Abstract
Background. Periodontitis is a chronic inflammatory disease of the supporting tissue surrounding the teeth. The disease is caused by specific bacteria, such as Porphyromonas gingivalis, which lead to the destruction of periodontal ligaments and alveolar bone.
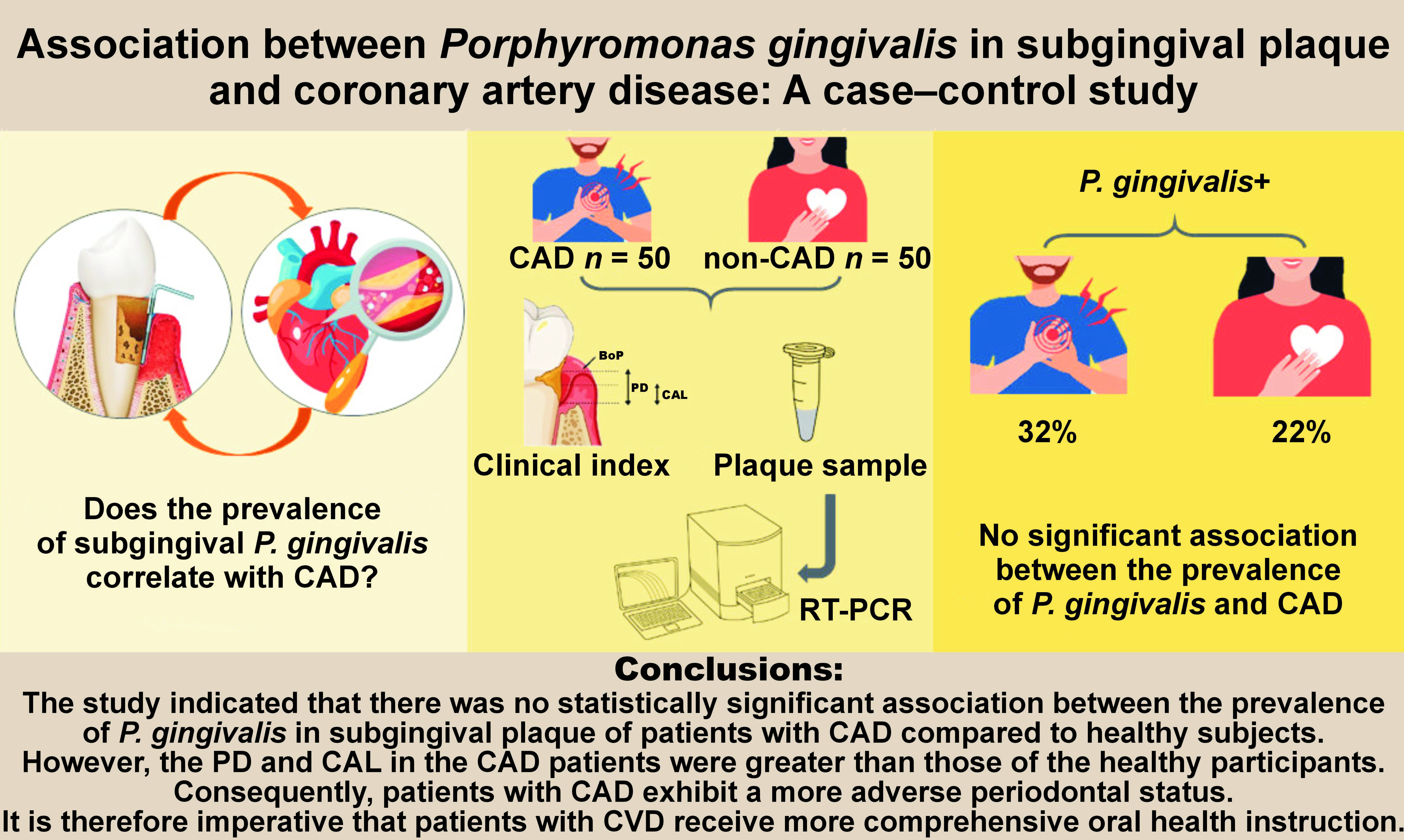
Objectives. The study aimed to evaluate the relationship between the prevalence of P. gingivalis in subgingival plaque and coronary artery disease (CAD).
Material and methods. Fifty patients with CAD and 50 healthy controls (non-CAD) participated in this case–control study. The periodontal health in the groups was evaluated through the assessment of the pocket depth (PD), clinical attachment loss (CAL) and bleeding on probing (BoP). The presence of P. gingivalis in subgingival plaque samples was determined through real-time polymerase chain reaction (RT-PCR). The data was analyzed using the χ2 test and the Mann–Whitney U test.
Results. The mean PD was 3.30 ±1.55 mm and 3.56 ±0.97 mm in CAD patients and non-CAD subjects, respectively (p = 0.028). No significant differences were observed in the CAL (p = 0.858) and BoP (p = 1.000) between the groups. The RT-PCR results revealed the presence of P. gingivalis 16S rDNA in 32% and 22% of the subgingival plaque of patients with CAD and non-CAD, respectively, with a mean concentration of 7.7 × 106. No statistically significant association was observed between the prevalence of P. gingivalis and CAD (p = 0.260). The results of the multiple logistic regression analysis showed an association between CAD and male sex (p = 0.004, odds ratio (OR): 4.163), as well as age (p = 0.011, OR: 1.067).
Conclusions. The findings of this study indicated that there is no statistically significant correlation between the prevalence of P. gingivalis in subgingival plaque and CAD.
Keywords: periodontitis, Porphyromonas gingivalis, coronary artery disease
Introduction
Periodontitis is a highly prevalent multifactorial chronic inflammatory disease of the teeth-supporting tissue.1 It is caused by the activity of dental plaque bacteria in the oral cavity and is the 6th most common human disease, with an overall prevalence of 45–50%. The most severe form of periodontitis affects 11.2% of the global population.2
There is an association between severe periodontitis and several non-communicable diseases (NCDs), including diabetes, chronic kidney disease (CKD), cardiovascular disease (CVD), and chronic obstructive pulmonary disease (COPD).2 As an inflammatory condition, CVD comprises coronary heart diseases, atherosclerotic, cerebrovascular, and peripheral vascular diseases.3 Over the last decades, a significant body of evidence has indicated a correlation between chronic periodontitis and an increased risk of developing CVD.4, 5, 6
It is hypothesized that in patients with periodontitis, an acute inflammatory immune response is involved in the transition from a symbiotic microbiota that is compatible with the host to an incipient dysbiotic microbiota, which supplies bacteria with resources from tissue breakdown and initiates a self-replicating pathogenic cycle. This cyclical interaction can persist for years in non-susceptible individuals, but it can develop quickly in those who are sensitive, leading to overt dysbiosis accompanied by an inefficient, protracted inflammatory or immune response.7, 8
The tissue destruction caused by periodontitis increases the amount of cytokines involved in the development of cardiovascular diseases. For example, matrix metalloproteinase-8 (MMP-8) has been identified as a key stimulatory and activating factor of pro-inflammatory mediators in the development of both cardiovascular diseases and periodontitis.9, 10
Indeed, periodontal pathogens or harmful endotoxins and exotoxins may penetrate from the oral cavity into the bloodstream during chewing or eating via damaged periodontal pocket epithelium. Therefore, bacterial dissemination and systemic infection lead to an inflammatory response, establishing a link between periodontitis and CVD.11, 12, 13
Periodontal pathogens are capable of directly invading the cardiovascular system. Reports indicate the presence of periodontal pathogens in human cardiac tissue, heart valves, pericardial fluids, and atherosclerotic lesions.1 The periodontal pathogens often identified in subgingival plaque of patients with chronic periodontitis include Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, and Treponema denticola.14 Among these, P. gingivalis is the principal pathogen in the initiation and development of chronic periodontitis.15 Additionally, it may act as a risk factor for several diseases, including CVD.16 For example, a study by Holmlund et al. demonstrated that the level of immunoglobulin G (IgG) antibodies against P. gingivalis increased in patients with myocardial infarction.17 Thus, assessing the clinical risk of oral infection with P. gingivalis is essential in patients with coronary artery disease (CAD). However, the implementation of oral hygiene training and the early diagnosis and treatment of periodontal problems can reduce the prevalence of CAD and its associated consequences. The aim of this study was to investigate the prevalence of P. gingivalis in subgingival plaque of patients with periodontitis and CAD, diagnosed by angiography as the gold standard due to the lack of adequate studies on the subject among the Iranian population. The hypothesis of the study was that the prevalence of P. gingivalis is greater in patients with CAD.
Material and methods
Study design
A total of 100 individuals were randomly selected from those referred to the Fatemeh Zahra Hospital (Mazandaran Heart Center), Mazandaran University of Medical Sciences, Sari, Iran, for inclusion in this observational study. The patients were divided into 2 groups of 50 individuals each, with one group serving as the case group (CAD group) and the other as the control group (non-CAD group). Only individuals with CAD as their sole systemic condition were included in the case group. Additionally, the study’s inclusion criteria encompassed the willingness to participate in the study and the absence of any other systemic illnesses. Individuals with artificial valves, those on immunosuppressive medications, those with infective endocarditis, pregnant women, those who declined to participate in the study, and edentulous patients were excluded from the study.18 Following the provision of informed consent, subjects were invited to participate in the study. Individuals who underwent angiography were divided into 2 groups based on the results of the procedure: patients without CAD; and patients with CAD involving 1, 2 or 3 arteries. All procedures were conducted in accordance with the ethical standards set forth by the Medical Ethics Committee of Mazandaran University of Medical Sciences and in alignment with the 1964 Helsinki Declaration and its subsequent amendments. The study was approved by the Medical Ethics Committee of Mazandaran University of Medical Sciences (ethics code: IR.MAZUMS.REC.1396.3183).
Sample size
According to the study conducted by Hyvärinen et al., the prevalence of P. gingivalis was 65.2% in patients with CAD and 37.5% in patients with normal coronary arteries.19 Based on a confidence interval (CI) of 95% and a study power of 80%, the requisite sample size for our study was determined to be 100 (i.e., 50 subjects in the CAD group and 50 subjects in the non-CAD group). The sample size was calculated using G*Power software (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower).
Periodontal examination
The researchers obtained pertinent information about the patients, including their family history of heart disease, diabetes, age, and smoking status. A trained periodontist performed examinations on the subsequent day following the angiography. The bleeding on probing (BoP), periodontal pocket depth (PD) and clinical attachment loss (CAL) were recorded in 6 teeth based on the method proposed by Ramfjord20 with a manual UNC-15 periodontal probe (Medisporex Company, Sialkot, Pakistan).
A diagnosis of periodontitis was based on the presence of at least 1 site with a PD ≥ 3 mm and a CAL ≥ 2 mm.21
Subgingival plaque samples
Subgingival bacterial samples were obtained from the deepest pockets in the 4 quadrants. After the removal of the supragingival sample, the site was isolated from saliva using cotton rolls. The paper cones were then inserted into the periodontal pocket for 30 s.18, 22 Subsequently, they were placed in a sterile microtube containing phosphate-buffered saline (PBS) for storage at −20°C until analysis for bacterial identification.
DNA extraction
According to the manufacturer’s instructions, the bacterial DNA was isolated from the samples using a G-spin™ Total DNA Extraction Mini Kit (iNtRON Biotechnology DR, Seongnam, South Korea). Briefly, 200 µL of PBS was added to the microtube containing the paper cones, vortexed for 10 s, and centrifuged at 19,000 g for 2 min. Finally, the supernatant was discarded. The protocol included treatment with RNase A and proteinase K, which were incubated at 56°C for 10 min. After isolation, the DNA was eluted in 100 µL of elution buffer. The quality, quantity and integrity of the purified DNA were analyzed using a nano-spectrophotometer (NanoDrop Spectrophotometer; DNA Technologies Core, Davis, USA) and 1% agarose gel.
Polymerase chain reaction for the detection of P. gingivalis
This study employed real-time polymerase chain reaction (RT-PCR) for the detection of P. gingivalis. The DNA samples were analyzed to determine the presence of P. gingivalis by means of a 16S rRNA-based RT-PCR detection method.23 The sequences of the 16S rRNA-specific primers were as follows: forward 5’-ACCTTCAACCAATTCTCCTTA-3’; and reverse 5’-GGTAATAATCGGCGTCTGA-3’. Amplifications were conducted in a final volume of 25 µL, containing 0.2 µL of Taq DNA polymerase, 1 µL of deoxynucleotide triphosphate (dNTP), 1 µL of each primer, 1 µL of template DNA, 1.7 µL of MgCl2, 2.5 µL of PCR buffer, and 16.6 µL of H2O. The PCR temperature profile was as follows: an initial denaturation at 94°C for 4 min; annealing at 60°C for 60 s; and extension at 72°C for 45 s. After 38 cycles, the PCR products underwent electrophoresis through 1% agarose gel in Tris/acetic acid–ethylenediaminetetraacetic acid (EDTA) buffer. The gel was stained with a green viewer and visualized under ultraviolet (UV) light.
Quantitative measurement of P. gingivalis by RT-PCR
The RT-PCR assay was performed using the PrimeScript™ RT Master Mix (Takara Bio Inc., Shiga, Japan). The specific primers were designed based on the 149bp sequence of the 16S rRNA gene. The primer sets comprised the forward primer (5’-GGGCGATACGAGTATTGCAT-3’) and the reverse primer (5’-TTCACCGCTGACTTACCG-3’). The amplification of the samples was conducted in duplicate using the ABI StepOne RT-PCR system (Applied Biosystems, Foster City, USA). A master mix without isolated DNA was used as a negative control. The absolute quantification of P. gingivalis was performed using the cycle threshold (Ct) of the samples and its comparison with the Ct of the standard samples.
Statistical analysis
The collected data was analyzed using the IBM SPSS Statistics for Windows software, v. 22.0 (IBM Corp., Armonk, USA). The χ2 test was applied to assess the prevalence of P. gingivalis in grouped variables. The Mann–Whitney U test was used to ascertain the differences between the CAD and non-CAD groups. The mean and standard deviation (M ±SD) were employed for quantitative data. Finally, a multiple logistic regression analysis was utilized to analyze the relationship between CAD and periodontal disease. The calculations were based on a 95% CI and a p-value of less than 0.05.
Results
In this case–control study, 100 subjects (50 CAD and 50 non-CAD patients) with a mean age of 54.86 ±9.59 years were analyzed. Of these, 47 were female and 53 were male. The prevalence of CAD differed significantly between male and female patients (p = 0.028). Specifically, 32 males (60.4%) and 18 females (38.2%) were diagnosed with CAD.
As illustrated in Table 1, a significant difference was observed in the mean age between CAD patients (57.46 ±10.18 years) and non-CAD individuals (52.26 ±8.27 years), with a p-value of 0.006. Moreover, the mean PD was 3.30 ±1.55 mm in CAD patients and 3.56 ±0.97 mm in non-CAD individuals, indicating a significant difference between these 2 groups (p = 0.028). On the other hand, no significant difference was observed in the mean CAL between the CAD group (4.16 ±1.93 mm) and the non-CAD group (4.02 ±1.37 mm) (p = 0.858).
The prevalence of periodontitis in the CAD patients was 34%, while this proportion was 32% in the non-CAD patients, indicating that there was no significant relationship between periodontal problems and CAD (p = 0.674). The 16S rDNA of P. gingivalis was identified in 16 (32%) and 11 (22%) subgingival plaque samples obtained from patients with CAD and non-CAD individuals, respectively (Table 2). Moreover, 27 patients exhibited the presence of P. gingivalis, with a mean concentration of 7.7 × 106, as identified by RT-PCR. Therefore, no statistically significant association was observed between the prevalence of P. gingivalis and CAD (p = 0.260).
Finally, multiple logistic regression analyses were conducted to examine the association between CAD and periodontitis, along with some potential risk factors (i.e., age and sex). There was a statistically significant association between CAD and male sex (p = 0.004, odds ratio (OR): 4.163), as well as age (p = 0.011, OR: 1.067) (Table 3).
Discussion
Cardiovascular disease is responsible for 17.9 million deaths and accounts for 45% of non-communicable disease-induced mortality worldwide.2 Several risk factors have been identified for CVD, including smoking, dyslipidemia, altered glucose metabolism, and hypertension.2
In recent decades, many studies have focused on the role of chronic infections, such as periodontitis, in the pathogenesis of CVD. Cumulative evidence from several studies has supported the role of periodontitis as an independent risk factor for CVD.1 Two meta-analyses have investigated the potential association between periodontal disease and CVD.24, 25 The studies concluded that periodontal disease is associated with an increased risk of cardiovascular events, including stroke and coronary heart disease.1
The purpose of this study was to determine the association between periodontitis and the prevalence of P. gingivalis in patients with CAD diagnosed by angiography. A significant difference in PD was observed between the groups. The mean CAL of patients with CAD was observed to be higher in comparison to non-CAD patients; however, this difference was not statistically significant. These results indicate that CAD patients exhibited poor oral and periodontal health.
In line with our report, Akbari et al. identified a significant difference only in PD between CAD and non-CAD cases.26 They reported that individuals with CAD had poorer periodontal health than those with normal angiography.26 Bateni et al. presented similar results, indicating that CAL and PD were elevated in the CVD group compared to the control group.27
Saliva plays a significant role in sustaining oral health by maintaining the integrity of dental tissues and preventing caries due to its biological functions, such as lubrication of oral tissue, antimicrobial and cleansing activities, removal of food debris and sugars, buffering capacity, control of plaque pH, remineralization of enamel with calcium and phosphates, and tissue repair. Moreover, oral hygiene is severely affected by a decrease in the salivary flow rate, dilution capacity, self-cleansing, and pH.28 Multiple factors can affect the salivary flow rate, including aging, pharmacological agents, certain health conditions, and stress.29 Therefore, poor periodontal status in patients with CAD may be attributed to the use of medications for the treatment of CAD.30 The use of statins and angiotensin II receptor blockers (ARBs) has been observed to result in a higher prevalence of periodontal disease.30 In addition, periodontium tissue changes and immunological alterations due to the aging process contribute to the causation and perpetuation of periodontal disease.31
This study revealed a prevalence rate of 32% for P. gingivalis in CAD patients and 22% in non-CAD cases, with no statistically significant differences between these groups. Similarly, Ardakani et al. have indicated that there is no meaningful relationship between the prevalence of P. gingivalis in subgingival plaque and the incidence and severity of atherosclerosis in experimental groups.18 The results demonstrated the presence of rDNA of P. gingivalis in 71.4% of Iranian patients diagnosed with periodontitis and atherosclerosis.18
In another study, the prevalence rate of P. gingivalis was reported at 61% in gingival sulcus plaque of patients with CAD and chronic periodontitis in the Iranian population.32 The contradictory results can be due to differences in the severity of periodontitis, sampling methods and racial differences.
The results of our study indicate a significant association between CAD and sex and age, which aligns with the findings of Bazile et al.33 The multiple logistic regression analysis demonstrates that age and male sex are comparable to other CAD-associated coronary risk factors. This study presents an OR of 1.067 (95% CI: 1.015–1.122, p = 0.011) and 4.163 (95% CI: 1.585–10.934, p = 0.004) between age and male sex, respectively, with CVD. Thus, it can be concluded that there is a close relationship between these variables.
Aging is recognized as a potential independent risk factor for CVD. Additional factors, such as obesity, diabetes and frailty, complicate and increase the cardiovascular risk factors associated with advanced age.34 The study performed by Mosca et al. indicates that the prevalence of coronary heart disease is higher in males within every age group until after 75 years of age compared to females.35 The estrogen hormone plays a cardioprotective role in females against coronary heart disease by regulating several metabolic factors, including lipids, the coagulation system and inflammation markers.35
The limitation of this study was its sample size. It is recommended that further research be conducted with a larger number of samples.
Conclusions
The results of this study indicated that there was no statistically significant association between the prevalence of P. gingivalis in subgingival plaque of patients with CAD compared to healthy subjects. However, the PD and CAL in the CAD patients were greater than those of the healthy participants. Consequently, it can be concluded that patients with CAD exhibit a more adverse periodontal status. It is therefore imperative that patients with CVD receive more comprehensive oral health instruction.
Ethics approval and consent to participate
All procedures were conducted in accordance with the ethical standards set forth by the Medical Ethics Committee of Mazandaran University of Medical Sciences and in alignment with the 1964 Helsinki Declaration and its subsequent amendments. The study was approved by the Medical Ethics Committee of Mazandaran University of Medical Sciences (ethics code: IR.MAZUMS.REC.1396.3183) and written informed consent was obtained from all patients.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.