Abstract
Background. Biological synchronized rhythmicity is a critical physiological process. The lack of synchronized rhythms, mainly those showing a circadian basis, like sleep, the heart rate (HR) and arterial blood pressure (BP), often leads to several organic challenges, usually associated with adverse outcomes.
Objectives. The aim of the study was to investigate whether the intensive care unit (ICU) environment favors clock genes and cardiorespiratory changes.
Material and methods. A total of 22 critically ill patients (16 males; 72.73%) with a mean age of 60.82 ±20.07 years and well-established cardiovascular conditions were selected from ICU. Blood samples were obtained, and total RNA was isolated and reverse-transcribed into complementary DNA (cDNA). A quantitative polymerase chain reaction (qPCR) was performed to assess the target gene expression levels. The urinary concentration levels of melatonin (MEL) were assessed. The heart rate, BP (systolic – SBP, diastolic – DBP and mean – MBP) and the oxygen saturation (SpO2) levels were assessed as continuous variables.
Results. The urinary MEL and Brain and muscle Arnt-like protein-1 (BMAL1) levels were shown to have a non-linear relationship with HR (coefficient (coef): 2.318, p = 0.032; coef: 2.722, p = 0.006, respectively) and SBP (coef: 1.000, p = 0.008; coef: 2.000, p = 0.037, respectively), with an explanatory power of up to 50.3% and 39.7% of the HR and SBP variability, respectively. Melatonin, but not BMAL1, was also shown to have a non-linear relationship with MBP (coef: 1.000, p = 0.007), with an explanatory power of up to 31.3% regarding the MBP variability. The HR and SBP oscillatory dynamics was shown to be related to changes in the genetic expression of BMAL1 and the urinary MEL concentrations. To a lower degree, MEL also impacted the variation of MBP.
Conclusions. Our results suggest that not only are circadian functional matrices crucial for the dynamics of vital parameters in critically ill patients, but also that routinely assessed cardiovascular parameters like HR and BP may constitute important markers for the circadian timing system function. These parameters are easy to assess and have a relevant prognostic value regarding recovery outcomes, as well as the morbidity and mortality rates in ICU.
Keywords: ICU, circadian disruption, clock genes, chronodisruption, cardiorespiratory functions
Introduction
Biological synchronized rhythmicity is a critical physiological process. The lack of synchronized rhythms, mainly those showing a circadian basis, like sleep, the heart rate (HR), and arterial blood pressure (BP), often leads to several organic challenges, usually associated with adverse outcomes. Sleep itself, as an independent regulator of many crucial body functions, should preferentially occur with minimum interference to optimize its role in structural and functional recovery and regeneration. Patients mostly benefit from the optimal circadian rhythmicity and sleep, which translates into better prognoses and reduces hospital discharge times.1 Meanwhile, the hostile intensive care unit (ICU) environment is typically linked to a negative impact on both the circadian timing system and sleep regulation centers, with important implications in the recovery of critically ill patients.1, 2, 3 Despite some of the most common disruptors being identified,1 it is largely unknown how they interact with the molecular matrix of the body’s internal time and, therefore, whether they might have a major contribution to a persistent deleterious role associated with the worsening of the main clinical condition, or increased morbidity and mortality.4 We, therefore, hypothesized that the circadian timing system and cardiorespiratory functions may interact in such a way that the disrupted circadian rhythmicity, typically occurring in ICU patients, would influence the crucial cardiovascular and respiratory parameters, like HR, BP and the oxygen saturation (SpO2) levels.
Material and methods
The present study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Provincial Research Ethics Committee of Cordoba (approval No. 277; ref. 3878). All patients were older than 18 years and gave their consent to participate voluntarily in the study.
A total of 22 critically ill patients (16 males; 72.73%) with a mean age of 60.82 20.07 years and well-established cardiovascular conditions (45.45% – cardiovascular surgery; 27.27% – ST-elevation myocardial infarction (STEMI); 4.55% – non-rheumatic mitral valve disease; 4.55% – out-of-hospital cardiac arrest; and 18.18% – cardiogenic shock) were selected from all the patients admitted with these characteristics to ICU. Specifically, accepting an alpha risk of 0.05 for a precision of ±0.15 units in a two-tailed test, with an estimated standard deviation (SD) of 0.35 and an estimated replacement rate of 0.10, a random population sample of 22 subjects was required, assuming that the available population in the cardiovascular pathology area of ICU during the recruitment period was 400 subjects. Data collection was performed from the 3rd quarter of 2020 until the end of the 2nd quarter of 2021. The participants were required to be over 18 years old and be treated in ICU for at least 48 h. Pregnant women, patients admitted to the restricted access modules, or those with scores above 13 in the Acute Physiology and Chronic Health Evaluation II (APACHE II) scale at baseline were excluded.
Prior to inclusion in the study, the patients were provided with an information sheet, and written informed consent was obtained from all participants. If the patients were unable to give their consent due to their clinical condition, consent was requested from their families. An A-weighting filter was applied during the different work shifts, and the following data on the patients’ physiological state was collected using a physiologic monitor (IntelliVue MP5; Philips, Amsterdam, the Netherlands): HR; the respiratory rate (RR); BP; and SpO2. Human whole blood samples were obtained from the 22 patients at 4 time points along 2 circadian cycles (8 h, 13 h, 18 h, and 23 h). Total RNA was isolated, and each blood sample was subsequently reverse-transcribed into complementary DNA (cDNA). A quantitative polymerase chain reaction (qPCR) was performed to assess the target gene expression levels (Brain and muscle Arnt-like protein-1 – BMAL1, circadian locomotor output cycles kaput – CLOCK). The urinary concentration levels of melatonin (MEL) were assessed at 12 consecutive time points along 2 circadian cycles (8 h, 10 h, 12 h, 14 h, 16 h, 18 h, 20 h, 22 h, 00 h, 2 h, 4 h, and 6 h). The heart rate, BP (systolic – SBP, diastolic – DBP and mean – MBP) and the SpO2 levels were assessed as continuous variables. A proficient researcher recorded the physiological variables at 15-minute intervals, and these measurements were also programmed into the physiologic monitor for continuous monitoring. The descriptive data of the studied patient group is gathered in Table 1.
To model the non-linear trends of the vital parameters of the patients in ICU, the generalized additive mixed models (GAMMs) were used following a two-fold rationale: a) the variation of the repeated measurements within and between the subjects should be taken into account, as vital parameters are repeatedly assessed over time for the same patient; and b) the non-linear effect of time on vital parameters could be captured using smoothing splines. This type of model is frequently used to study both the variation of the repeated measurements within and between the subjects with random effects, as well as the non-linear effects of covariates on the response variable with the smooth function of the covariate. Likewise, these flexible smoothing techniques help to capture small increases and decreases in the outcome variable during the follow-up period.
Results
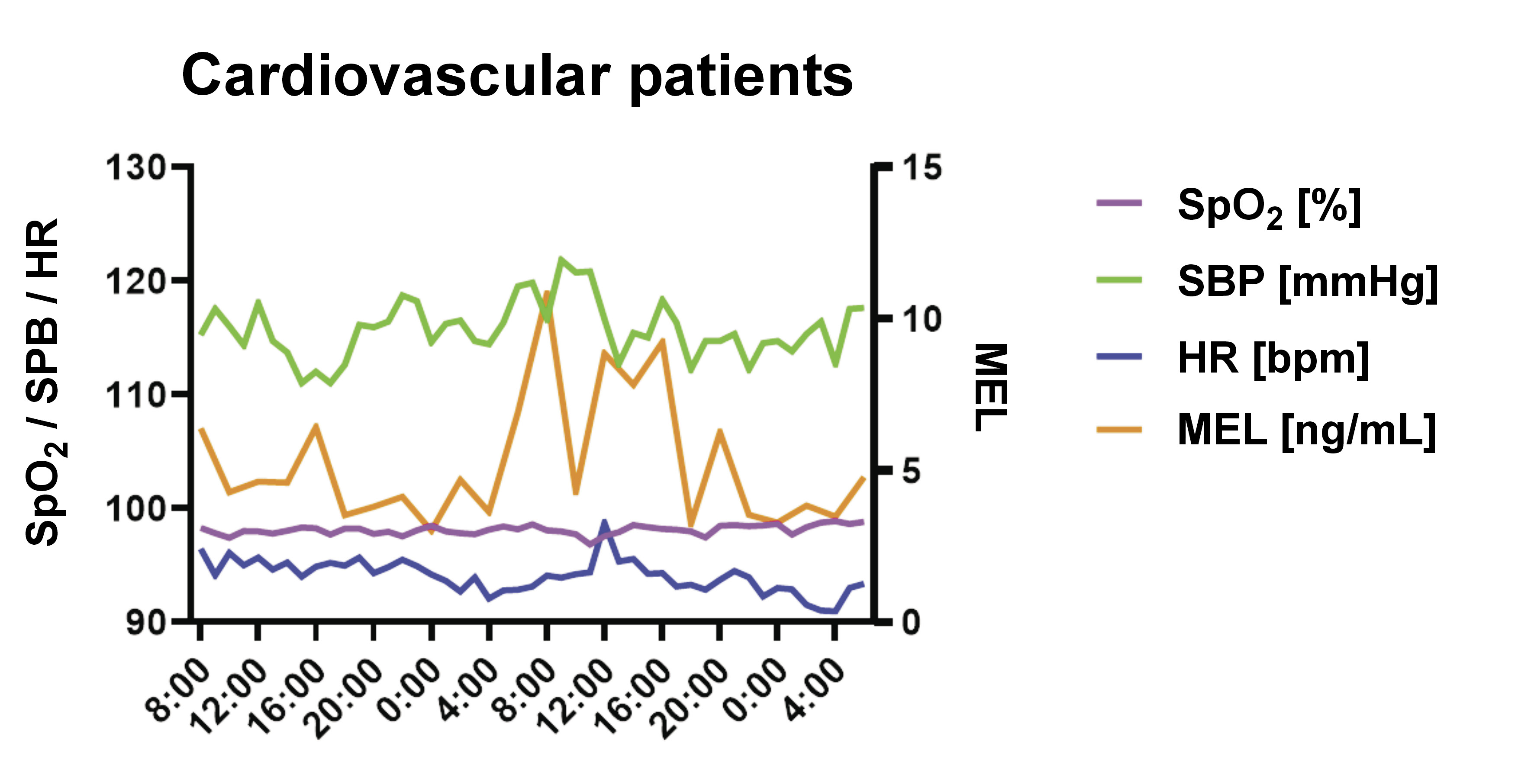
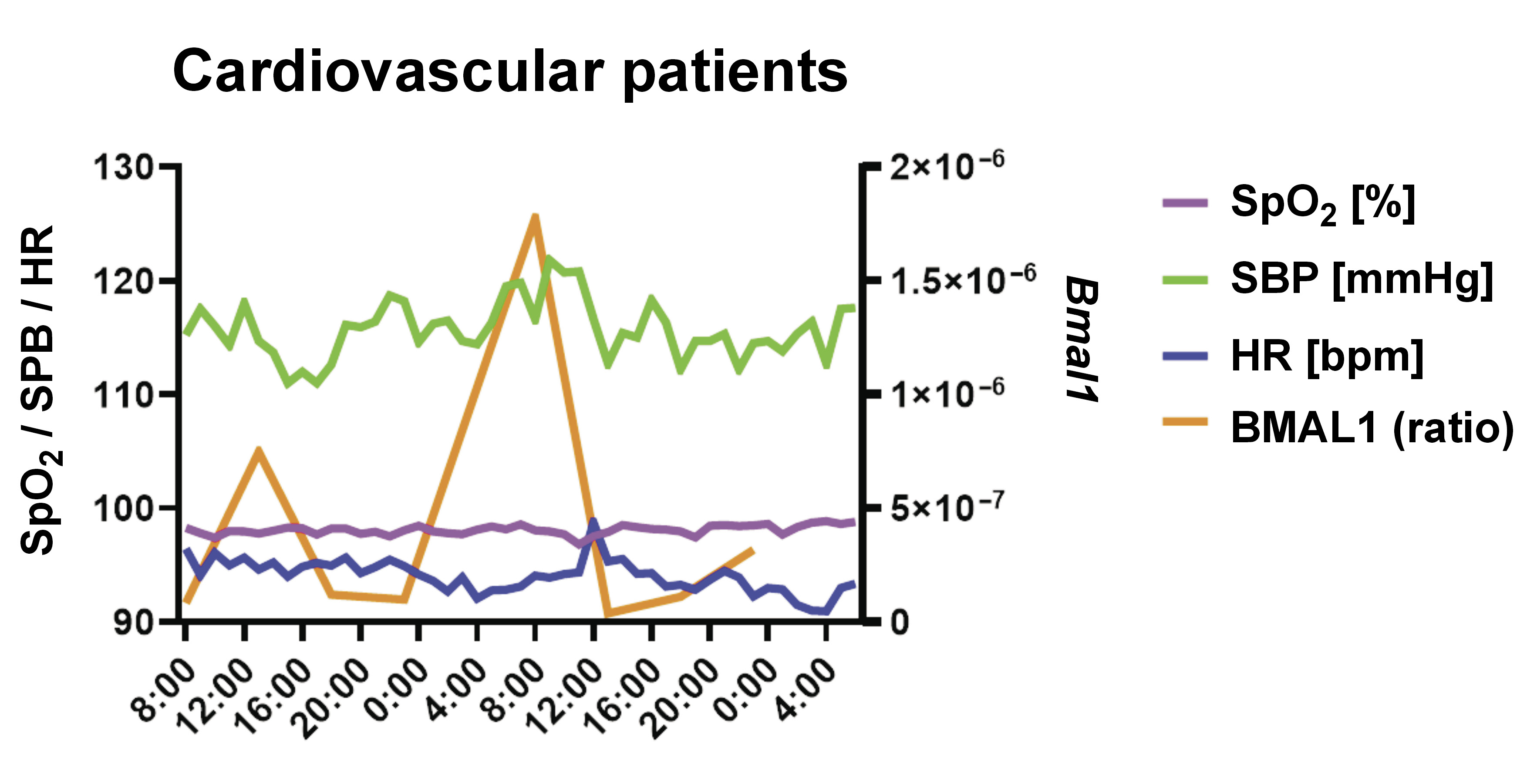
Both MEL and BMAL1 (but not CLOCK) were shown to have a non-linear relationship with HR (coefficient (coef): 2.318, p = 0.032; coef: 2.722, p = 0.006, respectively) and SBP (coef: 1.000, p = 0.008; coef: 2.000, p = 0.037, respectively), with an explanatory power of up to 50.3% and 39.7% of the HR and SBP variability, respectively. Melatonin, but not BMAL1, was also shown to have a non-linear relationship with MBP (coef: 1.000, p = 0.007), with an explanatory power of up to 31.3% regarding the MBP variability. The HR and SBP oscillatory dynamics was shown to be related to changes in the genetic expression of BMAL1 and the urinary MEL concentrations. To a lower degree, MEL also impacted the variation of MBP (Figure 1 and Figure 2).
Discussion
Together, the results suggest that not only are circadian functional matrices crucial for the dynamics of vital parameters in critically ill patients, but also that routinely assessed cardiovascular parameters like HR and BP may constitute important markers for the circadian timing system function. These parameters are easy to assess and have a relevant prognostic value regarding recovery outcomes, as well as the morbidity and mortality rates in ICU. Still, it is crucial to recognize that while patient-related factors are undoubtedly significant contributors to sleep disruption, the influence of the ICU environment should not be underestimated, and may be influencing the genetic and molecular aspects of circadian dysrhythmias, and chronobiological disruption.
From this work, a significant relationship between certain molecular markers of the biological clock and circadian rhythms and cardiovascular parameters like HR and BP was noted in ICU patients, implying that disruption in circadian rhythms can have a direct effect on these cardiovascular parameters, and may lead to the worsening of the ICU patient’s condition. Circadian disruption is common in ICU patients; several studies demonstrate that environmental conditions in ICU, especially excessive noise, particularly during patients’ rest periods, and exposure to artificial light at times of the day when they should be in darkness,5 can disrupt patients’ sleep and circadian rhythms, in turn worsening and delaying recovery. Indeed, emerging evidence indicates a link between disruption in circadian rhythms and poorer clinical prognoses.
Our findings may be of particular importance with regard to the subgroups of critical patients, like those with sleep dysfunctional complaints, or manifestations like insomnia or obstructive sleep apnea (OSA), both quite prevalent in ICU,6, 7 impacting the circadian timing system8 and causing cardiometabolic impairment.9 Furthermore, as they frequently co-occur (as in the case of Co-Morbid Insomnia and Sleep Apnea (COMISA)), exacerbating the cardiovascular and associated metabolic risk,9, 10 such pathological dynamics would plausibly have a negative effect on the circadian rhythmicity, sleep maintenance and general outcomes of this critical population. Interestingly, clock-related neuronal PAS domain protein-2 (NPAS2) has been recently found to be lower in OSA patients (with shortened sleep time) as compared to otherwise healthy individuals.11 As NPAS2 has been shown to be implicated in metabolic abnormalities, such as insulin resistance, it might be questioned whether the circadian-mediated cardiometabolic risk in OSA and perhaps insomnia could, in part, be explained by circadian-induced genetic changes. Still, other pathways and mechanisms could also support such interplay between circadian changes, sleep disturbance and the poor cardiometabolic outcomes often observed in ICU, such as some electrolytes, like calcium (Ca) and magnesium (Mg). From our findings, one could speculate about the putative contribution of Mg to the circadian disruption-related cardiometabolic risk in OSA patients. Some arguments behind this hypothesis are: 1) there is a well-established 24-hour cycle in the Mg flux12; 2) recent studies have found a lower Mg concentration in hypertensive OSA patients13; and 3) it is accepted that Mg is implicated in the metabolic dysfunctional status, therefore participating in the development of metabolic disorders.14 The knowledge of such interactions would increase awareness and likely benefit future research, as well as provide methods of adopting care that would favor the positive outcomes and survival rates of critically ill patients.
Felten et al. explored whether preserving or reinstating circadian regularity could enhance outcomes for patients in critical care settings, such as ICU and neonatal ICU.15 Strategies encompass adjustments to the environment to align with natural light-dark cycles and interventions such as medication timing and feeding schedules.15 The same authors proposed that advanced machine learning algorithms might enhance the analysis of clinical routine data on rhythmic patterns, using tools like cosinor analysis, therefore leading to a better understanding of how chronodisruption may affect important clinical outcomes. These innovations may enable real-time rhythmicity assessment and evaluation in the ICU environment, improving both the prediction of critical outcomes and therapeutic decisions.15 These molecular markers of circadian rhythms could also be used as biomarkers to establish the presence or absence of circadian dysregulation, and somehow support a prediction for patient recovery rates. Specifically, MEL could be an ideal candidate,16 as it is one of the central components of circadian rhythm regulation, and, as we have seen in our work, it has a non-linear relationship with HR, SBP and MBP. Melatonin may also be implicated in deregulatory mechanisms among distinct sleep disorders and cardiovascular diseases17; therefore, studying its levels in ICU patients could provide valuable information, obtained non-invasively, about the circadian status and sleep-associated performance of patients. The use of MEL could be studied as a possible intervention for ICU patients with circadian dysregulation, to attempt to restore normal circadian cycles, and greatly promote patient improvement and recovery.18 In addition to restoring circadian rhythms, and consequently positively affecting physiological parameters, the use of MEL as therapy may have a beneficial effect on other common complications of ICU patients, such as sepsis,19 one of the leading causes of death following traumatic injury.20
Limitations
There are certain limitations to our study, the main one being the high diversity and variability of the included conditions. This methodological constraint, which is related to the high heterogeneity of the recruited patients, was assumed from the initiation of the study design, since a more stringent criterion would critically affect the sample size. On the other hand, while these results do not allow generalizations to any particular model of disturbance, they point to a major hypothesis converging on distinct pathophysiological pathways commonly occurring in critical patients and, therefore, of great clinical interest. In the future, studies following the same research hypothesis should try to overcome this drawback.
Conclusions
In conclusion, a non-linear relationship was found between MEL and the clock gene Bmal1 with HR and SBP, and between MEL and MBP, indicating that the dysregulation of clock gene expression and MEL due to the hostile ICU environment may lead to disturbances in the cardiovascular variables of ICU patients.
Ethics approval and consent to participate
The present study was approved by the Provincial Research Ethics Committee of Cordoba (approval No. 277; ref. 3878). All patients gave written informed consent to participate voluntarily in the study.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.