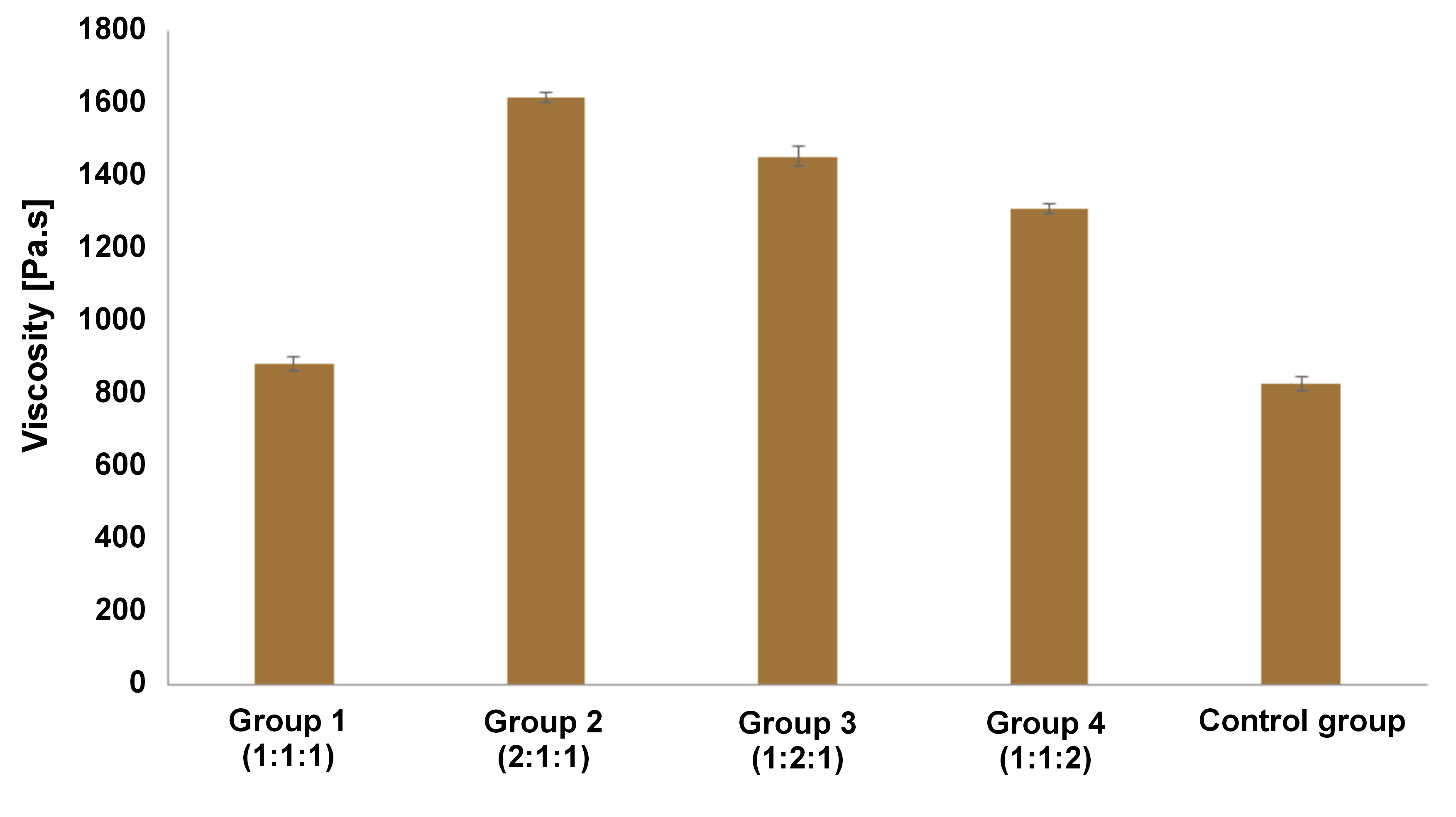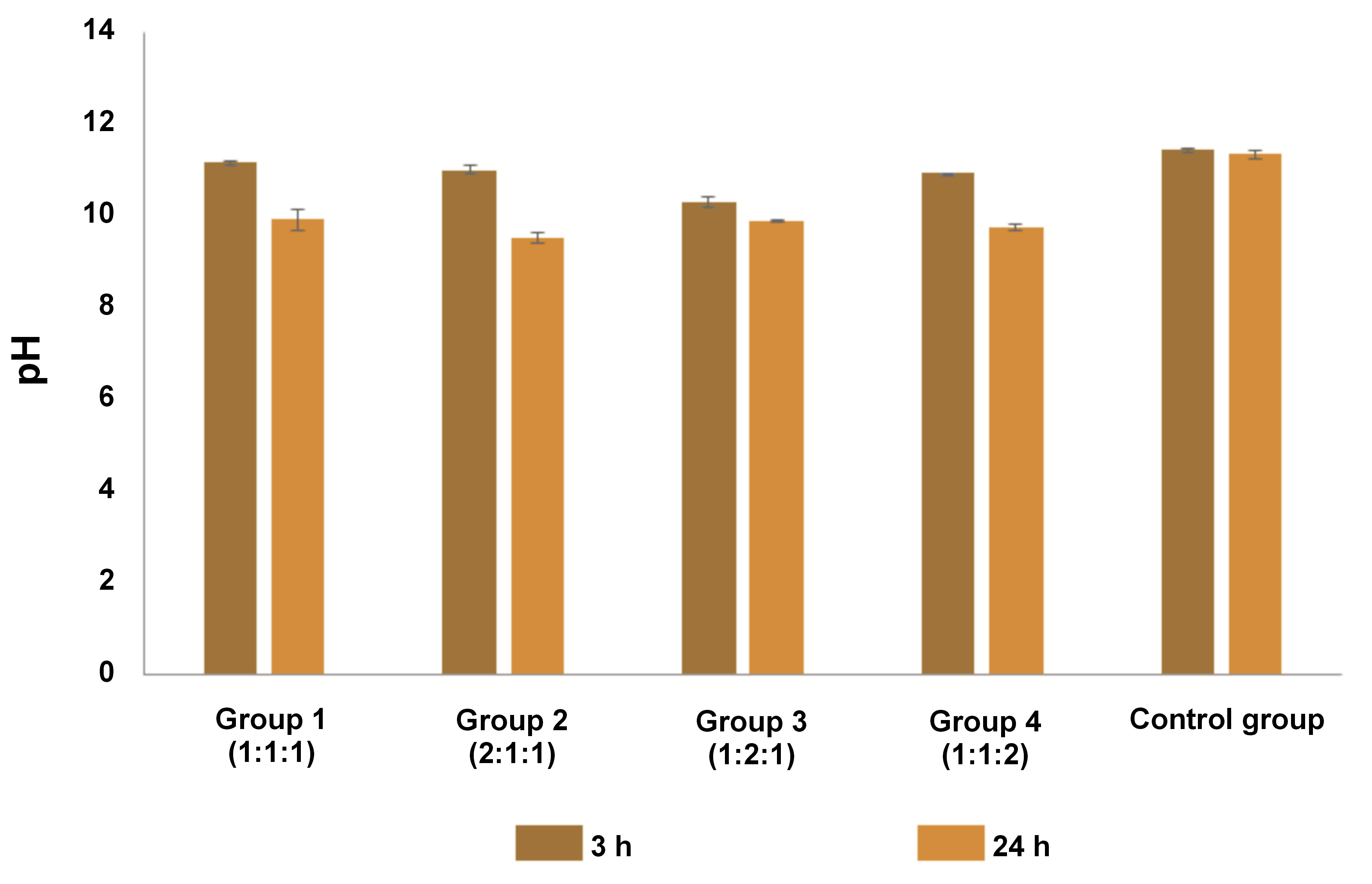Abstract
Background. Calcium hydroxide is used as a reparative dentin inducer. The combination of cocoa pod husk and calcium hydroxide has also been studied as a reparative dentin inducer, whereas anchovy was identified as a good calcium source.
Objectives. The aim of the study was to investigate the optimal ratio of a nano-combination of calcium hydroxide, cocoa pod husk extract and anchovy powder as a reparative dentin inducer by analyzing their material, antioxidant and cytotoxic properties.
Material and methods. The ratios of the 3 elements were established and their performance was compared in terms of viscosity, setting time, pH, and solubility rate. The optimal ratio was then examined for its antioxidant capacity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Additionally, its cytotoxicity against human dental pulp stem cells (hDPSCs) was evaluated through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Results. A ratio of 1:1:1 of calcium hydroxide, cocoa pod husk extract and anchovy powder was identified as the optimal ratio. It exhibited the greatest flowability, the shortest final setting time, had a pH range of 8.5–10.5, and the second highest solubility rate in comparison with other ratios. Moreover, it demonstrated active radical scavenging activity and stimulated the proliferation of hDPSCs.
Conclusions. Based on the material, antioxidant and cytotoxic properties, a ratio of 1:1:1 of nano-calcium hydroxide, cocoa pod husk extract and anchovy powder was determined to be optimal for use as a reparative dentin inducer.
Keywords: good health and well-being, reparative dentin inducer, novel nano-combination
Introduction
Direct pulp capping is a difficult dental procedure that aims to preserve the vitality of the pulp. The selection of cases and an optimal healing environment are required to achieve a favorable outcome. Complications may arise due to this treatment, including damage to odontoblast cells in the predentin layer, pulpal bleeding, bacterial contamination, and biomaterial properties.1, 2, 3 Direct pulp capping is performed to stimulate the formation of hard tissue from the inflamed pulp, thus forming a closure on the perforation in the residual pulp. The success of the treatment depends on the materials used in the procedure.4 For several decades, calcium hydroxide has been the most commonly implemented material, as it exhibits antimicrobial and mineralizing properties that facilitate pulpal healing. These properties are unparalleled among currently available biomaterials.5, 6 Additionally, calcium hydroxide is preferred due to its favorable pH and anti-inflammatory effect.4 However, the limitations of calcium hydroxide include the formation of multiple tunnel defects on the induced reparative dentin. In order to address this issue, a number of new biomaterials have been developed.
Mineral trioxide aggregate (MTA) is a biomaterial under consideration as a replacement for calcium hydroxide in the context of reparative dentin induction. Mineral trioxide aggregate can induce reparative dentin with a structure similar to that of primary dentin while causing no pulp inflammation.7, 8 However, MTA is not easy to use, and the acidic environment affects its setting time. In the previous study, MTA showed more cervical discoloration than calcium-enriched mixture cement,9 which could be considered a disadvantage for clinical application. Additionally, MTA is expensive.10 A systematic review of dental pulp capping agents found that pure calcium hydroxide powder has the same biocompatibility as MTA. However, another study reported failures when calcium hydroxide cement was used.11 Additionally, calcium hydroxide powder and its modifications, including varying particle size and combination with a natural source to enhance its antioxidant activity, have been shown to reduce pulp tissue inflammation and increase the calcium properties of materials.12, 13, 14
The efficacy of calcium hydroxide as a reparative dentin inducer can be enhanced through the implementation of various optimization techniques. These include a nanoscale escalation of anti-inflammation, calcium release and particle reduction. Calcium hydroxide dissolves into calcium ions and hydroxyl ions. The hydroxyl ions produce an alkaline environment, neutralizing the acidic pH caused by inflammation. Furthermore, they aid in the resolution of inflammation and the progression of dentin reparative formation. However, due to the high alkalinity of the environment, calcium hydroxide also causes coagulation necrosis.6, 12, 13 Cocoa pod husks are a natural product that has been studied for their flavonoid and methylxanthine content, as well as their ability to induce reparative dentin when combined with calcium hydroxide. A previous study found that the combination of cocoa pod husks with calcium hydroxide resulted in the formation of a greater quantity of reparative dentin than when calcium hydroxide was used alone.14
Calcium ions (Ca2+) play a regulatory role in the initiation of cellular events that lead to tertiary dentinogenesis. After dental pulp cells differentiate into odontoblast-like cells, they secrete extracellular matrix (ECM) and protein. A large influx of Ca2+ and phosphorus (P) ions is necessary for the mineralization of the collagenous ECM and the formation of hydroxyapatite.15 Mineral trioxide aggregate has advantages over calcium hydroxide in terms of the quantity and consistency of calcium released, which provides biocompatibility and sealing ability.6 The addition of anchovy powder can optimize the calcium release of calcium hydroxide. Anchovies are a rich source of calcium and are distinct from other calcium sources in that they do not contain substances that interfere with absorption or cause allergic reactions.16
The use of nanoscale combination materials has been shown to increase surface area, reaction rate, dissolution rate, and bioavailability.17 Reports have indicated that the application of nanotechnology facilitates the distribution of active molecules, lowers the inflammation rates and enhances dental pulp cell differentiation.18
In our study, we examined the characteristics of a nano-combination of calcium hydroxide, cocoa pod husk extract and pure anchovy powder. The optimal formulation was identified based on the viscosity, setting time, pH, solubility, antioxidant activity, and cytotoxicity.
Material and methods
Preparation of a nano-combination of calcium hydroxide, cocoa pod husk extract and anchovy powder
Nano-calcium hydroxide (80–100 nm) (Nanoshel, Punjab, India) was used in the experiment. The cocoa pod husks were obtained from a local producer (Kampung Coklat, Blitar, Indonesia). The fresh anchovies were procured from the fish market in Muara Karang, Jakarta, Indonesia. The cocoa pod husk extract and the anchovy powder used in the research were in nanoscale particles. Each of the natural materials was prepared to optimize the ability of calcium hydroxide to induce reparative dentin.19 The nanopowder of the cocoa pod husk extract was prepared using ultrasonic-assisted extraction with 80% ethanol, followed by freeze-drying for 48 h to produce a particle size of 107.8 ±27 nm. Similarly, the anchovy nanopowder was prepared by high-energy milling for 24 h, resulting in a particle size of 789.3 ±170.7 nm. The mixture of nano-calcium hydroxide, cocoa pod husk extract and anchovy powder was prepared in weight ratios of 1:1:1 (group 1), 2:1:1 (group 2), 1:2:1 (group 3), and 1:1:2 (group 4), as well as in the pure nano-calcium hydroxide solution (control group). The materials were weighed on an analytical balance (FS-AR210; Fujitsu, Tokyo, Japan). The powder mixtures were homogenized using mixer mills (MM 400; Retsch, Munich, Germany). For each of the subsequent tests, the combination powder from each group was mixed with sterile aquadest (Sigma-Aldrich, St. Louis, USA) into a paste. The ratio of powder to liquid was 1:1.17 To ensure thorough homogenization, the powder and liquid were mixed for 30 s in a mixing capsule using an amalgamator (Ultramat 2; SDI Limited, Victoria, Australia).20
Viscosity test
The viscosity test was performed with the use of a viscometer (Brookfield HAT; Anderen Ltd, Stoke-on-Trent, UK). The glass beaker was filled with approx. 200 mL of the paste and was placed beneath the tool. The tool was lowered until the spindle was fully submerged. The test was carried out at various rotation speeds. The reading on the dial was multiplied by the correction factor in order to calculate the viscosity.21 The test was repeated 6 times for each group. The viscosity was necessary for the low value.
Setting time examination
The initial and final setting times were examined using Gillmore needles (Dental Material and Testing Center of Research and Education, Faculty of Dentistry, Universitas Trisakti, Jakarta, Indonesia). The tool consisted of 2 arms with needles that differed in weight and tip diameter. A needle with a weight of 100.0 ±0.5 g and a tip diameter of 2.0 ±0.1 mm was used to determine the initial setting time. A 456.0 ±0.5 g and 1.0 ±0.1 mm-tip-diameter needle was used to evaluate the final setting time. The sample paste was initially placed in a ring mold (10.0 ±0.1 mm internal diameter, 2.0 ±0.1 mm thickness), and the mold was then positioned beneath the initial needle. The needle was lowered until it was fully submerged in the paste, held for 5 s, and then raised. The needle was lowered every 30 s, and the initial setting time was obtained when the needle was unable to make a full circular indentation in the inspected sample. The investigation was continued in the same manner with the second needle to obtain the final setting time.22 The test was repeated 6 times for each group. The optimal setting time was identified as the shortest setting time.
pH determination
The sample was placed in a cylindrical mold with a diameter of 10.0 ±0.1 mm and a height of 2.0 ±0.1 mm. Six specimens were created for each group. Each specimen was placed in a vial containing 10 mL of distilled water and incubated at 37°C.23 The pH of each group was determined at 3 h and 24 h using a pH meter (Oakton pH 2700 Benchtop Meter; Cole-Parmer, Vernon Hills, USA). The electrode was calibrated before being immersed into the sample paste. The displayed value was recorded. The test was conducted in triplicate for each sample, and the mean data was recorded as the pH value. The optimal pH was determined for materials with high alkalinity.
Solubility test
The samples were prepared and placed in 6 molds with dimensions of 20.0 ±0.1 mm in diameter and 1.5 ±0.1 mm in height. The mold was placed on a glass slab, filled with the paste, and flattened with a second glass slab. Six specimens were created for each group. The specimens were incubated at 37°C for 24 h and then exposed to air for 15 min. Then, they were weighed in triplicate using an analytical scale (FS-AR210; Fujitsu), with the mean data used as the sample weight (S). Bottle glasses were prepared and weighed 3 times, with the mean data utilized to calculate the bottle weight (W0). The specimen was placed in a bottle glass containing 5 mL of distilled water and incubated at 37°C for 24 h. This was followed by a drying process, which commenced with rinsing the bottle containing the specimens with distilled water and then drying in an oven at 105°C. The bottle and residues were weighed on the analytical scale (FS-AR210; Fujitsu) 3 times for each specimen. The mean data was used to calculate the W0 and the residue weight (Wt). The solubility was calculated by dividing the difference between Wt and W0 by S and multiplying the result by 100. The optimal solubility was obtained if the value was less than 3%.23
Antioxidant assay
The antioxidant assay was conducted at the Research Center for Chemistry (Indonesian Institute of Sciences, Jakarta, Indonesia) using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. A total of 4 mg of each sample was weighed and diluted with methanol to create a 1000-L solution. The tests were carried out with various concentrations of 10%, 50%, 100%, and 200%. To the solution, 0.5 mL of DPPH was added, mixed and allowed to stand for 30 min. The blank solution was prepared by adding 0.5 mL of methanol to 2 mL (0.2 mM) of DPPH solution. An ultraviolet-visible (UV-Vis) spectrophotometer (UH5300; Hitachi, Tokyo, Japan) was used to measure the absorbance at 517 nm. The difference between the blank and the sample was used to calculate sample inhibition. The analysis was conducted in duplicate. The antioxidant activity was classified as inactive (IC50 > 500 μg/mL), weak (>250‒500 μg/mL), moderate (>101‒250 μg/mL), active (50‒100 μg/mL), or highly active (<50 μg/mL).24
Cytotoxicity assay
The cytotoxicity of the optimal ratio to human dental pulp stem cells (hDPSCs) was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay at PT Prodia StemCell (Jakarta, Indonesia). First, the mixture powder (calcium hydroxide, cocoa pod husk extract and anchovy powder) was combined with distilled water in a 1:1 ratio to achieve the optimal ratio combination. The paste was then placed into a cylindrical mold (25 mm in diameter and 5 mm in height) and kept at room temperature for 24 h. Following this period, the sample was removed from the mold, placed into a 1-mL culture medium, and incubated at 37°C for 24 h. Subsequently, the medium of the samples was filtered (0.1 μL) and stored at −20°C. An indirect cytotoxicity assay was performed according to the ISO 10993 standard.25 The hDPSCs were placed onto 96-well plates at a density of 1 × 104 cells per well and cultured in a growth medium for 24 h. A series of dilutions of the sample medium was prepared at concentrations of 100%, 50%, 25%, and 10%. After 24 h, an MTT assay was performed, and the percentage of cellular growth was calculated. The cells in the growth medium served as the control group.25
Statistical analysis
The data was presented as mean (M) and standard deviation (SD). A one-way analysis of variance (ANOVA) was employed for each test using the IBM SPSS Statistics for Windows software, v. 25.0 (IBM Corp., Armonk, USA). The level of significance was set at p < 0.05. After obtaining the results of the statistical analysis, the group with the optimal ratio was selected for the assessment of the antioxidant and cytotoxic properties.
Results
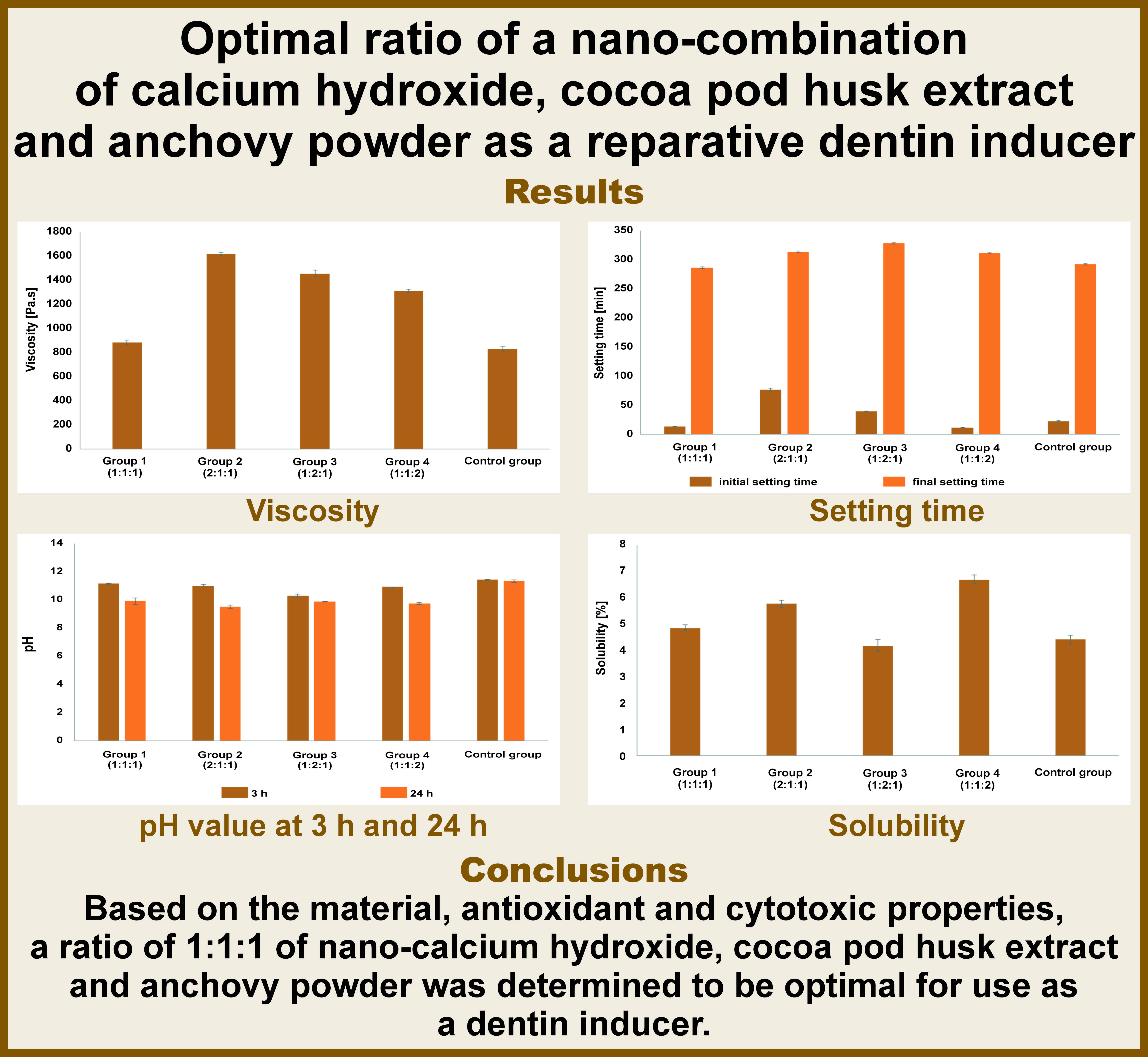
Viscosity
As shown in Figure 1, the control group (nano-calcium hydroxide) exhibited the lowest viscosity, followed by group 1. One-way ANOVA revealed statistically significant differences between the groups (p < 0.01).
Initial and final setting times
The initial and final setting times of the material are shown in Figure 2. Group 1 and group 4 showed a shorter initial setting time than the control group. Groups 2 and 3 demonstrated longer initial and final setting times than those observed in the control group. The mean value of the initial and final setting times for group 1 was consistently lower than that of the control group. The one-way ANOVA indicated a significant difference between the initial and final setting times in all groups (p < 0.01). Furthermore, Tukey’s test showed significant differences between the groups in the initial and final setting time results (p < 0.01). However, no statistically significant difference was observed between groups 2 and 4 in the final setting time (p = 0.107).
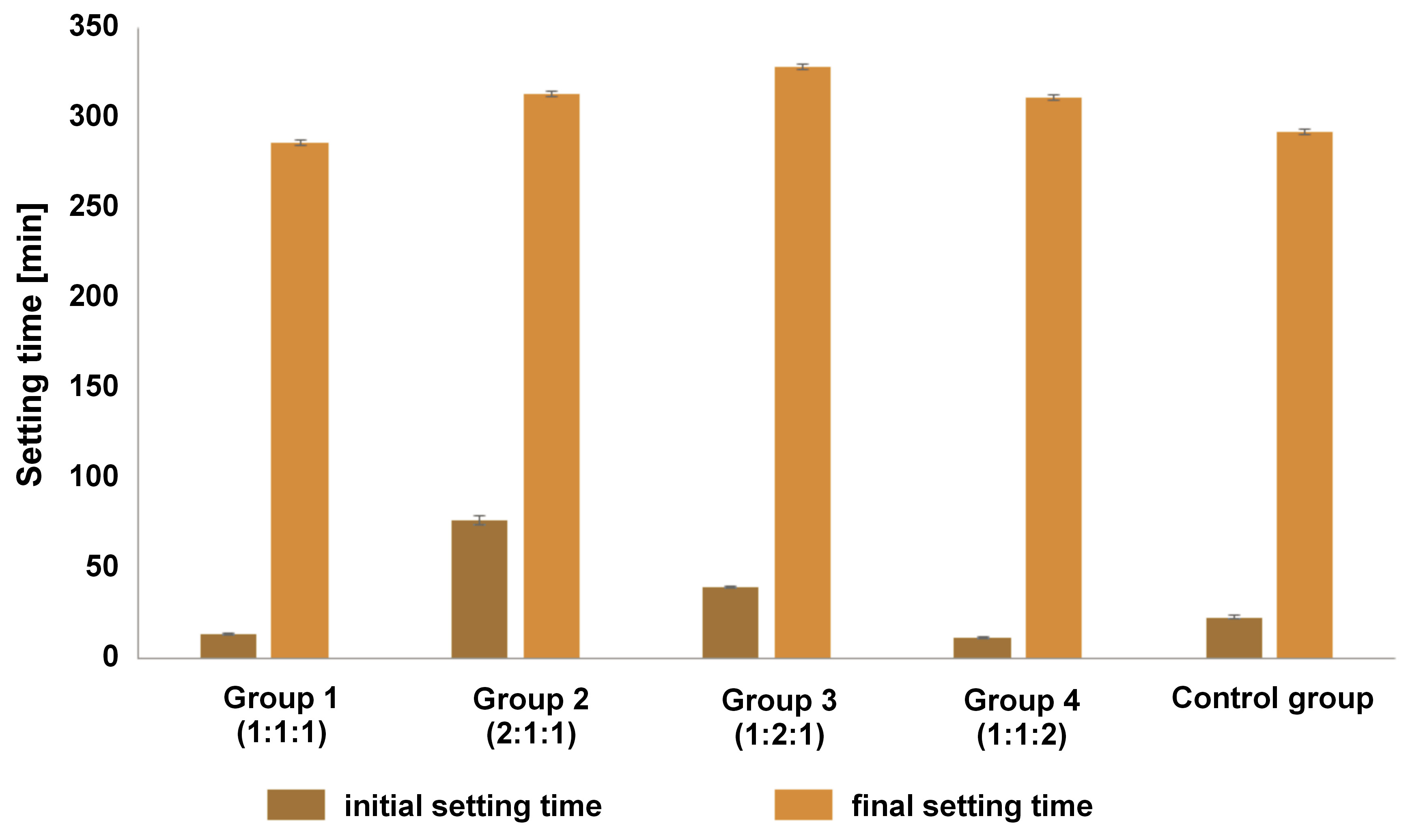
pH values
Figure 3 illustrates the comparison of pH values of the samples after 3 h and 24 h. The control group showed the highest pH values, followed by group 1. One-way ANOVA indicated a statistically significant difference in pH between 3 h and 24 h in all groups (p < 0.001). Furthermore, Tukey’s test did not reveal any significant differences between groups 2 and 4 after 3 h (p = 0.311). Additionally, no significant differences were observed between the 24-h pH values in groups 1, 2 and 3 (p > 0.05). All the tested materials exhibited pH values above 8.5.
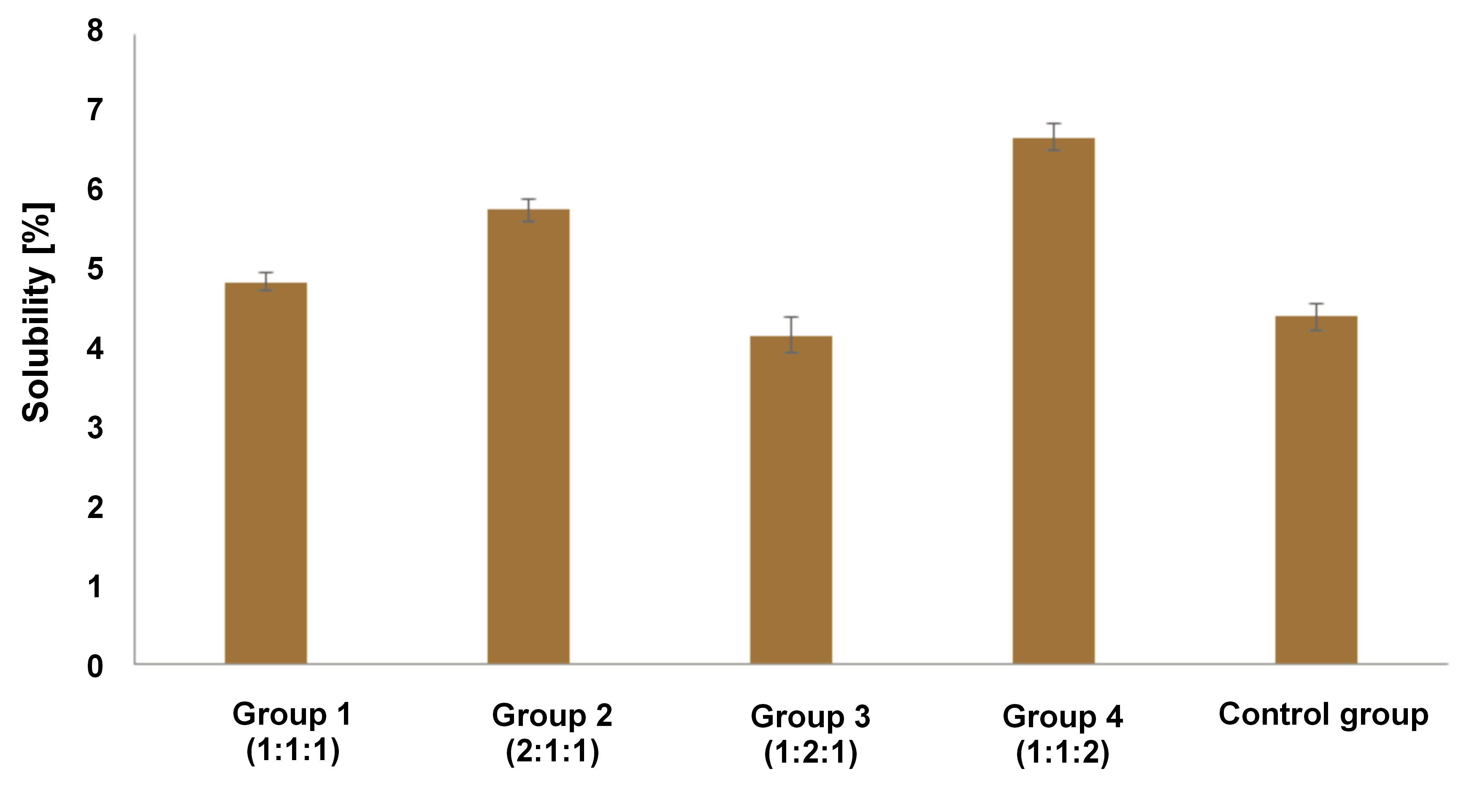
Solubility
As shown in Figure 4, the solubility of the control group was 4.41%. Group 3 had a lower solubility than the control group. In contrast, the remaining groups demonstrated a higher solubility than the control group. One-way ANOVA followed by Tukey’s test revealed that there were no statistically significant differences between group 3 and the control group (p = 0.113). The solubility rate of all tested materials exceeded 3%.
Selection of the group exhibiting the optimal properties
According to the evaluation of the viscosity, setting time, pH, and solubility, group 1 possessed the most favorable properties. The 1:1:1 ratio displayed the lowest viscosity, the shortest final setting time, a 24-h pH range of 8.5–10.5, and was second in the solubility rate compared to the other groups. Accordingly, the ratio was considered optimal and was subsequently evaluated for its antioxidant and cytotoxic properties.
Antioxidant property of the optimal ratio
The DPPH assay at the half maximal inhibitory concentration (IC50) of the optimal ratio combination of 3 nanoscale materials was determined to be 62.21 ±0.10 μg/mL. Given its strong antioxidant activity, quercetin was used as a comparison standard, displaying a scavenging activity of 4.50 ±0.00 μg/mL.
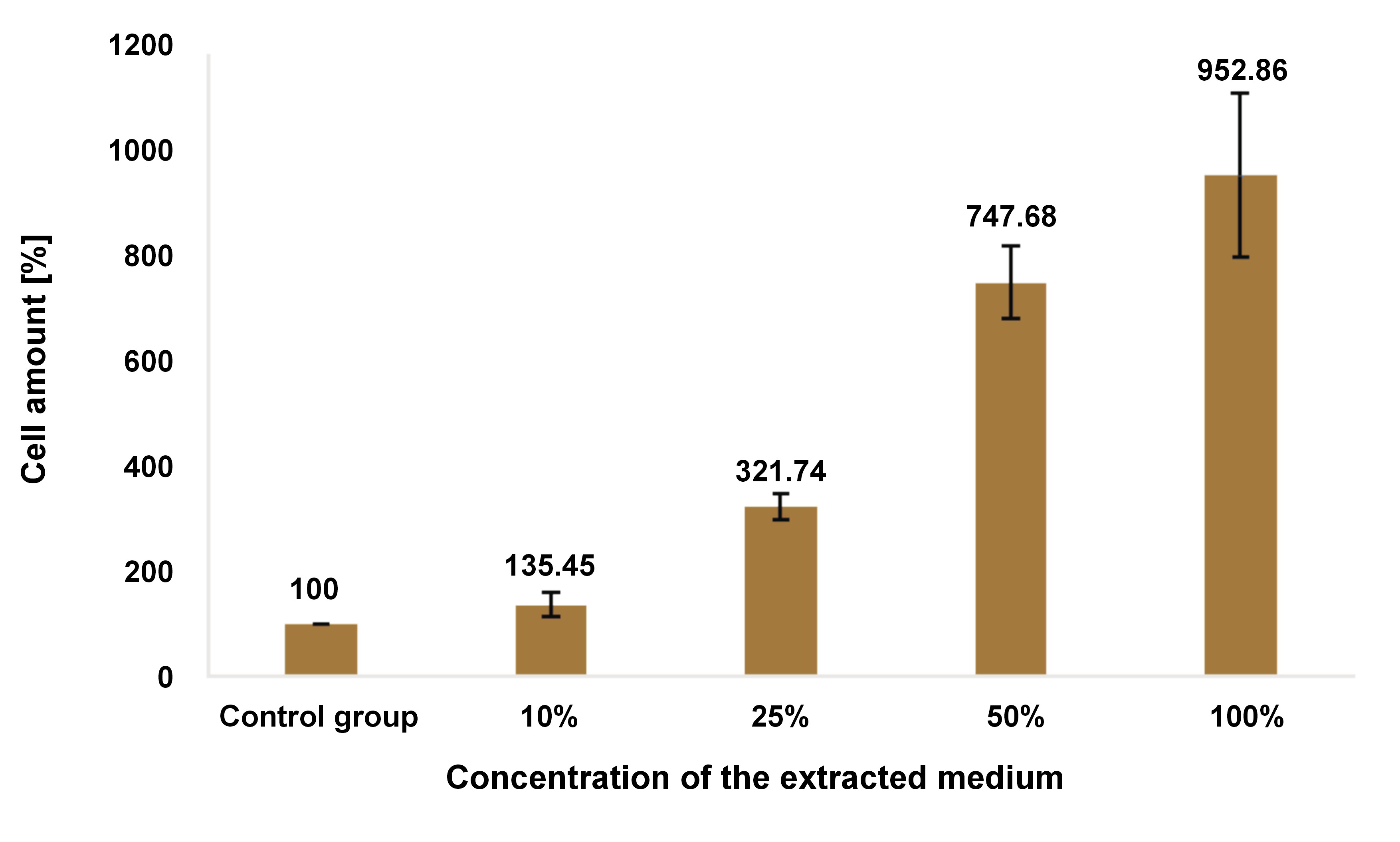
Cytotoxicity of the optimal ratio
Figure 5 and Table 1 present the results of the viability test. All concentrations of the optimal ratio combination showed no evidence of cytotoxicity. Conversely, the more concentrated the sample, the more proliferative the activity of the hDPSCs that were induced. There was no statistically significant difference between the 10% concentration and the control group (p > 0.05).
Discussion
In the presented research, we conducted an analysis to identify the optimal ratio of the nano-combination of calcium hydroxide, cocoa pod husk extract and anchovy powder. Each of the natural elements had the potential to enhance the calcium hydroxide capacity by facilitating the formation of the reparative dentin of a good quality. The flavonoids and theobromine found in cocoa pod husks have antioxidant properties, which result in an anti-inflammatory effect. Additionally, they function as an antibacterial agent.25, 26, 27, 28, 29, 30, 31 In a study that compared the application of calcium hydroxide and cocoa pod husk combination to pulp perforation in rats, the calcium hydroxide effect was improved.14 The study demonstrated that the combination resulted in the formation of a greater quantity of reparative dentin than when pure calcium hydroxide was used alone.14 Another approach used in the present study was the addition of anchovy powder. The application of anchovy has been shown to induce odontoblast proliferation in rat dental pulp, whereas reports have indicated that anchovy cream application resulted in the formation of more reparative dentin than when pure calcium hydroxide was used alone.32, 33 Nanoscale materials were used to optimize the desired effect. It is established that nanotechnology can optimize the distribution of bioactive molecules necessary for reducing inflammation and inducing the differentiation of pulp cells.18, 34 Nanoscale materials range in size from 1 nm to 1000 nm, although many scientists restrict this range to <100 nm.35
Previous research showed that direct pulp capping materials require a setting time, solubility and pH properties.22 Another study concentrated on the pH and solubility characteristics.23 Therefore, we focused on the above properties when analyzing the nano-combination of calcium hydroxide, cocoa pod husk extract and anchovy powder.
The viscosity of the testing materials should be low, given that they are intended to serve as reparative dentin inducers. Additionally, the materials must exhibit good flow properties to adapt to irregular cavity floors. The low viscosity of the material will prevent the formation of gaps and postoperative sensitivity. It should be noted that there is currently no standard viscosity for such materials.36 The mean data from group 1 (1:1:1 ratio) revealed the lowest viscosity (884.17 ±20.59 Pa.s) among the tested groups, with the exception of the control group. The obtained viscosity result was considered high due to the nanoparticle size of the materials. The standard powder and liquid ratios were analyzed in this study. Similarly, nanosized particles yielded a greater number of particles than microsized particles and increased the viscosity of the testing materials.37
The setting time was divided into 2 parts: initial and final. The initial setting time started with the operator beginning to mix powder and liquid components and concluded when the first Gillmore needle was unable to make a full indentation. The final setting time was calculated by adding the initial setting time to the time required for the second Gillmore needle to make a full indentation.22 The results revealed that the materials exhibited a prolonged setting time. As previously reported, there is currently no standard for this parameter.36 However, knowledge of the setting time allows for the anticipation of clinical procedures and the prediction of the working time. In our study, group 4 (ratio 1:1:2) showed the shortest initial setting time (11.42 ±0.49 min), and group 1 (ratio 1:1:1) showed the shortest final setting time (286.33 ±1.40 min). The initial setting time was shown to be shorter than MTA (70 min), as reported by previous study.20 According to the same study, the initial and final setting times were not fixed when the restoration procedure was performed, as they were influenced by material rheology. In the case of MTA, restoration can be completed in 9.5 min before the initial setting time.20 The high viscosity of the MTA may reduce the time required for the restoration procedure.
The pH value of the testing materials affects the signaling of molecules in inflamed tissues. It is acknowledged that high-pH calcium hydroxide causes coagulation necrosis on the tissue surface upon contact with the material.6, 13 The results of the present study demonstrate a pH value for calcium hydroxide of 11.43 ±0.07, which was consistent with the range reported in other studies (11.5 ±0.2).38 In our study, no significant difference was observed between the 3-h and 24-h time points, indicating that the pH value remained relatively stable during this period. All tested materials exhibited a pH value greater than 8.5. The high pH level activated the transient receptor potential ankyrin 1 (TRPA1), which promoted dentinogenesis in the odontoblast. Even in the absence of Ca2+, the high alkalinity (pH 9) improved store-operated Ca2+ entry (SOCE).39 In another report, dental pulp cells cultured at pH 7.5 caused growth arrest or cell death, whereas those cultured at pH 9 showed moderate proliferation.40 This implies that all tested materials had a favorable pH value.
In a study by Poggio et al., the material solubility was expected to be less than 3%.23 However, the authors showed that the solubility was above 3%. Although all tested materials did not meet the specified parameters, the standard was more applicable to ready-made materials. The solubility of pure calcium hydroxide or its modifications promoted the dissolution of bioactive material into the tissue.23 In our study, the lowest solubility was observed in group 3 (1:2:1) (mean: 4.17 ±0.22%), followed by group 1 (1:1:1) (mean: 4.85 ±0.11%). Group 1 demonstrated the most optimal ratio of nano-calcium hydroxide, cocoa pod husk extract and anchovy powder based on the 4 parameters.
The antioxidant property of the material was evaluated based on the subsequent anti-inflammatory reaction. Prior research has shown that the inflammatory process augments the release of reactive oxygen species (ROS) and inflammatory mediators, which in turn increases signal transduction and mediates critical cellular stress responses. Reactive oxygen species are produced throughout the process, resulting in cell destruction and subsequent impact on cell viability. The antioxidant aids in disrupting the cycle and creates a balanced environment for repair.41 In our study, the IC50 of a nano-combination of calcium hydroxide, cocoa pod husk extract and anchovy powder was 62.21 ±0.10 µg/mL. The antioxidant activity of the cocoa pod husk was determined, and the result was consistent with those previously reported for the cocoa pod husk (58.014 ±0.004 µg/mL).26 The antioxidant activity was confirmed.24
Unlike pure calcium hydroxide, the nano-combination of calcium hydroxide, cocoa pod husk extract and anchovy powder was expected to stimulate cell proliferation. The expectation was based on reports related to anchovy powder42 and the high pH. The results of the cytotoxicity assay were unexpected. In comparison to the control group, 10% concentration of the nano-combination induces 1.3-fold proliferation, 25% induce 3-fold proliferation, 50% induce 7-fold proliferation, and 100% induce 9-fold proliferation.
The antioxidant and cytotoxic properties of this nano-combination powder require investigation. The findings encourage further research into the potential use of a nano-combination of calcium hydroxide, cocoa pod husk extract and anchovy powder as a reparative dentin inducer.
Conclusions
The novel nano-combination of calcium hydroxide, cocoa pod husk extract and anchovy powder, proposed in this study at a 1:1:1 ratio, was found to possess the requisite properties to be used as a reparative dentin inducer. The formulation promotes the increased rate of restoration placement after treatment, the short initial setting time (13.5 min) and a high pH at 24 h (9.91), thereby promoting tissue healing. The antioxidant properties were identified as active, and the materials demonstrated excellent biocompatibility, as evidenced by the MTT assay. Further basic follow-up tests should be performed in order to prepare the combination, such as an antibacterial potential test. In the future, in vivo research should be conducted to examine the impact of the novel nanostructured material on the dentinogenesis marker. Further evaluation is required to explore the potential enhancement of the material’s properties when each component is combined with calcium hydroxide.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.