Abstract
The complex interplay between the gut microbiota, cancer treatments and patient characteristics has emerged as a significant area of research. This study sought to examine these relationships in the context of colorectal cancer (CRC).
A comprehensive search of relevant studies was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. The studies included a variety of treatment modalities and microbiological parameters. A data extraction form, designed specifically for this review, was used to assess a range of variables across all studies.
The analysis revealed a multifaceted interaction between the gut microbiota, genetic factors and treatment outcomes. Elderly patients with CRC frequently received single-agent chemotherapy, with outcomes that were comparable to those of younger patients. The presence of tumorigenic bacteria, including Escherichia coli and Bacteroides fragilis, was associated with early colon neoplasia. Additionally, an abundance of Fusobacterium spp. was observed in colonic adenomas, contributing to a pro-inflammatory environment. Although the FcγRIIIa-158 V/V genotype was associated with higher cetuximab-mediated antibody-dependent cellular cytotoxicity (ADCC), no direct influence of FcγR polymorphisms on treatment response was noted. Furthermore, the combination of programmed cell death protein-1 (PD-1), BRAF and MEK inhibition showed favorable response rates. The gut microbiome, especially the presence of Fusobacterium spp., had a notable influence on the therapeutic response in CRC.
These findings underscore the role of the gut microbiota and genetic factors in cancer treatment outcomes, emphasizing the potential of a holistic approach to cancer management. Future research should exploit these findings in order to develop microbiota-modulating strategies and personalized medicine approaches for the purpose of improving the efficacy of cancer treatment.
Keywords: gut microbiota, genetic polymorphisms, cancer treatment outcomes, Fusobacterium, tumorigenic bacteria
Introduction
In recent decades, cancer has emerged as a major public health issue, representing a significant global burden with complex, multifactorial etiologies contributing to its onset and progression.1 With the cancer mortality rate rising, resulting in the loss of millions of lives worldwide,2 there has been minimal progress in reducing this mortality. Accordingly, a comprehensive understanding of the factors that modulate cancer progression is essential for the development of effective therapeutic strategies. The emerging research has begun to elucidate the intricate role of the human microbiome in health and disease, creating a new paradigm in our comprehension of carcinogenesis.2
The relationship between microbial entities and neoplastic cells within the bodily ecosystem can be viewed through the lens of evolutionary dynamics.3 Specifically, the mutualistic interactions between these 2 cellular populations, which enhance their proliferative capacities and their ability to evade immune surveillance, could potentially confer an evolutionary advantage.4 This suggests that the physiological environment may often favor the survival and propagation of microbial and neoplastic cells that engage in cooperative behaviors, thereby outcompeting those that do not partake in such synergistic interactions. Such cooperation can be stabilized through evolutionary processes, such as positive assortment or partner selection.5, 6, 7, 8
Colorectal cancer (CRC) is one of the most prevalent malignancies globally, with significant morbidity and mortality rates.3 The progression of the disease is multifactorial and influenced by genetic, environmental and lifestyle factors. Among these, the role of the gut microbiota, the complex community of microorganisms that inhabit the human gut, has recently received considerable attention in the field of colorectal carcinogenesis.
The gut microbiota plays an integral role in maintaining homeostasis, including nutrient metabolism, the protection against pathogens and the modulation of the immune system.4 Dysbiosis, defined as an imbalance or alteration of the gut microbiota, has been associated with various pathological conditions, including inflammatory bowel diseases and metabolic disorders. Recent studies have suggested a potential correlation between gut microbiota dysbiosis and CRC.5, 9, 10, 11
The emerging evidence indicates that gut microbiota dysbiosis may contribute to colorectal carcinogenesis through several mechanisms, including the promotion of chronic inflammation, the production of carcinogenic metabolites and the alteration of host immune responses. However, the exact role of gut microbiota dysbiosis in the progression of CRC remains unclear and is a subject of ongoing research.12, 13, 14
In addition to the role of the gut microbiota, the treatment modality for CRC can also significantly influence the disease progression. The impact of common treatments such as surgery, chemotherapy and radiation therapy, as well as more recent approaches like immunotherapy, on the course of CRC can vary considerably.15 The interaction between these treatments, the gut microbiota, and their cumulative effect on CRC progression is a complex interplay that is yet to be fully understood.16
Despite the growing body of evidence, our understanding of the microbiological aspects of cancer progression remains fragmented.5 Previous studies have often focused on specific types of cancer or microbial species,9, 10, 11 which has limited our ability to fully map the overall landscape of microbial influence on cancer progression. Furthermore, the inherent complexity of the microbiome, coupled with the influence of various confounding variables such as diet, antibiotic usage and host genetics, introduces additional layers of complexity to these investigations.
In light of the aforementioned context, we conducted this systematic review with the objective of synthesizing the existing literature on the role of gut microbiota dysbiosis in the progression of CRC and the influence of different treatment modalities. This review aims to collate and analyze the current evidence in order to shed light on the diverse ways in which microorganisms may modulate cancer progression.
Material and methods
PRISMA protocol
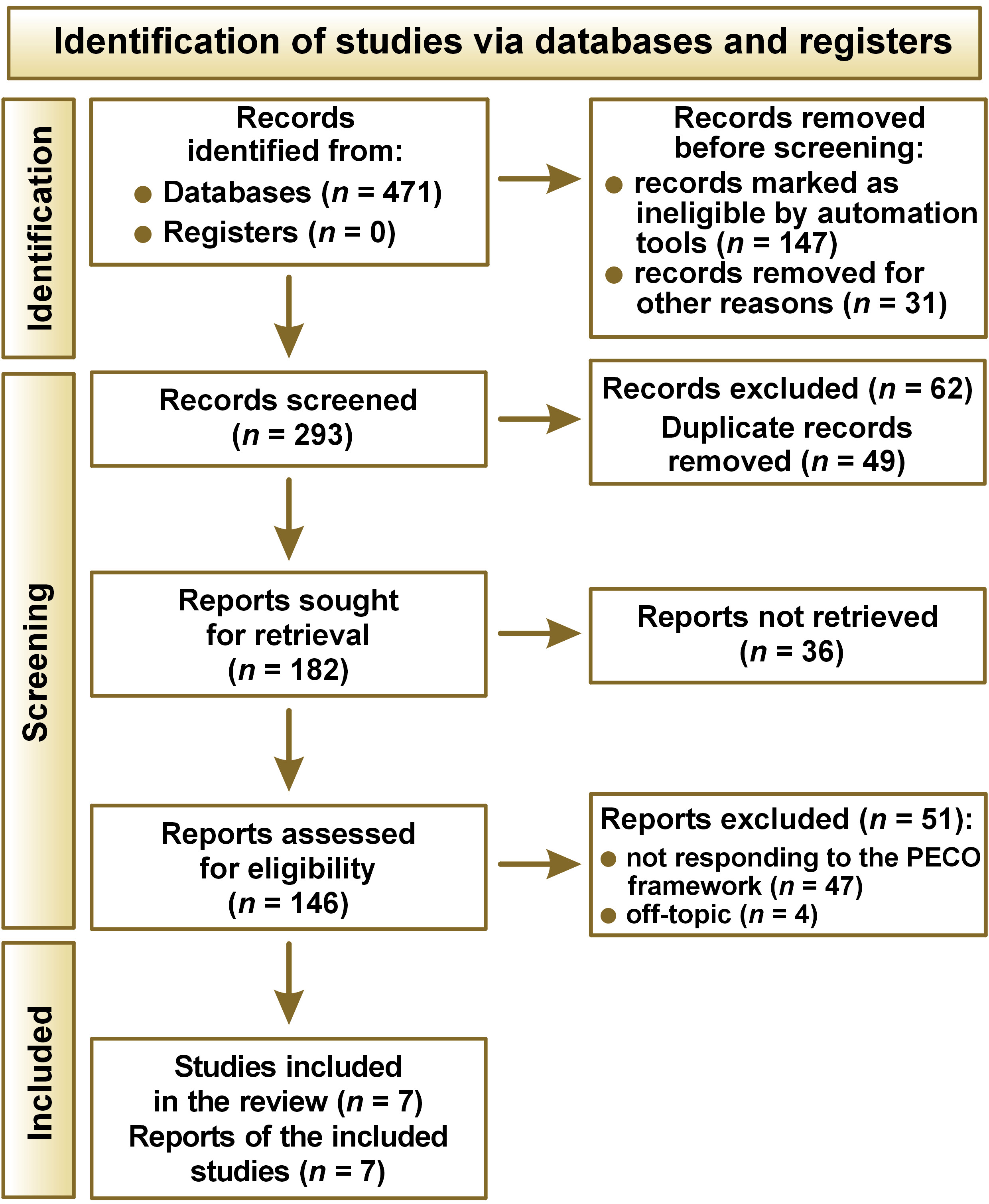
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 protocol17 was employed to guide the review process, the schematics of which are shown in Figure 1.
PECO framework
The Population, Exposure, Comparison, Outcome (PECO) framework was utilized to define the research question and direct the search strategy:
– Population (P): adult patients (>18 years old) diagnosed with CRC;
– Exposure (E): presence of gut microbiota dysbiosis identified through fecal microbiota analysis (e.g., 16S rRNA gene sequencing, metagenomics);
– Comparison (C): adult CRC patients with normal gut microbiota composition and/or those undergoing different treatment modalities (e.g., surgery, chemotherapy, radiation therapy, immunotherapy);
– Outcome (O): progression of CRC measured by validated clinical staging systems.
Database search protocol
The search strategy for this systematic review was designed to identify all relevant studies exploring the relationship between gut microbiota dysbiosis and the progression of CRC. A comprehensive search of relevant studies was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. The search was performed across 8 databases, namely PubMed/MEDLINE, Embase, Web of Science, Scopus, the Cochrane Library, CINAHL, APA PsycINFO, and Google Scholar. The search strategy was then adapted to align with the syntax and subject headings of the other databases, using Medical Subject Headings (MeSH) terms and Boolean operators, as shown in Table 1.
Inclusion and exclusion criteria
The inclusion criteria were as follows: original research studies; studies examining the association between microbiological factors and cancer progression; studies conducted in human subjects; and studies published in English. The following studies were excluded from the review: case reports, case series or animal studies; studies lacking sufficient data on cancer progression; studies not focused on microbiological factors; and reviews, editorials or commentaries.
Data extraction
Two independent reviewers conducted the data extraction using a pre-designed form. Any discrepancies were resolved through discussion or, if necessary, by consulting a third reviewer. The data extraction form captured the following information: the first author’s name; the year of publication; the study design; the country where the study was conducted; the sample size; the patient demographics (age and sex); details on CRC diagnosis; the methods used to measure and classify gut microbiota dysbiosis; a description of the comparison group (normal microbiota and/or different treatment modalities); the type of treatment modalities examined; the outcome measures (cancer progression and survival rates); and the main findings.
To assess the agreement between the 2 reviewers during the data extraction process, the inter-rater reliability was calculated using Cohen’s kappa statistic. The values of the kappa statistic range from −1 to 1, with 1 indicating perfect agreement, 0 indicating no more agreement than would be expected by chance, and −1 indicating total disagreement. The kappa statistic was found to be 0.85 in this review, indicating a high level of agreement between the 2 reviewers. The high level of agreement reinforced the robustness and reliability of the data extraction process.
Bias assessment
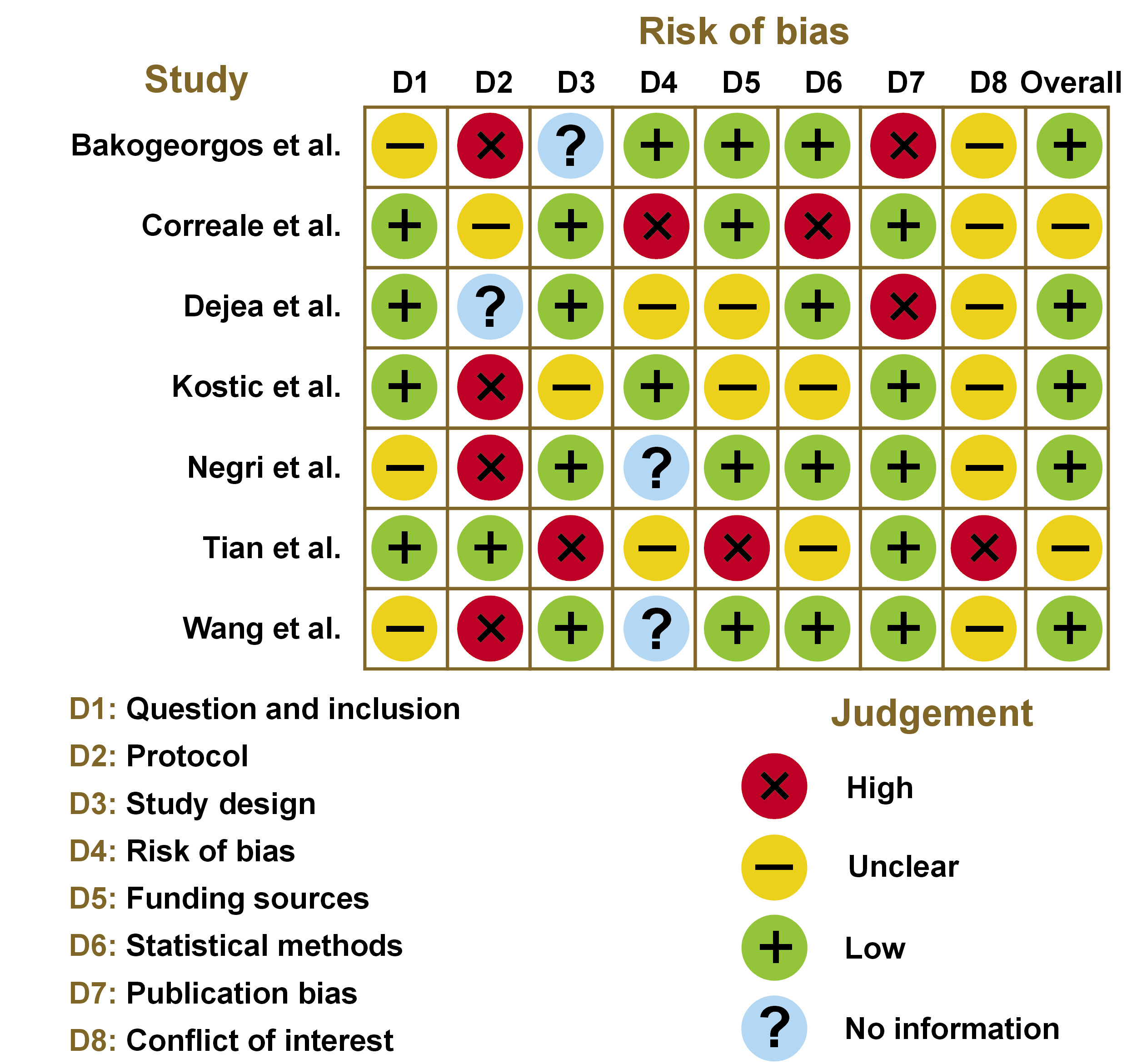
The Newcastle–Ottawa Scale (NOS)18 was used for the assessment of the quality of non-randomized studies, as illustrated in Figure 2.
Results
Study selection process
A total of 471 records were initially identified from various databases, while no records were found in the registers. Prior to the screening process, a number of records were removed due to the exclusion criteria: 69 were review articles; 78 were case reports or editorials; and 31 were not written in English.
Additionally, 62 records were excluded due to the absence of a full-text version, and 49 duplicate records were removed, leaving 293 records for screening. Of these, 182 reports were sought for retrieval, although 36 could not be retrieved. This resulted in a total of 146 reports being assessed for eligibility. Further exclusions were made on the grounds that 51 reports did not respond to the PECO approach or were considered to be off-topic. Following a rigorous screening and evaluation process, 7 studies were included in the review for further synthesis.19, 20, 21, 22, 23, 24, 25
Demographic characteristics
Table 2 presents the papers selected for inclusion in this review. Collectively, these studies highlight the microbiological role of cancer progression and its correlation with the gut microbiome. The papers exhibited variable sample sizes, ranging from 6 to 120. Several microbiological parameters were assessed in relation to CRC and its treatment.19, 20, 21, 22, 23, 24, 25 These parameters included the details of treatment delivery and chemotherapy toxicity and efficacy,19 the immunobiological activity of chemoimmunotherapy regimens,20 and the presence of bacterial biofilms and oncotoxin-encoding genes in patient samples.21 Some studies focused on particular microbial entities, such as the enrichment of Fusobacterium spp. in human colon and stool samples.22 Other studies examined genetic factors, including FcγR polymorphisms and their role in cetuximab-mediated antibody-dependent cellular cytotoxicity (ADCC).23 Additionally, clinical trials evaluating combined treatment strategies for CRC were documented,24 along with the gut microbiome analysis in the context of CRC treatment.25
Overall results
Upon analysis, a significant association was observed between CRC and gut microbiota dysbiosis. Patients with CRC demonstrated consistent alterations in the composition and diversity of their gut microbiota when compared to both the normal gut microbiota group and those undergoing different treatment modalities. Dysbiosis was characterized by changes in the relative abundance of specific microbial taxa, which may be indicative of a distinct microbial profile associated with CRC.
Discussion
The reason for focusing our investigation on the microbiology of CRC was driven by a multitude of compelling factors. The human gut, the primary site of CRC, is home to a complex and diverse microbial ecosystem. The gut microbiota is essential for maintaining the health of the host and has been linked to several pathological conditions, including CRC.9 The intricate relationship between the gut microbiota and CRC represents a promising field for exploration. The existing evidence indicates that dysbiosis of the gut microbiota, defined as an imbalance in the regular microbial community, may contribute to the onset and progression of CRC.8 Nevertheless, the precise mechanisms and extent of this involvement remain unclear. Moreover, CRC is among the leading causes of cancer-related deaths globally.1, 4 A deeper understanding of the role of the microbiota in CRC could provide insights into the pathogenesis of the disease, prognosis and potential treatment options.
The progression of cancer is a complex process influenced by a multitude of factors, including genetic, environmental and lifestyle aspects.1 Recently, there has been a growing recognition of the role of microbiological elements, specifically the role of the microbiota, in this process. The microbiota, particularly the gut microbiota, plays a critical role in maintaining the balance within the human body. Disruptions to this balance can contribute to disease, including cancer.4, 5 Dysbiosis, or an imbalance in the composition of the microbiota, can lead to an environment that promotes cancer progression. For instance, certain bacteria may produce toxins that damage DNA and promote cellular mutations, leading to cancer. Additionally, other bacteria may contribute to the development of cancerous changes in cells by promoting inflammation.10
In the study by Bakogeorgos et al., it was observed that elderly patients tended to receive single-agent chemotherapy more frequently than other known interventions.19 The rate of severe toxicities did not differ significantly between the 2 groups. Furthermore, the overall response rate (ORR), progression-free survival (PFS) and overall survival (OS) were similar across the groups, suggesting comparable efficacy regardless of age and treatment intensity.19 The study by Correale et al. demonstrated the superior effectiveness of the GOLFIG regimen over FOLFOX, as evidenced by an improved PFS and response rate.20 However, the experimental arm displayed a higher incidence of non-neutropenic fever, signs of autoimmunity and changes in immune cell counts, indicating an elevated immune response.20
As evidenced by the findings of Dejea et al., tumorigenic bacteria, specifically Escherichia coli and Bacteroides fragilis, have been associated with the early stages of colon neoplasia.21 The co-colonization of these bacteria was demonstrated to accelerate tumor onset, with the formation of biofilms in the colonic mucosa and a notable enrichment of oncotoxin-encoding genes.21 In the study by Kostic et al., Fusobacterium spp. was found to be enriched in human colonic adenomas and was associated with increased tumor multiplicity.22 This enrichment was demonstrated to promote the recruitment of tumor-infiltrating immune cells, thereby creating a pro-inflammatory environment that is conducive to the progression of colorectal neoplasia.22 Negri et al. observed that peripheral blood mononuclear cells harboring the FcγRIIIa 158 V/V genotype exhibited significantly higher cetuximab-mediated ADCC.23 However, no correlation was observed between FcγR polymorphisms and the response rate or time to progression following cetuximab-based therapy. This finding suggests that other factors may influence the treatment response.23
In the study by Tian et al., the primary endpoint was met, with a confirmed response rate that was favorable relative to historical controls of BRAF-targeted combinations alone.24 Notably, single-cell RNA sequencing showed a greater induction of tumor cell-intrinsic immune programs and more complete MAPK inhibition in patients with a better clinical outcome.24 Wang et al. reported an ORR of 15.2% and a disease control rate of 36.4% in evaluable patients.25 Patients with liver metastases had a lower ORR than those without. Furthermore, the examination of the gut microbiome revealed a significantly increased relative abundance and positive detection rate of Fusobacterium spp. in non-responders when compared to responders. Patients with high levels of Fusobacterium spp. exhibited a shorter PFS than those with low levels, underlining the potential influence of the microbiota on treatment outcomes.
The findings from our analysis are in close alignment with the observations reported by Wong and Yu and Villéger et al., further emphasizing the potential of the gut microbiota as an influential factor in CRC treatment and prognosis.26, 27 Similar to our findings, the review by Wong and Yu highlighted the role of Fusobacterium nucleatum, E. coli and B. fragilis, and underscored the significance of these bacteria in colorectal carcinogenesis and treatment outcomes.26 Furthermore, their review emphasized the potential clinical applications of gut microbiota analysis, including its use as a screening, prognostic or predictive biomarker, as well as the possibility of modulating the microbiota for CRC prevention or treatment. These propositions are in accordance with the conclusions drawn from our study, which underscores the potential for integrating microbiota considerations into cancer treatment strategies. In comparison, Villéger et al. focused on the potential of microbial markers for non-invasive early diagnosis and/or prognostic assessment of CRC and advanced adenomas.27 While our analysis did not explore this aspect in detail, the observed disruption in the gut microbiota balance and the alteration in the fecal metabolome of CRC patients resonates with our findings on the role of the gut microbiota in influencing cancer treatment outcomes. Furthermore, Villéger et al. proposed the use of microbial variation markers as predictors of treatment response,27 which is consistent with our study’s findings on the potential influence of the gut microbiota on treatment effectiveness.
However, while both studies extensively discussed the potential use of the gut microbiota for CRC screening and prognosis,26, 27 our study additionally highlighted the potential role of genetic factors, such as specific genetic polymorphisms, in modulating treatment efficacy. This underscores the need for a holistic approach that considers both the microbiota and genetic factors in CRC management.
Certain bacteria can cause chronic inflammation, which has been associated with various types of cancer. A persistent cycle of cell damage and repair resulting from chronic inflammation increases the likelihood of DNA replication errors and, consequently, mutations. For instance, chronic inflammation caused by the Helicobacter pylori infection is known to increase the risk of gastric cancer.4 Arthur et al. demonstrated that inflammation increases the abundance of E. coli and alters its genes, potentially promoting tumor development.2 Rhee et al. showed that the B. fragilis toxin induces colitis and histopathological changes in mice, and suggested that it may lead to subclinical colitis in humans.4 Yu and Schwabe claimed that the gut microbiota may promote the progression of liver disease and hepatocellular carcinoma via mechanisms such as gut leakiness and bacterial dysbiosis.9 Wu et al. discovered that B. fragilis triggers colitis and induces tumors via a STAT3- and TH17-dependent pathway, thereby providing insights into colon carcinogenesis.5 Ma et al. demonstrated that the gut microbiota can impact the effectiveness of cancer drugs, potentially affecting chemotherapy and immunotherapy outcomes.3
The microbiota can modulate the body’s immune response, which plays a vital role in identifying and eliminating cancer cells.28 Some bacteria may suppress the immune response, allowing cancer cells to evade detection and destruction by the immune system.29, 30, 31 Other bacteria may enhance immune responses, potentially leading to an overactive immune system and chronic inflammation, both of which may contribute to the progression of cancer.32 Certain bacteria can cause metabolic changes that promote cancer.33 For instance, some gut bacteria are capable of metabolizing dietary components into carcinogenic compounds. An example is the conversion of dietary choline and carnitine into trimethylamine by gut bacteria, which is further converted into a proatherogenic compound, trimethylamine N-oxide (TMAO), in the liver.33, 34, 35
In certain cases, bacteria or their products may translocate from the gut to other parts of the body, leading to inflammation and potentially promoting cancer.36 This phenomenon is often observed in the context of leaky gut syndrome, where the integrity of the intestinal barrier is compromised.4 Microbes can also influence the effectiveness of cancer therapies. Some bacteria are capable of metabolizing chemotherapeutic agents, reducing their effectiveness.37, 38, 39, 40
Limitations
When interpreting the findings of this study, several limitations must be acknowledged. Firstly, the heterogeneity among the studies included in the analysis represents a significant constraint. The analyzed studies employed different methodologies, treatment regimens and patient cohorts, which inherently introduce variability in the results and limit the ability to draw definitive conclusions. The disparities in the sample size and the lack of uniformity in the assessment parameters across the studies may have influenced the outcomes and subsequent interpretations. Secondly, although the study highlighted the role of specific gut microbiota, including Fusobacterium spp., E. coli and B. fragilis, in influencing cancer treatment outcomes, the complexity of the gut microbiota extends beyond these identified species. The gut microbiome is a complex ecosystem comprising a vast array of microbial species, and the collective interactions and functions of these species could influence therapy response. However, this study exhaustively explored this topic. Moreover, the role of genetic polymorphisms was evaluated in a limited context, focusing on FcγR polymorphisms and cetuximab-mediated ADCC. A more expansive range of genetic factors may exert an influence on the response to various cancer treatments, which were not addressed in this study. Lastly, the study focused primarily on CRC, which may limit the generalizability of the findings to other types of cancer. The relationship between the gut microbiota, genetic factors and treatment outcomes may vary across different types of cancer due to the specific genetic and microenvironmental characteristics of each cancer type.
Conclusions
The observed alterations in microbial composition indicate a potential association between the gut microbiota and the progression of CRC. This was particularly evident in the modulation of drug efficacy through a multitude of mechanisms, including direct metabolism of the therapeutic agents, immunomodulation, bacterial translocation, enzymatic degradation, reduction in microbiota diversity, and ecological variability. This finding emphasizes the importance for further investigation into the role of the gut microbiota in CRC pathogenesis. Such research could facilitate the development of targeted interventions aimed at modulating the microbiota to influence disease progression and treatment outcomes in these patients. Further research is warranted to elucidate the mechanisms underlying this association and to explore the therapeutic implications of modulating the gut microbiota in the context of CRC management. However, it was also inferred that despite the compelling evidence indicating the role of the gut microbiota in oncogenesis and cancer treatment, numerous intricacies remain to be elucidated. A deeper understanding of the complex interactions between the host, the microbiota and cancer is essential to fully recognize the therapeutic potential of modulating the gut microbiota. This underscores the necessity for further research employing robust experimental designs and longitudinal studies to elucidate the temporal and causal relationships involved.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.