Abstract
Background. Avelumab is a human antibody that targets the programmed cell death ligand-1 (PD-L1) protein in cancer cells. Novel anticancer therapies for renal cell carcinoma (RCC) consider cluster of differentiation 15 (CD15) and interleukin 17 receptor A (IL-17RA) as potential targets. Notably, the expression of PD-L1, CD15 and IL-17RA is dependent on signal transducer and activator of transcription 3 (STAT3).
Objectives. The aim of the study was to investigate whether targeting PD-L1 with avelumab alters the expression levels of CD15 and IL-17RA, and to assess the STAT3-mediated regulation of CD15 and IL-17RA.
Material and methods. We applied immunocytochemistry (ICC) and confocal laser scanning (CLS) microscopy to assess the expression and localization of the immunotherapy targets in 3 renal cancer cell lines and 1 healthy renal cell line.
Results. After treatment with 20 ng/mL avelumab, renal cancer cells showed a reduction in STAT3 expression. The expression of CD15 increased in cancer cells that exhibited a high level of IL-17RA, and the membrane signal of CD15 was reduced. In other renal cancer cell lines, the expression of CD15 decreased. Conversely, the level of IL-17RA changed only in healthy renal cells after treatment with avelumab, with no impact on renal cancer cells.
Conclusions. Our study suggests that the targeting of PD-L1 with avelumab alters the expression of CD15 and IL-17RA, which play an important prognostic and therapeutic role in novel anticancer therapy.
Keywords: CD15, avelumab, IL-17RA, membrane antigen modulation
Introduction
Renal cell carcinoma (RCC) is a type of kidney cancer that originates in the lining of small tubes within the kidney.1 There are several subtypes of RCC, including clear cell RCC, papillary RCC and chromophobe RCC. Clear cell RCC is the most common subtype, accounting for 75% of all RCC cases.1, 2 Renal cell carcinoma is among the most lethal urological cancers due to its delayed diagnosis and poor response to therapies. Surgery is the primary treatment for localized RCC, with partial or radical nephrectomy being performed depending on the size and location of the tumor.1 For advanced RCC, systemic therapy with targeted agents such as sunitinib, pazopanib and axitinib is the standard of care.1, 2 Immunotherapy with nivolumab, ipilimumab and avelumab is also used in the treatment of advanced RCC.1, 2
Avelumab is a human monoclonal immunoglobulin G1 (IgG1) antibody that targets the programmed cell death ligand-1 (PD-L1) protein, which is expressed in cancer cells. It has been approved as a monotherapy for the treatment of patients with Merkel cell carcinoma and urothelial carcinoma. Avelumab is used in combination with axitinib for the treatment of RCC.
By acting on PD-L1, avelumab interferes with various downstream signaling pathways, affecting the expression of various proteins. Among the potential membrane antigens which are affected by anti-PD-L1 therapy, cluster of differentiation 15 (CD15) and interleukin 17 receptor A (IL-17RA) have been identified as novel targets for the anticancer therapy of various tumors and diseases.1, 3
The expression of CD15 and CD15s is linked to lymphatic and venous invasion, lymph node metastasis, distant metastasis, tumor stage, tumor recurrence, and overall survival in cancer.4 These antigens have the potential to serve as biomarkers for the diagnosis and prognosis in various types of cancer and may represent therapeutic targets.5 However, further research into the complex expression of CD15 and CD15s is needed to properly classify diagnostic parameters. The CD15 has demonstrated efficacy as a target for cancer therapy, affecting both cancer cells and immune myeloid-derived cells. This leads to an improved response to therapy as well as the inhibition of cancer growth and progression in tumor microenvironments.6 Experimental and clinical studies have demonstrated that targeting CD15 and CD15s is a promising treatment approach.7 However, ongoing efforts should focus on refining the clinical application of anti-CD15 therapy and assessing its safety profile.
Renal cell carcinoma is closely associated with immune mediators such as IL-17, which is an inflammatory cytokine that responds to tissue damage and external pathogens. The antigen IL-17RA is an interleukin receptor that is expressed in various kidney cells, including podocytes, mesangial cells and renal proximal tubular endothelial cells.8, 9, 10 The relationship between IL-17 and tumors is complex and has become an area of interest for numerous studies. Interleukin-17 plays a dual role in cancer development, as it can be both pro- and anti-tumorigenic.8 The interaction between IL-17 and IL-17RA has been shown to promote neoplasm metastasis and induce angiogenesis.11 A study by Dębiński et al. demonstrated the significance of lymphangiogenesis, especially intratumoral lymphatics, in aggressive cases of RCC.12 Additionally, IL-17 signaling is thought to similarly affect the tumor microenvironment.13
Escors et al. have previously described the effects of the PD-L1 intracellular signalosome in cancer cells.14 The cancer cells alter the protein expression via signal transducer and activator of transcription 3 (STAT3) phosphorylation.14 The effects of IL-17A in kidney epithelial cells are exerted via the ERK1/2 and STAT3 pathways.10, 15 The expression of CD15 has been observed to inversely correlate with STAT3 levels.16 When the level of CD15 increases, the level of STAT3 decreases. Therefore, the expression levels of both CD15 and IL-17RA depend on the expression of STAT3 and its phosphorylation level.
Avelumab, a PD-L1-targeting antibody, has shown efficacy in the treatment of various cancers. However, its impact on critical antigens such as CD15 and IL-17RA remains unexplored. The CD15, increasingly recognized in RCC prognosis, has an influence on invasion, metastasis and overall survival. Its potential as a diagnostic and therapeutic marker emphasises the need for comprehensive investigation. Concurrently, IL-17RA, which is expressed in renal cells, plays a dual role in cancer, influencing both metastasis and angiogenesis. The relationship between PD-L1, CD15, IL-17RA, and their downstream signaling pathways remains understudied. This research aims to address this gap in knowledge by investigating the effects of avelumab on the expression of CD15 and IL-17RA. A comprehensive understanding of the manner in which avelumab modulates these antigens is pivotal for advancing personalized therapies, unraveling novel biomarkers and enhancing our understanding of cancer antigen modulation by anticancer drugs. This short study focuses on the changes in CD15 and IL-17RA expression after treatment of the cells with avelumab, addressing a crucial knowledge gap and shedding light on the emerging role of cancer antigen modulation by anticancer drugs.17 Moreover, it establishes a foundation for more refined therapeutic strategies and improved patient outcomes.
Material and methods
Primary cell culture
Renal cancer cells (RC1, RC2, RC3) and healthy renal cells (HR) were derived from the renal cancer resections obtained from patients at the Department of Minimally Invasive and Robotic Urology (University Center of Excellence in Urology, Wroclaw Medical University, Poland). The corresponding part of the resected tissues underwent pathological examination. The RC1 cells were derived from a 68-year-old female patient with G1 clear cell RCC (primary tumor subtype 1a (pT1b)). The RC2 cells were derived from a 69-year-old male patient with G2 clear cell RCC (pT1b). The RC3 cells were derived from a 78-year-old female patient with G2 clear cell RCC (pT1b). The HR cells were derived from a 59-year-old male patient with G2 papillary cell RCC (pT1b). The use of patient-derived cells from the same individual provides a more clinically relevant and representative model for studying RCC and allows researchers to better understand the specific characteristics of both cancerous and healthy cells within the context of an individual’s unique biological profile. After the surgical removal of the tumor, 0.5 cm3 of cancer tissue was inserted into the 2-mL probe with phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, USA). The sample was immediately transported to the laboratory and further cut into small pieces. The tissue samples were incubated in a culture medium, which was replaced daily. The cells were cultured in 25-mL polystyrene cell culture flasks (Falcon®; Corning Life Sciences, Tewksbury, USA) with Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich) and antibiotic (streptomycin/gentamycin) in a humidified incubator (Heracell; Thermo Fisher Scientific, Waltham, USA) under standard culture conditions at 37°C in an atmosphere containing 5% CO2. When required, the cells were rinsed with PBS and removed by trypsinization (0.025% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA); Sigma-Aldrich). The cells were resuspended and transferred into new culture flasks. The flasks in which the cells exhibited consistent morphology were trypsinized, and a small number of cells were transported to the new culture flask.
Avelumab preparation and incubation
The experimental protocol involved seeding the renal cancer cells on glass coverslips and 10-well microscopy slides to facilitate subsequent analyses. Avelumab, sourced as Bavencio in a 20 mg/mL concentration, was prepared by dissolution in DMEM to attain concentrations of 20 ng/mL and 20 µg/mL. Subsequently, the requisite quantity of the drug solution was administered to the cells, initiating a 24-hour incubation period. Following this exposure, the cells were fixed using 4% paraformaldehyde. Then, immunocytochemical and fluorescent staining procedures were employed to visualize and assess the expression patterns of CD15 and IL-17RA for potential alterations induced by avelumab at the molecular level. This methodological approach aimed to capture and analyze the responses of RC cells to varying concentrations of avelumab, thereby laying a foundation for understanding its impact on CD15 and IL-17RA expression.
MTT assay
In this study, the assessment of cell viability and metabolic activity was conducted using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After cultivating renal cancer cells (RC1, RC2, RC3) and HR cells under specified culture conditions in polystyrene cell culture flasks, the MTT assay was employed to assess cellular health. Following the seeding of cells in 96-well plates, the cells were treated with the MTT reagent and incubated, allowing viable cells to enzymatically convert the dye into formazan crystals. Subsequently, the formazan crystals were solubilized, and a spectrophotometric measurement was conducted at approx. 570 nm, providing a quantitative readout of cell viability. The colorimetric assay served as a crucial tool for evaluating the impact of experimental variables on the metabolic activity of the renal cancer cells, offering insights into their proliferative and survival capacities. The MTT assay, a standard method in cell biology, facilitated a robust assessment of cellular responses and contributed valuable data to the characterization of RC and HR cells.
Immunocytochemical staining
The immunocytochemical staining was employed to semi-quantitatively assess the expression and distribution of CD15, IL-17RA and STAT3 in RC1–RC3 and HR cell lines following the incubation with avelumab. The cells were incubated for 24 h on 10-well microscopy slides, washed in PBS, and fixed in 4% formalin. Afterward, they were washed in PBS, and a peroxide block (ab80436 kit; Abcam, Waltham, USA) was added for 10 min. Subsequently, the cells were washed in PBS with 1% Triton X (Merck Life Science Sp. z o.o., Poznań, Poland). The protein block agent (Abcam) was incubated with the cells for 10 min. The cells were then incubated with the first-order antibodies (1:250, STAT3 (124H6); 1:250, IL-17R (49M4D2); 1:250, CD15 (sc-19648)) for 24 h at 4°C. Afterwards, the cells were washed with PBS and 1% Triton X, and the mouse complement agent (Abcam) was added. After 10 min, the cells were washed with PBS and 1% Triton X. Then, a 3,3’-diaminobenzidine (DAB) mixture (1 DAB:50 DAB substrate) was applied for 10 min in the dark. Next, the cells were washed for 10 min in distilled water and stained with hematoxylin for 1 min. The excess stain was removed by washing with water for 30 min, and the cells were dehydrated by incubation for 5 min in each of the ethanol solutions (50%, 60%, 70%, 80%, 90%, and 96%). In the final stage of sample preparation, xylene I and II were used to dehydrate the sample. Finally, the glass was mounted on the sample using dibutylphthalate polystyrene xylene (DPX) medium (Aqua-Med ZPAM-KOLASA, Łódź, Poland). The samples were observed under a light microscope (Olympus BCX43; Olympus, Tokyo, Japan) and the images were captured using a Plan-Apochromat 20× objective (Olympus). In each sample, the percentage of stained cells was recorded.
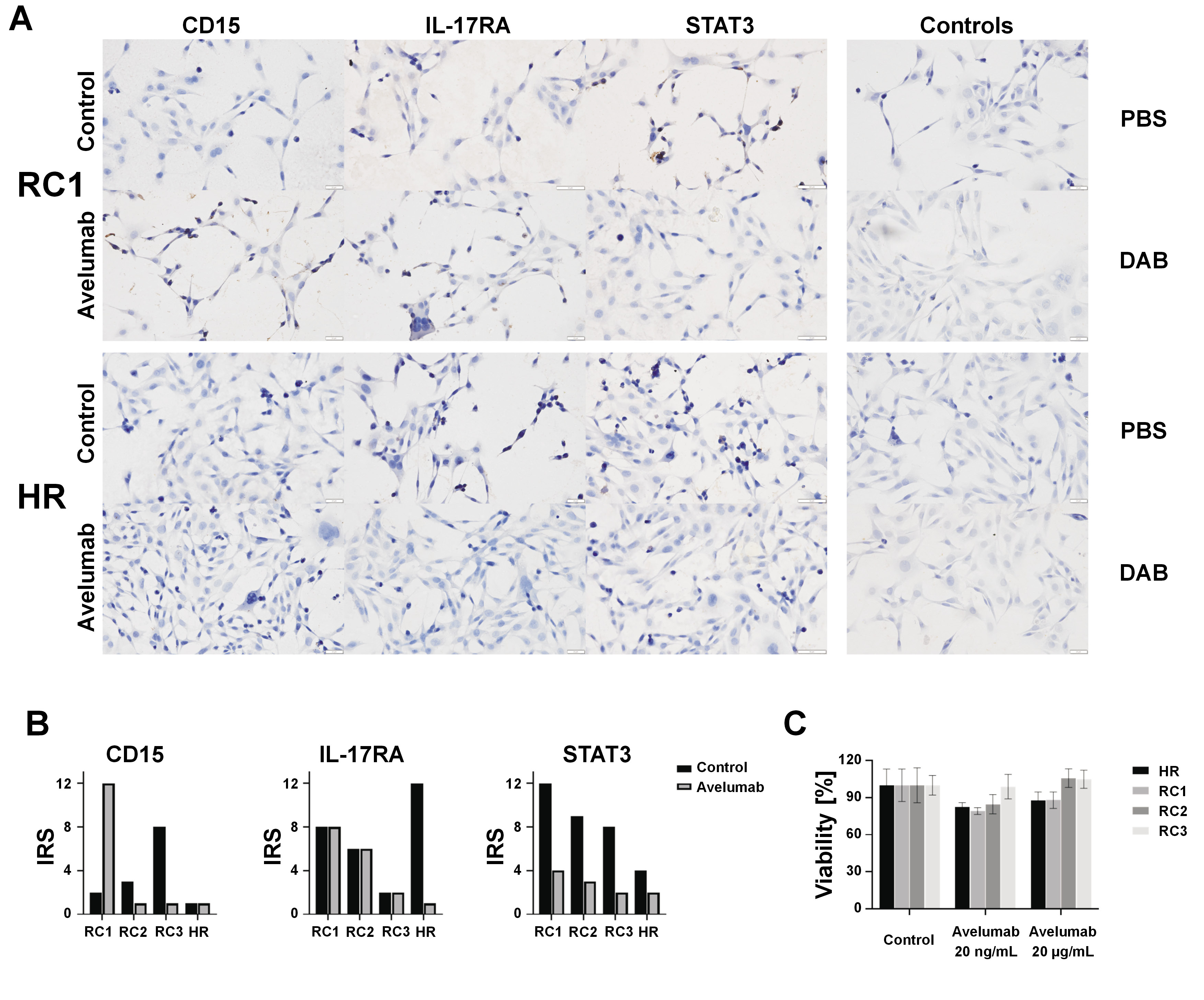
The immunoreactive score (IRS), developed by Remmele and Stegner, is a widely utilized method for the semi-quantitative assessment of immunohistochemical staining in histopathology.18 Introduced in 1987, the IRS integrates both the intensity and proportion of stained cells to provide a comprehensive score reflecting the overall immunoreactivity within a tissue sample. The IRS system assigns a numeric value to the staining intensity (ranging from 0 to 3) and another value to the proportion of positively stained cells (ranging from 0 to 4). These values are then multiplied, resulting in a final IRS ranging from 0 to 12. This scoring system allows researchers and pathologists to objectively evaluate the expression levels of specific antigens in tissues, thereby contributing to the understanding of disease processes and aiding in the development of targeted therapeutic approaches. The IRS has been particularly valuable in the field of cancer research, where the assessment of the extent and intensity of protein expression can provide insights into tumor biology and prognosis.
Fluorescent staining
To visualize and assess the expression ratio of STAT3 to phospho-STAT3 and IL-17RA to IL-17, confocal laser scanning (CLS) microscopy was used. The cells were incubated on covered glass slides within Petri dishes after 24 h and washed 3 times with PBS. The fixed cells were labeled with the first-order antibodies (1:250, STAT3 (124H6; Cell Signaling Technology, Inc., Danvers, USA); 1:250, IL-17R (49M4D2; Novus Biologicals, Centennial, USA); 1:250, CD15 (sc-19648; Santa Cruz Biotechnology, Dallas, USA)) for 1 h at 37°C, in accordance with the manufacturer’s protocol. Next, the cells were washed with PBS and stained with the second-order antibodies (Alexa Fluor™ 594 and Alexa Fluor™ 488 (1:500; A32731 and A32744, respectively; Abcam) for 1 h at 37°C. Fluoroshield™ with 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich) was applied for the visualization of nuclei and for mounting the cells. The cells were observed using the Olympus FluoView FV1000 CLS microscope (Olympus) and the images were captured using a Plan-Apochromat (60×) oil immersion objective (Olympus).
Statistical analysis
The viability and microscopy experiments were performed in at least 3 replicates. The data was expressed as mean ± standard deviation (M ±SD) and analyzed using one-way analysis of variance (ANOVA) (GraphPad Prism 8; GraphPad Software, Boston, USA). A p-value <0.05 was considered statistically significant. The immunocytochemical reaction was performed for each sample in triplicate.
Results
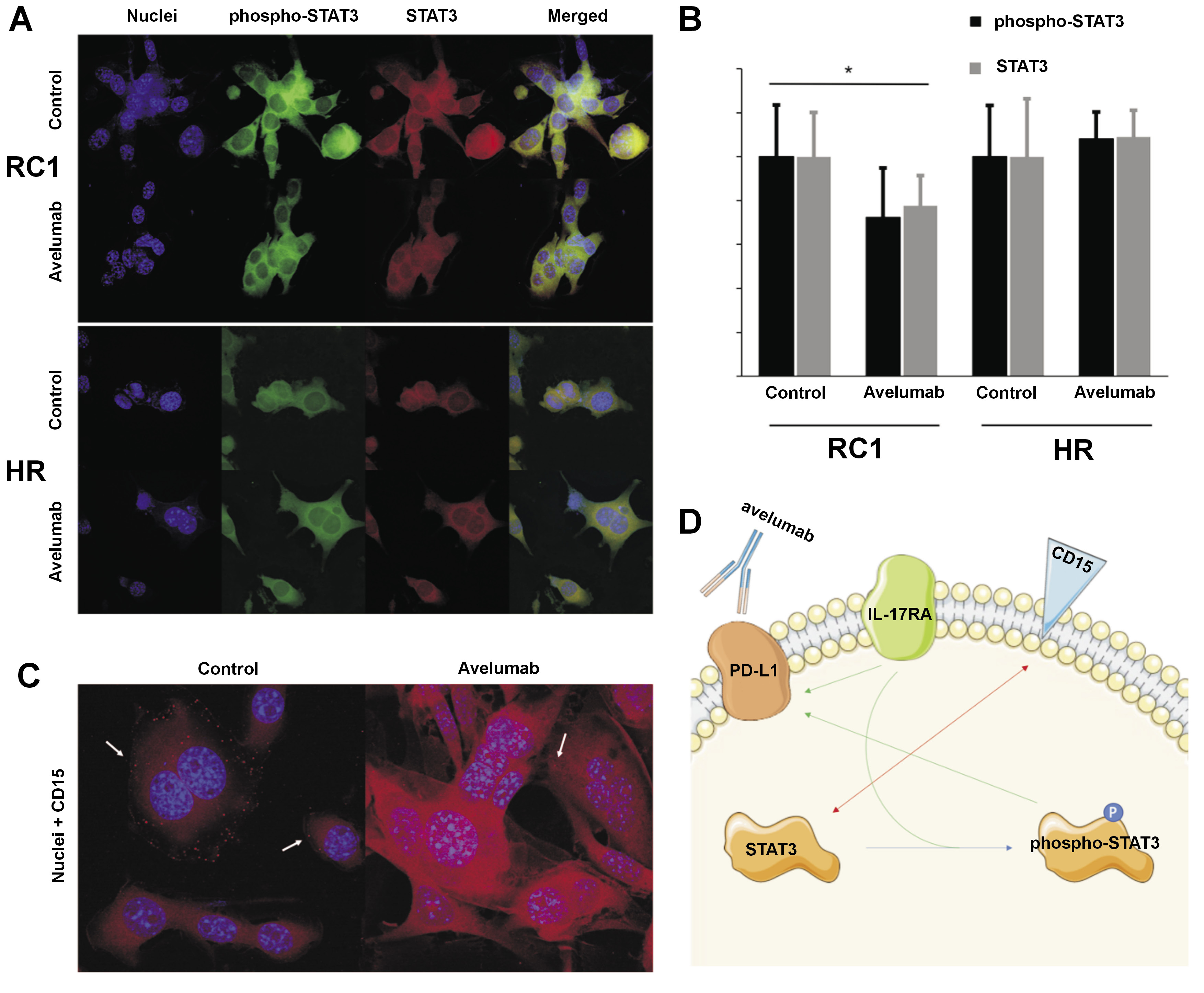
The results of our study demonstrate a profound and selective downregulation of STAT3 expression in renal cancer cells in response to avelumab, as illustrated in Figure 1A. This downregulation was specifically associated with both the total STAT3 protein and its phosphorylated form, as depicted in Figure 2A and Figure 2B. The HR cells did not exhibit a similar response to avelumab, indicating that the observed effect was specific to cancerous cells. Furthermore, despite the marked changes in STAT3 expression, no discernible morphological alterations were observed in the renal cancer cells following the incubation with avelumab.
Our investigations using immunocytochemistry (ICC) have revealed an intriguing antagonistic relationship between CD15 and IL-17RA expression, as illustrated in Figure 1B. Specifically, when the expression of CD15 was influenced by avelumab treatment, there was no concomitant change in the expression of IL-17RA. Upon exposure to avelumab, the RC1 cell line exhibited a shift of CD15 from the cell membrane to the membranous organelles inside the cells, as depicted with white arrows in Figure 2C. Despite the shift in the localization of CD15, the overall expression of CD15, as observed in both ICC and immunofluorescent studies, exhibited an unexpected increase.
Conversely, a decrease in IL-17RA levels in the HR cells following avelumab incubation was not accompanied by a corresponding change in the expression of the receptor in the renal cancer cells. This intriguing finding suggests that avelumab may exert distinct and cell-specific effects on IL-17RA expression, with HR cells responding differently than their cancerous counterparts. The differential response of renal cancer cells and healthy cells to avelumab underscores the complexity of the drug’s impact on signaling pathways and highlights the necessity for further exploration of its precise mechanisms of action.
The observed alterations do not affect the viability of the cells, as evidenced by the absence of statistically significant results (Figure 1C). Noteworthy, avelumab, a drug that affects the immune system, is not solely responsible for cytotoxicity in the cancer cell culture. These results contribute to a more comprehensive understanding of the molecular responses induced by avelumab in renal cancer cells. The selective downregulation of STAT3, the antagonistic relationship between the expression of CD15 and IL-17RA, and the differential responses in renal cancer cells and healthy cells provide valuable insights that may have implications for the development of targeted therapies in renal cancer. The absence of morphological changes in cancer cells following avelumab treatment further emphasizes the specificity of the observed molecular alterations. Our findings pave the way for future research into the intricate interplay of signaling pathways affected by avelumab in renal cancer, offering potential avenues for therapeutic interventions and personalized treatment strategies.
Discussion
The STAT3 signaling is responsible for the simultaneous alterations in CD15 and IL-17RA. Several studies have indicated that IL-17RA-mediated signaling leads to the phosphorylation of STAT3, which in turn activates the transcription of PD-L1.15, 19, 20 Yang et al. demonstrated that the expression of IL-17RA is associated with the promotion of cancer stem-like properties in cancer cells via the STAT3 pathway.21 The findings of the study conducted by Hevehan et al. showed that the expression of STAT3 remains strongest in CD15− cells and gradually declines upon granulocytic differentiation, thereby supporting the negative regulation of protein expression.22 Giordano et al. demonstrated that STAT1/STAT3-dependent phosphorylation does not cause CD15 overexpression, indicating that the shift from STAT3 to phospho-STAT3 does not independently affect the expression of CD15.23
A summary of the interaction between CD15, IL-17RA and STAT3 is shown in Figure 2D. A study by Zerdes et al. demonstrated that STAT3 promotes PD-L1 expression24 and another study showed that phospho-STAT3 increases the expression of PD-L1 through direct binding to the PD-L1 promoter.25 The targeting of IL-17A has been observed to inhibit the expression of PD-L1 in tumor cells.26, 27 Escors et al. discussed the impact of PD-L1 intracellular signalosome in cancer cells, which modifies protein expression through STAT3 phosphorylation.14 Meanwhile, IL-17A was found to affect kidney epithelial cells through the extracellular signal-regulated kinase 1/2 (ERK1/2) and STAT3 pathway,10, 15 with CD15 expression correlating to STAT3 levels.16 Interestingly, the levels of both proteins were observed to act in a reverse manner, with STAT3 levels decreasing when CD15 levels increased. Consequently, the expression levels of CD15 and IL-17RA were found to depend on the expression of STAT3 and its level of phosphorylation. There are currently no studies on the impact of avelumab on the expression of CD15 and IL-17RA. To address this gap in knowledge, we aimed to investigate the changes in CD15 and IL-17RA expression following treatment with avelumab, thereby deepening our understanding of the potential role of anticancer drugs in modulating cancer antigens.17
The findings of this study offer valuable insights into the effects of avelumab on the expression levels of PD-L1, CD15 and IL-17RA in renal cancer cells. However, further research is required to fully understand the underlying mechanisms and to optimize the use of avelumab and other immunotherapeutic agents in the treatment of RCC. Future studies may explore the impact of combination therapies targeting multiple signaling pathways and proteins involved in RCC, as well as the use of biomarkers to identify patients who are most likely to benefit from immunotherapy. In addition, the study of the effect of avelumab on other cell types, such as tumor-associated immune cells and stromal cells, may provide additional insights into the complex interactions between different cell types within the tumor microenvironment. Ultimately, continued research in this area has the potential to facilitate the development of more effective and personalized therapies for patients with RCC.
Limitations
While our study provides valuable insights into the impact of avelumab on the expression levels of PD-L1, CD15 and IL-17RA in renal cancer cells, it is important to acknowledge certain limitations that warrant consideration. Firstly, the lack of existing studies regarding the influence of avelumab on the expression of CD15 and IL-17RA necessitates a cautious interpretation of our findings. Further investigations are required to validate and expand upon our observations. Additionally, the inherent complexity of the tumor microenvironment introduces a degree of variability, as evidenced by the observed differences among patients (RC1–RC3) in our study. The high degree of variability observed in the results may also be attributed to inherent biological heterogeneity within the patient cohort and potential variations in the tumor microenvironment, genetic makeup and previous treatment histories. Recognizing the significance of this variability, future studies should aim to address and elucidate these patient-specific factors, employing larger cohorts and comprehensive patient profiling to enhance the generalizability and robustness of our findings. Despite these limitations, our study serves as a foundational exploration into the potential effects of avelumab on RCC. It provides a platform for further research and the refinement of therapeutic strategies in the pursuit of more effective and personalized treatment approaches.
Conclusions
In targeting renal cells with avelumab, our study unveils notable alterations in the expression of CD15 and IL-17RA antigens. Significantly, the complex interplay between STAT3 and the phospho-STAT3 pathway emerges as a pivotal mechanism orchestrating these molecular changes. While our findings underscore the potential of these antigens as promising targets in novel anticancer therapies, further investigations involving sophisticated in vivo studies and an expanded research cohort encompassing a greater number of patients in study samples are required to further corroborate our findings. These endeavors will not only serve to fortify the robustness of our results and hypotheses but also contribute to the development of more precise and personalized therapeutic regimens, ultimately advancing the clinical applicability of avelumab in RCC.
Ethics approval and consent
to participate
The study was approved by the Ethics Committee of Wroclaw Medical University (approval No. 755/2022).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.