region with elevated Rn exposure and to investigate any potential relationship between Rn exposure and Pb levels in the teeth. The average Pb concentration in the enamel was comparable to previous measurements of Pb in other regions of Finland, indicating that Rn decay did not cause a notable rise in Pb levels in the teeth.43
In a study conducted by Järvinen et al., the levels of Pb in the enamel of deciduous molars were analyzed using proton-induced X-ray emission.44 The results suggest that the general population of Finland is not currently subjected to significantly elevated levels of artificially introduced environmental Pb, whether in urban or rural settings. Naturally occurring environmental Pb remains a critical factor in cumulative long-term exposure experienced in Finland.44
Fosse and Justesen conducted an investigation into the levels of Pb present in the deciduous teeth of Norwegian children.45 The study aimed to assess the concentration of Pb in dental tissues in order to understand the extent of Pb exposure among children in Norway. The research sought to identify potential sources of Pb exposure and to evaluate the impact of environmental factors on Pb accumulation in teeth. The deciduous teeth were obtained from a variety of Norwegian counties, including urban centers, industrial zones, and rural as well as fishing communities. The findings indicated that both urbanization and industrialization resulted in increased Pb absorption. However, the average level of Pb detected in Norway was significantly lower than the levels typically observed in other nations. Additionally, automobile exhaust was dismissed as a major contributor to excessive Pb absorption.45
The assessment conducted by Arora et al. focused on examining the distribution of Pb in primary teeth as a means of assessing Pb exposure during the pre- and neonatal stages.46 The researchers employed laser ablation inductively coupled plasma mass spectrometry to measure the presence of Pb in both the enamel and dentin of 10 primary teeth. The study illustrates a valuable methodology for obtaining temporal data on environmental Pb exposure during the pre- and neonatal periods by analyzing the spatial distribution of Pb in the dentin of primary teeth.46
Modern human populations are increasingly exposed to chronic environmental heavy metal contamination due to rapid urbanization and extensive industrial activities. A bioindicator for elemental uptake is found in tooth dentin, where the absorption occurs during the processes of mineralization and formation, resulting in significant storage over many years. This uptake encompasses essential elements, primarily sourced from geogenic diets, along with non-essential elements introduced through environmental exposure. Asaduzzaman et al. examined 50 human teeth from different ethnic groups.47 It has been noted that concentrations of heavy metals tend to increase with age. A comparison of ethnic groups revealed that the teeth of ethnic Chinese individuals exhibited slightly higher metal concentrations than those of Malays and Indians. Additionally, female dentin demonstrated greater levels of metal concentrations than male dentin. The molars contained higher concentrations of Hg, Cu and tin (Sn), whereas the incisors showed elevated levels of Pb, Sr, antimony (Sb), and Zn. The increased levels of heavy metals in tooth dentin indicate pollution originating from industrial emissions and urbanization. This demonstrates that human tooth dentin serves as a reliable bioindicator of environmental pollution and can provide chronological data on exposure.47
Wychowanski and Malkiewicz conducted a study on residents of Central Poland (Mazowieckie province) to measure the concentration of metal ions present in the hard tissues of their teeth.48 They collected samples of enamel and dentin from participants living in urban and agricultural areas. The researchers used graphite furnace atomic absorption spectrometry to determine the concentration of Mn, Pb, Cd, and Cr in the enamel and dentin samples from retained teeth. A comparative analysis of the data revealed that the enamel and dentin of individuals living in industrialized areas exhibited significantly higher levels of Pb and Cd compared to those residing in agricultural areas. However, both groups exhibited comparable levels of Mn and Cr in hard tooth tissues. The results of this study confirm that the likelihood of exposure to heavy metals is dependent on both the place of residence and the extent of environmental pollution in that area.48
Nowak analyzed the presence of sodium (Na), Ca, K, and heavy metals in human teeth.49 The analysis was conducted in Katowice and Istebna in Poland between 1992 and 1993. The measurements were taken to determine the levels of heavy metals and Na, Ca and K in the teeth of individuals. Notably, in the relative unpolluted southern region of Poland, namely Istebna, the concentration of heavy metals in the teeth of the local population was significantly lower than in Katowice, an industrial hub in Silesia. The ratios of Pb:Fe and Pb:Mn detected in teeth could potentially serve as a measure of pollution. The analysis of the metal concentration levels in the teeth of individuals residing in Katowice and Istebna revealed significant differences in the mean concentrations of Pb, nickel (Ni), Cd, Zn, cobalt (Co), Mn, Cr, Na, and Ca.49
The focus of the study conducted by Nowak and Chmielnicka was to examine the correlation between Pb and Cd and essential elements present in the hair, teeth and nails of individuals living in the environmentally exposed Katowice District in Poland.50 The investigation aimed to assess the extent of environmental exposure to Pb and Cd between 1990 and 1997 in the residents of this district, which is known for its high levels of environmental exposure to these toxic metals. Additionally, the study examined the exposure to Fe, Zn, Cu, Mn, Ni, Cr, Ca, Na, and K based on the concentration levels found in hair, teeth and nails. The investigation aimed to ascertain whether the accumulation of Pb and Cd could have an impact on the concentration of essential metals, including Fe, Zn, Cu, and Ca. Additionally, a control group was included in the study, consisting of residents from the Beskid area in Poland. The aforementioned elements were analyzed with regard to sex, age and tooth type. Atomic absorption spectroscopy was utilized to determine the concentrations of the elements present in the media under examination. Notably, teeth samples obtained from individuals residing in the Katowice District exhibited elevated levels of Ni, Cr and Mn.
The purpose of the study conducted by Fischer et al. was to analyze the Pb content in various groups of teeth among inhabitants of the Silesian region in Poland.51 The highest concentration of Pb was identified in canines, while molars exhibited the lowest concentration of Pb among all groups of teeth. The study demonstrated that the accumulation of Pb in the hard tissues of the body increases with age. Additionally, individuals exposed to Pb emissions from industrial sources were found to have significantly higher levels of Pb present in their teeth.51
In their study, Malara et al. aimed to establish whether impacted mandibular teeth and the adjacent bones could serve as a form of biomonitoring media for evaluating the exposure to heavy metals.52 The research involved the utilization of impacted lower third molars and fragments of the cortical bone, which were extracted during the removal of wisdom teeth. The research participants were chosen from 2 locations: the relatively polluted Ruda Śląska region in Poland; and the Bielsko-Biała region in Poland. Atomic absorption spectrometry with flame atomization was used to determine the concentrations of Cd, Cr, Cu, Fe, Pb, Mn, and Zn in the samples. The inhabitants of the Ruda Śląska region exhibited significantly higher concentrations of Cd and Pb in their impacted third molars and the surrounding bones compared to those living in the Bielsko-Biała region. Furthermore, a significant positive correlation was observed between the concentrations of Cd in the impacted teeth and the surrounding bones. These findings suggest that the levels of Cd and Pb in the environment may be reflected in the impacted mandibular teeth and the surrounding bones of individuals.
A study that explored the concentration of toxic metals in impacted third molars and surrounding bone tissue was carried out by Bryła et al.3 The study was similar to a previous investigation, but the samples were collected from 2 distinct groups: residents of the Wroclaw district; and residents of Wroclaw city in Poland. The objective of the study was to determine the concentrations of several elements, including Pb, Cd, Cr, Ni, Fe, Mn, Cu, and Zn, in a portion of bone covering the third molars and in the teeth themselves. The levels of Cd, Pb and Mn in the samples demonstrated an increase in correlation with the patient’s age. The levels of Cd and Pb were found to be higher in the residents of Wroclaw, as compared to other locations. Additionally, the residents of Wroclaw exhibited higher levels of Cu in their teeth. It was observed that the concentrations of Cr in the teeth was 33% lower than those in the mandibular bone, and that the concentration of Ni decreased with age. In all hard tissues of the tooth and bone, Pb and Cd aggregates were identified, in contrast to bioelements, which demonstrated a greater tendency to aggregate, primarily within the dentin.
The primary source of Cr in the human body is dietary consumption. However, in cases where there is occupational exposure or residing in areas with high levels of Cr in the air, the absorption of Cr through the lungs may be greater than through ingestion. Fischer et al. conducted a study to investigate the correlation between Cr and other selected elements in impacted wisdom teeth.53 The objective of the study was to determine the levels of Cr, as well as other elements such as Fe, boron (B), Co, Cu, Zn, selenium (Se), Mo, and barium (Ba) in the mineralized tissues of impacted teeth in a population exposed to elevated levels of Cr in the air. The level of exposure to Cr compounds can be determined through the use of impacted wisdom teeth as a diagnostic tool. A comparison between the Cr content in impacted third molars and erupted permanent teeth reveals a lower amount of Cr in the former, indicating that the primary source of Cr in embedded teeth is the bloodstream. The concentration of Cr in hydroxyapatites of impacted wisdom teeth was found to be mainly influenced by the levels of Fe, B, Cr, Co, Se, and Mo.53
Rayad et al. conducted an in vitro assessment to measure the concentration of toxic metals in third molars obtained from residents of the Legnica–Głogów Copper District in Poland.1 The objective of the study was to identify the concentration of toxic metals, which included Mn, Cr, Ni, Cu, Fe, Cd, Pb, and Zn, in the extracted third molars of patients from the Legnica–Głogów Copper District, as well as to ascertain the risk factors that determine the accumulation of these metals. The patients were divided into 2 groups based on their place of residence: residents of the Legnica–Głogów Copper District; and a control group of residents from Wroclaw. The SpectraAA atomic absorption spectrometer was used to determine the concentrations of Pb, Cd, Cr, Ni, Fe, Mn, Cu, and Zn in an air-acetylene flame. The analysis of third molars among inhabitants of the Legnica–Głogów Copper District revealed elevated levels of Fe and Pb. The identification of major risk factors that could lead to the accumulation of harmful metals in the teeth has been established. The study demonstrated a strong correlation between the concentration levels of Cr, Cu and Zn and a person’s age. Additionally, a correlation was identified between the amount of Cr present and the concentration of vitamin D3 found in the bloodstream.1
In their research, Rayad et al. conducted an analysis of the Hg levels in impacted wisdom teeth obtained from individuals residing in the Legnica–Głogów Copper District.2 The primary objective of the study was to emphasize the impact of environmental pollution on the human body by determining the amount of Hg present in the impacted third molars of residents in this area. To ascertain the levels of Hg in the samples, AAS was employed. The accumulation of Hg in the teeth of individuals in the control group located in Wroclaw was also studied, with a focus on identifying the risk factors that contribute to this phenomenon. The final model examined a number of factors, including thyroid and parathyroid gland ailments, cardiac ailments, and interval-scale vitamin D3 concentration. Among these factors, the presence of cardiac diseases was found to be statistically significant in relation to an increase in Hg concentration in third molars. The concentration of Hg was found to increase with age and the duration of residence in the Legnica–Głogów Copper District.2
The impact of environmental pollution in the Legnica–Głogów Copper District on the incidence of tooth count disorders in adolescents residing in that region was investigated by Rzepnicka et al.54 The primary objective of their research was to ascertain the degree of hypodontia in adolescents from educational institutions in Legnica (Poland). The dental health of students from secondary schools in Legnica was evaluated. Of the 1,000 students evaluated, 7.7% had hypodontia. This finding is higher than in other parts of Poland, which could be a result of environmental pollution in the Copper Basin of Lublin, the Głogów Region of Poland.54
The study conducted by Malara et al. aimed to investigate the impact of environmental exposure on the coexistence of Cd and Zn in teeth.55 Specifically, the objective of their research was to determine whether the exposure to heavy metals in the environment affects the coexistence of Cd and Zn in human teeth. The study utilized a sample size of permanent teeth, with teeth sourced from residents of Bielsko-Biała and Ruda Śląska in Poland. The levels of Cd and Zn in the teeth were determined through the use of AAS. A negative correlation was observed between Cd and Zn levels in the teeth of residents from Bielsko-Biała, whereas a positive correlation was noted between the 2 metals in the teeth of residents from Ruda Śląska. The research findings indicated a notable discrepancy between the levels of Zn and Cd present in the teeth of residents from Ruda Śląska, in comparison to those from Bielsko-Biała. Additionally, the exposure to environmental sources of these metals influenced their interaction within the human body.55
The assessment of the exposure of a population to heavy metals from the environment can be determined through the analysis of biological samples. Teeth, in particular, can be utilized to estimate long-term environmental exposure due to the stability of their elemental composition. The objective of the study conducted by Malara and Kwapuliński was to identify the presence of physiological and toxic metals in the teeth of individuals residing in Ruda Ślaska and Bielsko-Biała in Poland.56 Additionally, the study aimed to explore the potential impact of environmental exposure to heavy metals on the occurrence and coexistence of these metals in teeth. The research material for this study consisted of the permanent teeth obtained from individuals between the ages of 20 and 68 from both cities. The levels of Cd, Cr, Cu, Fe, Mn, Pb, Zn, K, Na, Ca, and Mg were measured using AAS with flame atomization. The teeth of individuals residing in Ruda Śląska showed a significant increase in the levels of Cd, Cr, Cu, Fe, Mn, Pb, and Zn, accompanied by a decrease in the levels of Ca and Mg and elevated Pb:Ca and Pb:Mn ratios. An examination of the correlation matrix revealed differences in the co-occurrence of metals in the teeth of the 2 studied groups. The study confirmed that the exposure to heavy metals in the environment has a significant impact on the presence and coexistence of these elements in individuals’ teeth.56 Malara et al. conducted similar research on the effect of Pb on the content of other metals to investigate their interdependence.57 The permanent teeth extracted from the inhabitants of Ruda Śląska were analyzed. The content of Pb, Fe, Mn, K, Ca, and Mg was determined through the use of AAS with flame atomization. Furthermore, Pearson’s product moment correlation analysis demonstrated that Pb exerted an adverse effect on the content of Ca, Mn, Fe, and Mg, while having no impact on the content of Na and K. The tooth structure showed a strong interdependence between Ca, Fe and Mn.57 Studies have identified an inverse correlation between the presence of Pb and these 3 vital elements, which may be attributed to the process of substitution.
In their investigation, Fischer et al. conducted a detailed analysis of the concentration of Ba in the facial bones, deciduous teeth and impacted teeth of individuals residing in Poland.58 The research involved both children and adults (aged from 6 to 78 years) from an industrialized region. The condition of the teeth was assessed holistically, without any distinction between the dentin and enamel. To prepare the facial bones and teeth, a sequence of procedures was undertaken, including rinsing, desiccation, crushing in a ceramic mortar, measurement of the sample (approx. 0.2 g), and microwave mineralization in pure nitric acid with a spectral dimension. The concentration of Ba increased with age in bone tissue and in impacted teeth. In contrast, the level of Ba was observed to decline with age in the deciduous teeth.58
The study conducted by Fischer et al. involved an evaluation of the Se content in retained wisdom teeth.59 Specifically, the researchers analyzed the Se content in the teeth of 10 individuals residing in the Upper Silesian Industrial Region in Poland. The results indicated an average Se content of 0.77 ±0.25 μg/g in the teeth, which was notably lower in comparison to the concentration of Se found in other hard tissues, as reported by prior researchers. Notably, a high correlation was observed between the Se content and the content of other elements, such as As, Fe, Sb, and Hg, which suggests that the industrial emissions in the area were the primary source of these elements found in the teeth.59
Piekut et al. conducted an evaluation to determine the potential of primary teeth as an indicator of children’s environmental exposure to heavy metals.60 The objective of their study was to ascertain whether primary teeth could serve as an effective environmental indicator of Cd, Pb and Zn exposure in children residing in Bytom, Poland. The study involved an examination of the primary teeth of children between the ages of 2 and 14. Before the analysis, the teeth underwent a mineralization process. The concentration of Cd, Pb and Zn in the samples was determined using AAS. The results of the analysis demonstrated variability in the levels of Cd, Pb and Zn within the primary teeth. Younger children have been found to have higher levels of Cd and Pb than their older counterparts. This indicates that these metals may have been present during prenatal development. Additionally, the concentration of heavy metals identified in primary teeth differs between sexes, with boys showing higher levels of Pb and Zn than girls. The study conducted in Bytom analyzed primary teeth of children and found limited potential for these teeth to serve as an indicator of exposure to Cd, Pb and Zn in the environment. It appears that prenatal exposure may be a complicating factor in assessing the exposure to these metals in children.60
Wiechuła et al. performed a statistical examination of quantities of metals found in the teeth of individuals residing in the Silesian region of southern Poland.61 Specifically, the concentrations of 11 different metals, namely Cd, Cr, Cu, Fe, Mn, Pb, Zn, Na, K, Ca, and Mg, were measured in the teeth of 2 different groups of people residing in the Silesian region. The first group consisted of individuals living in the town of Katowice-Szopienice, situated in the center of the Upper Silesian Industrial Region, in close proximity to a Pb plant. The second group was comprised of residents of Strumień, an agricultural community. The results of the analysis showed that the residents of Katowice-Szopienice exhibited higher levels of trace metals in their teeth.61
Fischer et al. evaluated metal concentrations (Cd, Pb, Mn, Cu, Cr, Fe, Zn, Na, K, Mg, and Ca) in deciduous and permanent human teeth, specifically in the maxilla and mandible.62 The results showed significantly higher concentrations of metals in the deciduous teeth in comparison to the permanent teeth. Regression and principal component analyses revealed that the process of binding elements by hydroxyapatite in deciduous teeth is becoming more dynamic. Furthermore, the concentration of metals was observed to be higher in the permanent and deciduous teeth of the maxilla compared to those in the mandible.62
It is important to acknowledge that the exposure to toxic metals in the workplace leads to their accumulation in the human body. In a study conducted by Malara et al., the presence of certain metals in the teeth of coal miners was investigated in relation to age and duration of employment.63 The research consisted of an evaluation of Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn, K, Na, Ca, and Mg concentrations in different types of teeth, including incisors, canines, premolars, and molars. Atomic absorption spectroscopy was utilized to measure the concentration levels of these metals. The results showed that the concentration of Pb in molars had a positive correlation with the age of the coal miners, whereas the concentration of Na and K demonstrated a positive correlation with the duration of employment.63 Kwapuliński et al. also conducted a study on the prevalence of selected metals in the teeth of coal miners.64 The study aimed to investigate the metal concentrations in the teeth of coal miners and non-miners in the town of Ruda Śląska, which is situated in the Upper Silesian Industrial Region in Poland. The study subjects consisted of men between the ages of 20 and 50. The results of the study provide valuable insights into the prevalence of metals in the teeth of individuals exposed to environmental contaminants in both occupational and non-occupational settings. The findings revealed that the concentrations of Pb, Cr, Fe, Na, Ca, and Mg in the teeth varied depending on the tooth type. Although there were no statistically significant differences in the concentrations of elements present in the teeth of miners and non-miners, some isolated differences were observed in the context of specific elements and teeth, such as elevated levels of K in incisors, Pb in premolars, and Cr, Zn and Mg in molars.64
Poczatek et al. performed an investigation to assess the potential risks to workers in the industry by evaluating the levels of particular elements in their teeth and bodily fluids.65 The study involved measuring the levels of certain elements in the teeth and body fluids of Polish workers across 3 distinct industries with established production profiles: Rail Rolling Stock Repair Workshops, which specializes in repairing rail vehicles; Philips Lighting Poland, which produces lighting systems; and Metal-Plast, a factory that is involved in building and furnishing. As part of the study, the teeth were extracted, and the samples of various bodily fluids, including urine, blood and saliva, were collected during routine health check-ups. The study evaluated the levels of various elements, including Ca, Mg, fluorine (F), P (in the form of phosphates), K, Na, Fe, Zn, Cu, Cd, and Pb. To measure the elements, AAS was utilized. The study demonstrated that the concentrations of these elements present in the teeth and body fluids of the subjects varied across the industries. Significant differences were identified in the levels of Mg, phosphates, Zn, Na, and K in the teeth.65
Johnston et al. evaluated Pb and As in the shed deciduous teeth of children living near a lead-acid battery smelter.66 The assessment of prenatal and initial stage exposure to toxic elements can be achieved through the use of shed deciduous teeth to measure Pb and As. A community-based research approach was taken to analyze 50 shed deciduous teeth from 43 children who have lived within a 2-mile radius of a smelter for the entirety of their lives. Laser ablation inductively coupled plasma mass spectrometry was used for the evaluation of the concentrations of Pb and As in the teeth. In order to determine the concentration of Pb in the soil, the researchers utilized spatial kriging to analyze the soil’s surface. Findings of the research imply that the exposure to toxic metals during the prenatal and early life stages is linked to soil contamination caused by past industrial activities in an urban community located near a smelter.66
The detonation of bombs, bullets and other forms of ammunition in areas of conflict results in the release of several neurotoxicants into the environment. Currently, the Middle East is facing a considerable degree of environmental degradation as a result of extensive bombardments. Savabieasfahani et al. developed a method for elemental bioimaging to examine trace elements in the deciduous teeth of children from Iraq who were born with defects.67 Additionally, the authors analyzed naturally shed teeth from Lebanon and Iran for trace elements. The research confirmed that elevated levels of metal in deciduous teeth are correlated with increased levels of war activity.67
Haavikko et al. employed the proton-induced X-ray emission technique to assess Pb levels in the enamel and dentin of deciduous teeth of children from 2 Finnish towns.68 It was presumed that Helsinki, the capital, would indicate high Pb exposure, while the rural town of Kuopio in central Finland would represent low to moderate Pb exposure. In nearly all cases, with the exception of 2 teeth, the enamel exhibited greater Pb concentrations than the dentin. The ratio of Pb concentration between enamel and dentin was not consistent and showed significant variability.68
The study conducted by Shishniashvili et al. focused on the use of primary teeth and hair as reliable markers for measuring environmental pollution.69 The results of their study revealed that in areas with unfavorable environmental conditions, the levels of toxic elements such as Pb, Hg, Sn, and titanium (Ti) were significantly higher compared to areas with more favorable conditions. Furthermore, their research demonstrated that dental hard tissues in polluted regions of Tbilisi (Georgia) exhibited higher concentrations of toxic elements and lower levels of essential elements when compared to less polluted areas.69
Tsuji et al. reported increased levels of Pb in the dentin of deciduous teeth obtained from isolated First Nations communities in the western James Bay region of northern Ontario, Canada.70 The research findings indicated that the average concentration of Pb in shed teeth from this remote area was comparable to the levels observed in children living in urban environments or in proximity to smelting facilities. Furthermore, a notable percentage of the children exhibited elevated Pb levels in their dentin.70
van Wyk and Grobler assessed the Pb concentrations in the shed deciduous teeth from children residing in 2 selected urban areas of the Cape Peninsula.71 The children residing in proximity to 2 major industrial facilities exhibited the following average Pb levels: whole teeth at 20,419 ppm; enamel at 10,952 ppm; and dentin at 22,733 ppm. In contrast, the children living near light industrial facilities exhibited Pb levels of 16,556 ppm in whole teeth, 2,919 ppm in enamel and 19,926 ppm in dentin. These variations were statistically significant at the 1% level for teeth and enamel, and at the 5% level for dentin.71
Lyngbye et al. conducted a study of Pb exposure in children. In a low-exposure area, the researchers explored potential predictors of Pb burden in children.72 A total of 1,302 first-form school children from the municipality of Aarhus, Denmark, donated their deciduous teeth for the measurement of Pb concentration in the circumpulpal dentin. Families were interviewed regarding possible sources of Pb exposure. Children with a high Pb burden resided significantly more often in heavily-travelled streets than those with a lower burden, but only during their first 3 years of life. The relationship between traffic intensity and the risk of a high Pb burden was evident in a dose-response manner.72
Gomes et al. assessed the levels of Pb present in the superficial enamel of deciduous teeth in children aged 4 to 5 years.73 The findings revealed that children residing in industrial regions exhibited considerably higher concentrations of Pb in their enamel compared to those living in areas distant from industrial influences.73
It is important to highlight that the dental environment is becoming increasingly contaminated with metals from dental materials, primarily due to procedures that generate aerosols, which could compromise the long-term health of dentists, dental students and dental staff. The current pollution in dentistry includes metallic nanoparticles that are highly reactive and can easily become airborne, particularly those that detach from the bulk composition. Additionally, dental amalgam may release liquid Hg or Hg vapors. These issues give rise to concerns within the dental community.74
Smoking
The deposition of tar and toxic metals in the body represents a significant health concern associated with smoking. Any form of smoking, whether active or passive, can result in the accumulation of toxic metals in one’s teeth. In their analysis, Malara et al.75 evaluated the impact of smoking on the concentration of Pb and Cd in the hard tissue of teeth. The study was conducted on permanent teeth obtained from both smokers and non-smokers residing in Ruda Śląska in Poland. Atomic absorption spectrophotometry was used to determine the concentration of Cd and Pb. To gain further insight into the accumulation of these harmful metals, the Ca levels in the tooth samples were assessed, resulting in the calculation of the Cd:Ca and Pb:Ca quotients. The act of smoking cigarettes has been observed to affect the concentration of Pb and Cd present in the teeth of individuals. The teeth of smokers contained higher levels of these elements in comparison to non-smokers, with the discrepancy in Cd content being statistically significant.75 Additionally, studies have shown that the extent of the increase in Cd and Pb levels due to smoking is more pronounced in males than in females.75, 76 Inhalation of cigarette smoke represents a significant source of exposure to heavy metals. Passive exposure to cigarette smoke can also result in the accumulation of these substances. Deciduous teeth, with their stable chemical composition, are often utilized as an indicator of heavy metal exposure in children. In a study conducted by Malara et al., the impact of passive smoking on the concentration of specific metals in deciduous teeth was examined.76 The study utilized deciduous teeth as its primary research material, with some samples sourced from children exposed to cigarette smoke in their apartments. The levels of various elements, including Cd, Cu, Fe, Mn, Pb, Zn, Ca, and Mg, were measured using AAS with flame atomization. The results indicate that the exposure to cigarette smoke in children can lead to changes in the levels of both toxic and essential elements found in deciduous teeth. Specifically, higher levels of Cd, Cu, Pb, and Zn, which are permanent constituents of cigarette smoke, were observed, along with lower levels of Mn, Ca and Mg. Additionally, it was noted that children exposed to cigarette smoke in their apartments exhibited a disturbed gradient of Pb levels depending on the type of tooth.76 Malara et al. also evaluated the occurrence of Pb and Cd in deciduous teeth of children exposed to cigarette smoke in apartments.77 The study utilized shed deciduous teeth from children between the ages of 6 and 13 who had been exposed to tobacco smoke in their apartments, as well as from children in the same age group who lived in smoke-free apartments. The results showed that the deciduous teeth of children exposed to tobacco smoke in their apartments exhibited higher levels of Pb and Cd, accompanied by elevated Pb:Ca and Cd:Ca ratios, indicative of significant accumulation of these metals when compared to the teeth of children residing in smoke-free apartments.77
In several biological samples, there is an alteration in the coexistence pattern of elements (regardless of whether they are antagonistic or synergistic) when in the presence of toxic elements at harmful levels. The study conducted by Malara et al. aimed to determine whether there is a variation in the amount of Cd and Zn present in the hard tissues of retained wisdom teeth in smokers and non-smokers, and whether the exposure to cigarette smoke has an effect on the coexistence of these metals within the tissues.78 The materials used for this study were retained wisdom teeth from both smokers and non-smokers, and the Cd and Zn levels were determined using AAS. The results showed that the retained wisdom teeth of smokers exhibited higher levels of Cd and Zn compared to the non-smokers’ teeth. Furthermore, the coexistence pattern of Cd and Zn in teeth was influenced by exposure to heavy metals, exhibiting a strong synergistic relationship in smokers.78
The study conducted by Alhasmi et al. employed laser-induced breakdown spectroscopy to detect the presence of toxic elements in the teeth of smokers and non-smokers.79 The research investigated periodontal parameters associated with differences in exposure to these toxic elements. The aim of the study was to ascertain the impact of smoking on the accumulation of toxic elements in teeth and the associated changes in periodontal health. The study revealed that the concentrations of Pb, Cd and As were significantly higher in the teeth of smokers compared to non-smokers and the control group. Specifically, smokers exhibited elevated levels of these toxic elements compared to non-smokers, who also demonstrated higher concentrations than the control group. Overall, the control group exhibited the lowest levels of these elements.79
One aspect investigated by Olovčić et al. was the impact of smoking on the accumulation of toxic metals.80 The researchers analyzed the presence of 12 different metals in dental samples collected from 2 cities in Bosnia and Herzegovina, namely Bihać and Sarajevo. The research revealed statistically significant differences in the levels of Zn between the dentin samples of smokers and non-smokers.80
The study conducted by Fischer et al. evaluated the significance of passive smoking on the levels of Pb and Cr found in deciduous teeth.81 The study presented the changes in Pb and Cr content in deciduous teeth of children exposed to environmental tobacco smoke. The control group consisted of children whose deciduous teeth were not exposed to tobacco smoke at home. The analysis revealed that the Pb content was higher in the non-exposed population (13.81 μg/g) than in the passive smoking population (12.28 μg/g). Passive smoking resulted in a reduction in Cr content in deciduous teeth. The quotient of Pb and Cr contents was higher in passive-smoking boys and girls and in different types of deciduous teeth.81 Fischer et al. also evaluated the effect of passive smoking on the concentration of other metals in deciduous teeth.82 The increased concentration of toxic elements may disrupt the balance of trace elements which are crucial for the body’s physiology. The study involved the examination of deciduous teeth that were collected during the process of being replaced by permanent dentition. The content of various elements, including Mn, Fe, Cu, Ca, K, Na, Mg, Zn, Cr, Cd, and Pb, was determined through the use of AAS. The deciduous teeth of children who were not exposed to tobacco smoke exhibited higher levels of elements that are essential for the body’s physiological functions, such as Fe, Zn, K, Na, and Ca. In contrast, the deciduous teeth of children exposed to passive smoking exhibited lower levels of these essential elements. Furthermore, the study demonstrated higher levels of toxic metals in the hard tissues of the teeth of children who were exposed to passive smoking at home.82 It is possible that these toxic metals originate from tobacco smoke.
Toxic metals are a major contaminant in cigarette smoke, leading to the accumulation of these elements in calcified tissue of the teeth after entering the human body. This creates new conditions for the coexistence and occurrence of metals, which can be described using Czarnowski’s model of the equilibrium between biological and chemical cations.83 In their study, Malara et al. investigated the impact of smoking on the cationic equilibrium present in the teeth of men.83 The study aimed to determine whether the consumption of cigarettes had any effect on the levels of Cd, Cr, Cu, Fe, Mn, Pb, Zn, K, Na, Ca, and Mg present in the hard tissues of men’s teeth, as well as on the constant value of the cationic equilibrium model. The research material consisted of extracted permanent teeth from both smokers and non-smokers. The results indicated that smokers exhibited higher levels of Cd, Cr, Cu, Pb, and Zn in their teeth, whereas the concentrations of K, Na, Ca, Mg, Fe, and Mn were lower in smokers’ teeth than those found in non-smokers. Additionally, the constant value of cationic equilibrium in the teeth of smokers was observed to be lower than that of non-smokers.83 The abovementioned studies are summarized in Table 1.
Limitations
It is important to consider a number of potential biases in this review. One potential source of bias is the use of language. The review included only articles written in English and Polish. Another potential source of bias is publication bias, which can occur when only studies with significant findings are published. It is important to note that this narrative review is limited to studies published between 1978 and 2023. Consequently, research published outside of the specified time frame may have been excluded.
Conclusions
The accumulation of toxic metals in the human body is an important issue due to its potential to result in a variety of adverse health outcomes. A visible sign of exposure to such substances is the development of oral complications. Human teeth can be negatively impacted by environmental chemicals, drugs, or physical agents during both embryonic development and after they emerge in the oral cavity. According to the presented publications, the concentration of heavy metals in human teeth is influenced by both environmental and non-environmental factors. The findings of these studies suggest that teeth can serve as a valuable tool for monitoring the presence of pollutants in the environment. It is crucial to emphasize the urgent need to minimize exposure to hazardous metals. The accumulation of toxic metals, such as Pb, Cd and Hg, can lead to structural damage to the teeth and gums. The presence of these metals can result in the weakening of tooth enamel, thereby increasing the susceptibility of the teeth to the development of cavities and mechanical damage. The presence of toxic metals in the body can disrupt the balance of the oral microbiota, fostering the growth of cavity-causing bacteria and increasing the risk of tooth decay. Toxins can affect tooth mineralization, resulting in weakened teeth that are more susceptible to erosion and fractures. In children, toxic metals can impede the normal development of the teeth, resulting in complications with tooth eruption and deformities. Additionally, heavy metals can affect inflammation and gum health, potentially leading to chronic periodontal diseases, such as gingivitis and periodontitis. Toxins present in the body can affect the effectiveness of dental treatments and interactions with medications used for oral health issues.1, 2, 3 The presence of toxic metals in the body can lead to significant adverse consequences for overall health. Metals such as Pb and Hg are recognized for their detrimental effects on the nervous system. The exposure to Pb may result in cognitive impairments, developmental delays in children and various neurological disorders. On the other hand, the exposure to Hg can lead to tremors, memory loss and additional neurological complications. Heavy metals, including Cd and Hg, can accumulate in the kidneys, resulting in a gradual impairment of their functionality. This accumulation may cause kidney damage or disease, thereby compromising the body’s capacity to filter waste and regulate fluid balance. Additionally, toxic metals can affect cardiovascular health. For example, Cd has been associated with hypertension and atherosclerosis, while Pb exposure has been shown to increase the risk of heart disease. Prolonged exposure to specific metals can weaken the immune system, increasing susceptibility to infections and illnesses. This decline in immune function can adversely affect the body’s overall ability to combat diseases and sustain health. Certain toxic metals have the potential to interfere with endocrine function, impacting hormone equilibrium and resulting in complications such as thyroid dysfunction, reproductive issues and metabolic irregularities. Prolonged exposure to specific heavy metals has been associated with an increased risk of cancer development. Notably, As and Cd are recognized as carcinogens that play a role in the onset of different cancer types.1, 2, 3, 6 Reducing exposure to hazardous metals requires a multifaceted approach involving strict regulations, increased public awareness and enhanced monitoring systems. Governments and environmental agencies must implement and enforce rigorous guidelines to limit the release of toxic metals into the environment. Public education campaigns should raise awareness about the sources and risks of metal exposure, encouraging individuals to take preventive measures. Additionally, technological advances can improve the detection and analysis of metal concentrations in teeth, providing valuable data for environmental health studies. Furthermore, interdisciplinary collaboration among scientists, healthcare professionals and policymakers is crucial for the development of effective strategies to mitigate the impact of toxic metals on human health. By integrating findings from dental research with broader environmental health studies, we can gain a more comprehensive understanding of the impact of these metals on various aspects of health and well-being. This holistic approach will enable the formulation of targeted interventions to reduce metal exposure and the associated health risks.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.