Abstract
Movement disorders of the stomatognathic system include oromandibular dystonia (OMD), oral dyskinesia, sleep/awake bruxism, functional (psychogenic) stomatognathic movement disorders (FSMDs), tremors, and hemimasticatory spasm (HMS). Most patients first consult dentists or oral surgeons. The differential diagnoses of these involuntary movements require both neurological and dental knowledge and experience, and some of these movement disorders are likely to be diagnosed as bruxism or temporomandibular disorders (TMDs) by dental professionals. However, excepting movement disorder specialists, neurologists may find it difficult to differentially diagnose these disorders. Patients may visit numerous medical and dental specialties for several years until a diagnosis is made. Therefore, movement disorders of the oral region may represent a blind spot between dentistry and medicine.
The present narrative review aimed to describe the clinical characteristics and differential diagnoses of some movement disorders, as well as the problems bridging dentistry and medicine. Movement disorders have the following characteristic clinical features: OMD – task specificity, sensory tricks and the morning benefit; FSMDs – inconsistent and incongruous symptoms, spreading to multiple sites and the lack of sensory tricks; and HMS – the paroxysmal contraction of unilateral jaw closing muscles, the persistence of symptoms during sleep and the loss of a silent period. A careful differential diagnosis is essential for the adequate and effective treatment of each involuntary movement. Refining the latest definition of bruxism may be necessary to prevent the misdiagnosis of involuntary movements as bruxism.
Both dental and medical professionals should take an interest in the movement disorders of the stomatognathic system, and these disorders should be diagnosed and treated by a multidisciplinary team.
Keywords: differential diagnosis, bruxism, movement disorder, oromandibular dystonia, hemimasticatory spasm
Introduction
The stomatognathic system is an anatomical and functional unit comprising hard tissues (bones constructing the mandible and maxilla, teeth, and temporomandibular joints) and soft tissues (masticatory, tongue, lip, cheek, and lower facial muscles, as well as nervous and vascular supplies). This system plays an indispensable role in various important functions, such as mastication, swallowing, speech, breathing, and facial expressions. Dentists and oral surgeons who specialize in the stomatognathic system were the first healthcare professionals to observe patients exhibiting symptoms in this region. Various movement disorders exist within the stomatognathic system, including oromandibular dystonia (OMD), orolingual dyskinesia, bruxism, functional (psychogenic) stomatognathic movement disorders (FSMDs), hemimasticatory spasm (HMS), tremors, tics, and myokymia.1 Moreover, several other diseases exhibit symptoms in the stomatognathic system, including Parkinson’s disease, Down syndrome, Tourette syndrome, Rett syndrome, autistic spectrum disorders, Huntington’s disease, Wilson’s disease, chorea-acanthocytosis, Leigh syndrome, and Lesch–Nyhan syndrome.2, 3, 4 In one study, approx. 70% of patients with OMD saw dentists, and 60% visited oral surgeons.5 However, around 90% of these patients were not properly diagnosed5; instead, they were diagnosed with bruxism, temporomandibular disorders (TMDs) or psychiatric diseases.1, 5, 6, 7, 8 According to the current definition of bruxism,9 some patients with movement disorders may be misdiagnosed.1, 7 Patients continue to receive ineffective treatment for misdiagnosed bruxism or TMDs, which sometimes leads to the discontinuation of further treatment.1, 7, 8 Medical professionals may misdiagnose bruxism or TMDs as other involuntary movements, such as jaw closing dystonia or jaw deviation dystonia.1, 7 Thus, these professionals cannot always adequately diagnose and treat the movement disorders of the stomatognathic system.1, 7, 8
The diagnosis and treatment of involuntary movements in the stomatognathic region require both medical and dental knowledge and experience. Dental professionals often lack knowledge about involuntary movements, such as dystonia, whereas medical professionals typically lack familiarity with the anatomy and function of the stomatognathic region. Therefore, this condition represents a blind spot in both dentistry and medicine. Furthermore, many attending physicians are unaware of their inability to properly diagnose and treat involuntary movements in the stomatognathic region.
The purpose of the present review was to outline involuntary movements in the stomatognathic region, particularly movement disorders that need to be differentiated from bruxism, such as OMD, FSMDs and HMS, explain their clinical signs and differential diagnoses, and discuss related problems in dentistry and medicine.
Material and methods
The literature search embraced electronic medical literature databases (PubMed, Embase, the Web of Science, Google Scholar, the Japan Medical Abstracts Society, and Medical Online) and used the following keywords: “movement disorder”; “sleep bruxism”; “awake bruxism”; “temporomandibular disorder”; “oromandibular dystonia”; “jaw closing dystonia”; “oral dyskinesia”; “differential diagnosis”; “hemimasticatory spasm”; “functional movement disorder”; “etiology”; “epidemiology”; and “treatment”. Additionally, a manual search was conducted to evaluate the articles cited in the related resources. Reports prior to November 30, 2023, identified in the abovementioned databases or via a manual search, with no language restrictions, were screened by the author.9, 10 The exclusion criteria were records irrelevant to the purpose of this study. All studies were evaluated and assessed for eligibility, and reviewed by the author.
Clinical characteristics of involuntary movements in the stomatognathic system
Bruxism
Bruxism is classified as either sleep or awake bruxism, which are defined as masticatory muscle activities that occur during sleep and wakefulness, respectively.11 Sleep bruxism is further characterized as rhythmic or non-rhythmic, and awake bruxism is characterized by repetitive or sustained tooth contact and/or by bracing or thrusting of the mandible.11 Sleep and awake bruxism are considered different behaviors during sleep and wakefulness.11 Bruxism is an oral motor behavior that primarily includes teeth grinding and/or clenching. Sleep bruxism, primarily characterized by grinding, and awake bruxism, mainly manifesting as clenching, typically do not occur in the same individuals.12 Bruxism can be distinguished as primary/idiopathic, secondary, and iatrogenic bruxism.13 Primary/idiopathic bruxism is characterized by the absence of any identifiable medical or dental causes. However, secondary bruxism is associated with a medical/psychiatric condition such as a movement disorder, sleep-related disorder, or neurological or psychiatric disorder.13 Iatrogenic bruxism follows drug intake or withdrawal including chemical substances and medications.13
Presentation
Bruxism has been associated with tooth wear or cracked teeth, failure of dental restorations, prostheses, or implants, pain in the masticatory muscles, teeth, temporomandibular joints, TMDs, masseter muscle hypertrophy, tooth fracture, cheek or lip biting, hypersensitive teeth, and tension headaches. Indentations on the tongue, lips, and/or linea alba on the inner cheek are occasionally observed.
In otherwise healthy individuals, bruxism should not be considered a disorder but rather a behavior that can be a risk (and/or protective) factor for certain clinical consequences.11 Additionally, a grading system for bruxism was proposed to determine the likelihood that a certain assessment of bruxism actually yields a valid outcome: 1) possible sleep/awake bruxism (based only on a positive self-report); 2) probable sleep/awake bruxism (based on a positive clinical inspection, with or without a positive self-report); and 3) definite sleep/awake bruxism (based on a positive instrumental assessment, with or without a positive self-report and/or positive clinical inspection).11
Although bruxism is generally assessed using self-reports or clinical findings, instrumental assessments are more reliable for diagnosing definite bruxism. Polysomnography is used as an instrumental assessment of sleep bruxism and is considered the gold standard for diagnosis.14 Polysomnography with audio-video recording or ambulatory electromyography (EMG) devices has been applied for a definite diagnosis; future studies using ambulatory EMG instruments may shift focus to scoring the entire spectrum of masticatory muscle activity.15 There is no definitive examination method for awake bruxism, but recently, ecological momentary assessment (EMA) has been applied. The EMA with a smartphone-based strategy has allowed data collection on the frequency of different awake bruxism behaviors reported by individuals in their natural environment.16, 17, 18, 19, 20 Applications using EMA include BruxApp®,16, 17, 18, 19, 20 WhatsApp with a web-based survey program called Mentimeter®,21 and a time logger.22 Early data were obtained from university students,16 but subsequent studies have included data from the general population17 and patients with bruxism.22 A recent study applied simultaneous recording of EMA and EMG to patients with bruxism to obtain more definitive data on awake bruxism.22 In the study, the association between probable and definite bruxism was confirmed. In the bruxism group, 30 out of 64 participants were definitively diagnosed and assigned to the control group. Additionally, 11 out of 36 participants were classified into the probable control group diagnosed with definite bruxism.22
Epidemiology
Bruxism is a common condition, observed to some degree during the lifetime of approx. 85–90% of the general population; however, only 5% develop a clinical condition.23 In earlier studies, the estimated prevalence of sleep bruxism awareness was based on reports by parents or sleep partners. Reports on the prevalence of awake bruxism vary widely, depending on the diagnostic method (possible or probable bruxism) and the patients investigated. According to an umbrella review, the prevalence of awake bruxism was 22–30%, and that of sleep bruxism was 1–15%.24 Sex differences with respect to sleep bruxism are not obvious, and most studies report an equal prevalence in men and women.25 However, awake bruxism occurs more frequently in women than in men.26 Because other movement disorders occur more frequently (60–70%) in women, a higher incidence of awake bruxism in women suggests that other movement disorders may be diagnosed as awake bruxism. However, numerous reports on the epidemiology of bruxism are based on patient self-reports or clinical symptoms, and their reliability should be carefully evaluated.
Etiology
Although the etiology of bruxism is not fully understood, a multifactorial etiology has been postulated, including biological, psychological (anxiety and stress), and exogenous (drugs, caffeine, tobacco, and alcohol) factors.24, 27 Sleep bruxism is characterized as a repetitive sleep-related movement disorder mainly involving rhythmic masticatory muscle activity at a frequency of 1 Hz, with occasional tooth grinding.28, 29, 30 Most sleep bruxism episodes coincide with brief cardiac and brain reactivations known as micro-arousals. Rhythmic masticatory muscle activity results from a sequence of events associated with sleep micro-arousals.28, 29, 30
Bruxism can occur in conditions beyond neurological disorders. A review examining bruxism in movement disorders found the highest incidence in Rett syndrome (97%), Down syndrome (42%), and autism spectrum disorder (32%).3
Treatment
Bruxism should not be recognized as a disorder in otherwise healthy individuals but rather as a behavior that can be a risk (and/or protective) factor for certain clinical consequences.11 In most cases, active treatment is not necessary. Treatments for bruxism include medication, occlusal splints, physical therapy, biofeedback therapy, and cognitive behavioral therapy.24 Recently, botulinum toxin therapy has been clinically applied.1, 31, 32 Botulinum toxin therapy should be considered in severe cases when other traditional treatments are ineffective. Palpation, EMG, and occlusal force measurements are used to identify target muscles.1, 8, 33, 34 In most cases, botulinum toxin is injected into the masseter and temporalis muscles. If the effect diminishes and the medial pterygoid muscle is tender, botulinum toxin should be administered into the medial pterygoid muscle.1, 8, 33, 34 In cases with severe grinding, the lateral pterygoid muscle is often painful and may also be treated.1, 8, 33, 34 Prolonged, intense bruxism can lead to the development of excessive tendonous tissue at the anterior margin of the masseter muscle, resulting in masticatory muscle tendon-aponeurosis hyperplasia.35, 36, 37 In such cases, a coronoidotomy is necessary to improve limited mouth opening and muscle pain.35, 36
Oromandibular dystonia (OMD)
Dystonia is a hyperkinetic movement disorder characterized by sustained or intermittent muscle contractions that cause abnormal repetitive movements and/or postures.2 Dystonia is categorized as focal, multifocal, segmental, hemidystonia, and generalized.2 Oromandibular dystonia is a focal type of dystonia characterized by sustained, intermittent, or task-specific contractions of the masticatory, lingual, pharyngeal, and/or muscles of the stomatognathic system.1, 2, 4, 5, 6, 7, 8, 38
Presentation
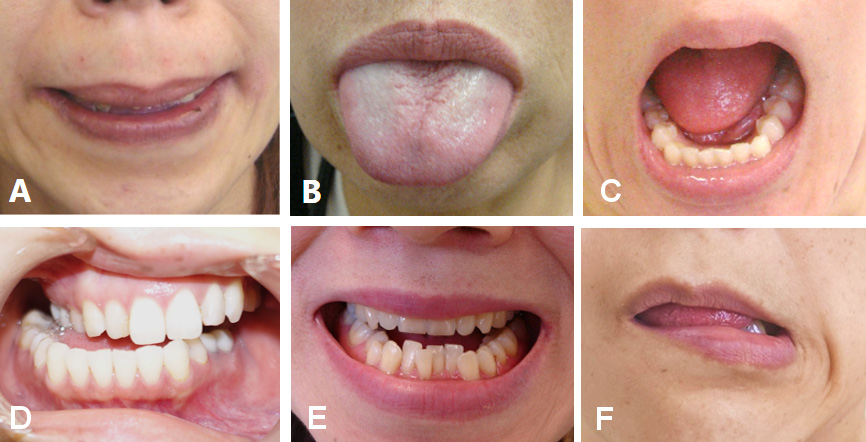
Symptoms of OMD include masticatory disturbance, tongue biting, cheek biting, limited mouth opening, muscle pain, dysphagia, dysarthria, upper airway obstructions,39 and temporomandibular joint dislocations.39, 40 These symptoms can significantly impair daily activities, cause social embarrassment and cosmetic disfigurement, and limit patients’ ability to work, thereby having a profound impact on quality of life.1, 6 Oromandibular dystonia is classified into 6 subtypes: jaw closing; jaw opening; lingual; jaw deviation; jaw protrusion; and lip dystonia (Figure 1).1, 6, 7, 8
Oromandibular dystonia is generally diagnosed based on characteristic clinical features of focal dystonia and EMG findings.1, 6, 7, 8 These features include stereotypy, task specificity, sensory tricks, co-contraction, morning benefit, and overflow phenomenon.1, 6, 7, 8, 38, 41 Patients with OMD exhibit distinct stereotypical patterns of muscle contraction depending on the subtype. For instance, stereotypy was observed in 95.8% of 385 patients with OMD in one study.7 During the initial phase, OMD symptoms often manifest only during specific tasks such as speaking, chewing, or mouth opening.38 Notably, 69.9% of patients with OMD demonstrated task specificity.7 Sensory tricks are sensory-based methods that can temporarily alleviate dystonia symptoms, such as chewing gum or candy.7, 41 Sensory tricks were observed in 51.4% of patients with OMD.7 Symptoms of dystonia tend to be milder in the morning. This is called morning benefit and was reported in 47.3% of patients with OMD.7 Co-contraction involves involuntary simultaneous contractions of agonist and antagonist muscles due to loss of reciprocal inhibition of muscular activities.1, 6, 7, 8 Dystonic contracture of masticatory muscles may extend to other muscles including the orbicularis oris, orbicularis oculi, or other facial, neck, and shoulder muscles, which is known as the overflow phenomenon.38
Epidemiology
The onset of OMD typically occurs in patients in their 50s.1, 42, 43, 44 However, drug-induced tardive OMD can occur in teenagers. Women are affected approx. twice as frequently as men.42, 43, 44 Oromandibular dystonia can occur in isolation; however, it may present with other comorbidities, such as segmental or generalized dystonia. The ratio of isolated OMD among all OMD cases differs considerably, as reported by neurologists (focal – 39%; segmental – 43%; and generalized – 10%)43 and oral surgeons (focal – 90.8%; segmental – 10.4%; and multifocal – 6.3%).43 This difference may be attributed to neurologists primarily assessing OMD cases associated with neurological diseases, whereas oral surgeons were able to identify numerous mild cases.44
The prevalence of OMD has been shown to be considerably higher than previously estimated.6, 43 A recent study reported the prevalence of OMD to be 9.8 per 100,000 persons, with an incidence of 2 per 100,000 person-years.43 The study suggested that OMD may have an equal or even higher prevalence than blepharospasm or cervical dystonia.43 Oromandibular dystonia is considered a rare disorder; however, in reality, several cases are incorrectly diagnosed.
Etiology
Oromandibular dystonia is part of the clinical spectrum of a wide variety of diseases. The causes of OMD can be idiopathic (unknown cause), inherited (dystonia of proven genetic origin), and acquired (dystonia due to a known specific cause).2 Numerous cases of focal or segmental isolated dystonia with onset in adulthood are idiopathic. Causes of inherited dystonia include autosomal dominant (DYT-THAP1, DYT-TAF1, DYT-ATP1A3, DYT-KMT2B, Huntington’s disease, neuroferritinopathy), autosomal recessive (pantothenate kinase-associated neurodegeneration, Wilson’s disease, chorea-acanthocytosis, Gaucher’s disease), X-linked (Lesch–Nyhan syndrome, McLeod syndrome), and mitochondrial (Leigh syndrome, deafness-dystonia syndrome).2, 4 Causes of acquired dystonia include perinatal brain injury (dystonic cerebral palsy, delayed-onset dystonia), infection (viral encephalitis, encephalitis lethargica, subacute sclerosing panencephalitis, human immunodeficiency virus (HIV) infection, tuberculosis, and syphilis), drugs (levodopa and dopamine agonists, neuroleptics, anticonvulsants, and calcium channel blockers), toxic (manganese, cobalt, carbon disulfide, cyanide, methanol, disulfiram, and 3-nitropropionic acid), vascular (ischemia, hemorrhage, arteriovenous malformation), neoplastic (brain tumor, paraneoplastic encephalitis), brain injury (head trauma, brain surgery, and electrical injury), and functional (psychogenic).2, 4
The pathophysiology of dystonia has not been fully elucidated. Dystonia was originally considered a basal ganglia disorder; however, it has been postulated to be a network disorder involving not only the basal ganglia but also the entire motor system, including the premotor and motor cortex, and the sensory system and thalamus. In addition, the cerebellum, which is directly connected to the basal ganglia, plays a significant role.45, 46 An autopsy study identified pathological markers in the striatum and cerebellum.47 The pathophysiology of OMD also remains unknown.48 Cortical negative shifts before voluntary movements, known as movement-related cortical potentials (MRCPs), reflect central motor control processes.48, 49, 50 Reduced amplitude of MRCPs has been reported in other types of dystonia such as cervical dystonia or writer’s cramp.48 A study comparing MRCPs between patients with OMD and normal participants found significantly reduced amplitudes of MRCPs over central and parietal areas for mouth opening and lateral movements in OMD patients, suggesting impaired cortical preparatory processes for mandibular movements.48 Further research with a larger sample is needed to elucidate the pathophysiology of OMD.
Injury to the peripheral nervous system has been associated with various movement disorders, including dystonia, hemifacial spasm, tremors, myoclonus, tics, and parkinsonism.51, 52, 53, 54 Although no confirmatory test exists to determine whether a movement disorder is genuinely induced by peripheral injury,53 even minor alterations in normal anatomy or physiology following dental procedures may result in peripherally induced movement disorders in predisposed patients.54
Treatment
If neurological diseases such as Parkinson’s disease have already been diagnosed and treated in a patient with OMD, OMD should be treated simultaneously by attending physicians.1, 8 However, if a neurological disease is suspected but not yet diagnosed, the patient should be referred to specialists.1, 8 Similarly, collaboration with a psychiatrist is necessary for the treatment of tardive dystonia.
The treatment of OMD must be multimodal and individualized, and current methods include pharmacological,4, 8, 38 botulinum toxin,1, 8, 32, 55, 56, 57 muscle afferent block,58, 59 occlusal splint (sensory trick splint),41 and surgical therapies (coronoidotomy).34, 35, 36 Deep brain stimulation has been increasingly applied in patients with other types of dystonia. However, patients with OMD have responded unsatisfactorily.60 Chemodenervation with botulinum toxin, that is, botulinum toxin therapy, is considered the first-line treatment for OMD. Botulinum toxin therapy methods for OMD or other movement disorders or conditions have already been reported in detail.1, 33, 55, 56, 57 Due to the number of muscle spindles, muscle afferent block therapy is more effective for jaw closing dystonia than for jaw opening dystonia.58, 59 A sensory trick splint is especially successful in patients with hyperactivity of the jaw closing muscles. In one study, 83.7% of the responders with splints presented with jaw closing dystonia.41 Coronoidotomy is only indicated for the most severe type of jaw closing dystonia associated with extremely limited mouth opening.35, 36 However, ⅓ of patients who underwent operation required additional botulinum toxin injections into the masseter and/or medial pterygoid muscles.36
Functional stomatognathic movement disorders (FSMDs)
Functional stomatognathic movement disorders are part of a spectrum of functional neurological disorders and are among the most common causes of neurological disability.61 The term “functional” is more commonly used than “psychogenic”.62 These disorders are considered to be caused by a complex interplay of biopsychosocial vulnerabilities triggered by psychosocial and/or physical factors.63 Functional movement disorders often have characteristic clinical features, particularly in the orofacial region.64 Therefore, a diagnosis should rely not on the exclusion of organic diseases or the presence of psychological symptoms but on the observation of characteristic clinical features.63, 65 Misdiagnosis of FSMDs as awake bruxism or a psychogenic disease is frequent.66
Presentation
In a previous study,66 a 10-item set of inclusion criteria for FSMDs was formulated based on previously reported criteria for functional movement disorders63, 65 or clinical features in facial functional dystonia,64, 66 aimed at comprehensively assessing 58 patients (42 women, 16 men; mean age: 46.2 years) with FSMDs. The criteria comprised 10 symptoms, with the prevalence of each as follows: rapid onset (74.1%); static course (60.3%); paroxysmal symptoms (86.2%); spreading to multiple sites (89.7%); spontaneous remission (58.6%); inconsistent symptoms (93.1%); distractibility (67.2%); incongruous symptoms (91.4%); the lack of sensory tricks (81.0%); and suggestibility (63.8%).65 The characteristic and distinguishable features of FSMDs included rapidly repeating lateral or tapping jaw and tongue movements (27.6%), which fluctuated considerably in speed and direction.66
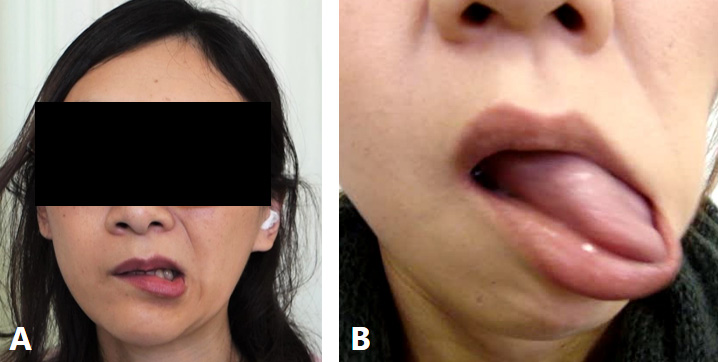
The most prevalent complaint of FSMDs was muscle pain (50%).66 Depression (38%), dysarthria (27.6%), and masticatory disturbances (15.5%) were also observed.66 Although specific electrophysiological tests or a gold standard for diagnosis are absent, functional movement disorders should be diagnosed with clinical certainty based on the available criteria.65 Functional movement disorders often exhibit distinctive clinical features in the orofacial region, such as tonic spasms involving the lip, eyelids, perinasal region, and forehead.64, 66 The most common phenotype is tonic jaw deviation involving ipsilateral downward and lateral lip pulling, observed in 84.3% of patients with facial functional movement disorders.64 Uni- or bilateral orbicularis oculi and platysma contraction are also frequently associated.63 Common patterns of FSMDs included jaw deviation (74.1%), jaw closing (50%), lip pulling (34.5%), and tongue movement (31%).65 The classic phenotype (unilateral lip pulling and jaw deviation) was observed in 44.8% of patients (Figure 2A).66 Characteristic features of FSMDs, such as repeated rapid jaw (lateral or tapping) and/or tongue movements, were observed in 22.4% of patients (Figure 2B).67
Epidemiology
The most common presentations of functional movement disorders are tremors, dystonia, myoclonus, and gait disturbance.63, 65 These symptoms were observed in 5–20% of patients in a movement disorder clinic, with functional dystonia being particularly prevalent.68 Functional neurological disorders have an estimated prevalence of 50 per 100,000 population based on a community registry.61 Functional movement disorders affecting the orofacial region are more prevalent in women (91.8% in one study64 and 72.4% in another66). The mean age at onset across these two studies was 37 years64 and 46.2 years,66 respectively. In one study, out of 1,720 patients with complaints of involuntary movements or muscle contractures in the masticatory, lingual, and/or lower facial muscles, 58 were diagnosed with FSMDs.66 Therefore, the prevalence of this condition may be relatively low.
Etiology
The etiology of functional movement disorders is likely multifactorial. Emerging data suggest that regional blood flow and activation patterns on positron emission tomography and functional magnetic resonance imaging are impaired in patients with FSMDs.69 Neurobiological abnormalities include hypoactivation of the supplementary motor area and abnormal connectivity with areas involved in movement selection or inhibition.63
Frequent precipitating events in patients with FSMDs included dental treatment (44.8%) and physical trauma (12.1%).66 Injury to the oral region or changes in anatomy or physiology following trauma or dental procedures may lead to FSMDs. However, the underlying mechanisms remain unclear.
Treatment
The first step in treating functional movement disorders should be explaining the diagnosis and confirming patient understanding.63 Various kinds of treatment include antidepressants, psychological therapy, cognitive behavioral therapy, and transcranial magnetic stimulation.63, 65, 69 Depression, anxiety, and pain may be treated pharmacologically. Based on a recent survey of members of the International Parkinson’s and Movement Disorder Society, the most effective therapeutic options are avoiding iatrogenic harm and educating patients about their diagnoses.70
In a study of 58 patients with FSMDs, symptomatic therapies for presenting symptoms included medication, muscle afferent block therapy, botulinum toxin therapy, and occlusal splint use.66 When patients with FSMDs showed obvious muscle hyperactivity, botulinum toxin therapy successfully improved symptoms.66 For typical cases of jaw or tongue deviation, botulinum toxin should be injected into the lateral pterygoid33 or tongue muscles.57 Additional targeted muscles may include the platysma, orbicularis oris, risorius, mentalis, and depressor anguli oris muscles.33 Occlusal splints occasionally prove effective for patients experiencing a sensory trick in the oral cavity.41 When therapies resulted in ineffective responses, patients were referred to psychiatrists or movement disorder experts for potential cognitive behavior therapy71 or physiotherapy.72
Hemimasticatory spasm (HMS)
Hemimasticatory spasm is characterized by intermittent paroxysmal contractions of the unilateral jaw closing muscles, resulting in brief twitches and/or prolonged spasms.73, 74, 75, 76, 77
Presentation
Involuntary movements can cause masticatory muscle pain, tongue or cheek biting, and temporomandibular joint dislocation,73, 78, 79 leading to masticatory disturbances or dysarthria. Hemimasticatory spasm is occasionally accompanied by hemifacial atrophy or localized scleroderma.76, 77, 78, 80 Scleroderma is a chronic connective tissue disease that manifests with skin lesions. A recent comprehensive review of HMS patients reported involvement of the masseter (97.4%), temporalis (48.3%), medial pterygoid (6%), and tongue (1.7%) muscles.81 Common triggers precipitating spasms include chewing, talking, and laughing.81 Brief twitches and often painful spasms can last from a few seconds to minutes.
Epidemiology
However, the prevalence of HMS has not yet been reported. It is assumed that the majority of patients without significant hemifacial atrophy or scleroderma visited dentists or dental surgeons and were diagnosed with bruxism. Thus, the actual prevalence of HMS may be much higher than expected. A recent comprehensive review reported that the mean age at onset was 37.1 years. Hemimasticatory spasm occurred more frequently in women (61.5%) than in men (38.5%).81
Etiology
However, the underlying mechanisms of HMS remain unclear. Linear scleroderma can involve the lower face (Parry–Romberg syndrome) and the upper face (en coup de sabre), named for its resemblance to “a stroke from a sword”.82 Deep tissue changes resulting from linear scleroderma can cause localized injury to the motor fibers of the trigeminal nerve.76, 79 Parry–Romberg syndrome is a rare craniofacial disorder characterized by progressive hemiatrophy of the skin, subcutaneous tissue, fat, and, in severe cases, underlying muscle, cartilage, and bone.82 There is a reported close relationship between Parry–Romberg syndrome and linear scleroderma en coup de sabre.83 Parry–Romberg syndrome and scleroderma complicated 17.9% and 23.9% of patients with HMS, respectively.81 Deep tissue changes from Parry–Romberg syndrome or scleroderma may lead to compression and focal demyelination of the motor branch of the trigeminal nerve.74, 77 Several cases of HMS have been reported following severe dental inflammation.72 Recent reports indicate HMS onset after dental or oral surgical treatments.81 Some cases of HMS worsened during pregnancy but improved after childbirth.84, 85 Pregnancy-related hormonal changes may influence the mechanism underlying HMS.
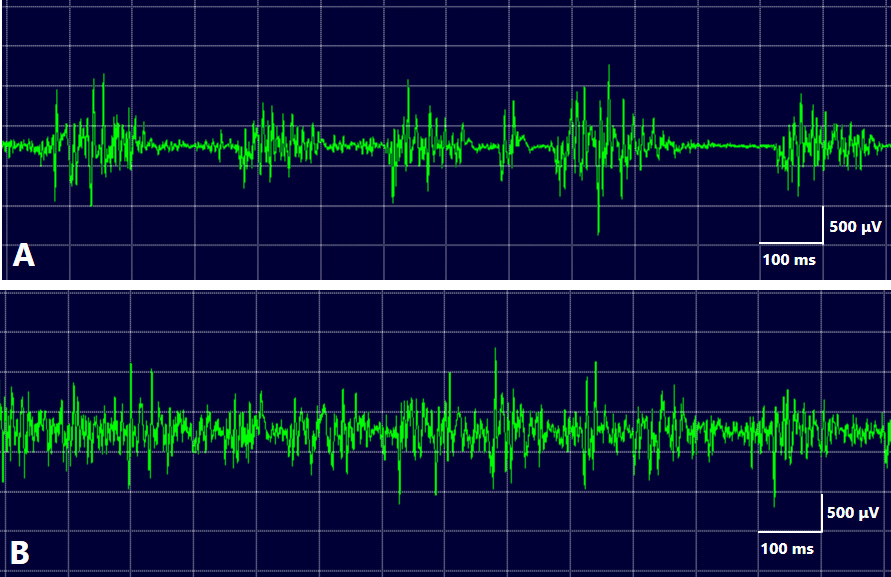
The EMG data of HMS showed irregular bursts of motor unit potentials correlated with twitches or spasms. Individual motor unit potentials showed high-frequency activity, up to 200 Hz, suggesting a peripheral origin of the abnormal activity.73 Unlike unilateral OMD, HMS does not exhibit a co-contraction or overflow phenomenon. Masseter reflexes were either absent or delayed in all examined patients. A distinctive electrophysiological finding of HMS was the absence of silent periods during spasms (Figure 3). Complete efferent block to the muscles is an exceptional and unique finding in HMS.76 Based on these electrophysiological findings, HMS is hypothesized to originate from either the motor root or the motor nucleus of the trigeminal nerve.74, 75, 76
Treatment
There are several treatment modalities available for patients with HMS. Surgical interventions, such as microvascular decompression, may be effective in definitively diagnosing patients with vascular compression.86, 87 Botulinum toxin therapy for jaw closing muscles is highly effective in improving spasms in patients with HMS. However, repeated injections of botulinum toxin may cause facial hemiatrophy due to masseter muscle atrophy and masticatory disturbance from reduced bite force.1, 34 As an alternative, muscle afferent block therapy blocks muscle afferents for the treatment of focal dystonia,88 and intramuscular injection of lidocaine reduces the efficacy of muscle spindle afferents without causing unwanted weakness.58, 59 Involuntary HMS contractions persist during sleep, causing some patients to stay awake due to contractions and related pain. Therefore, inserting an occlusal splint during sleep can be beneficial for patients with HMS. Opening the mouth stretches jaw closing muscles, thereby affecting signals from the muscle spindles.81 Increasing the occlusal vertical dimension with a splint may slightly stretch jaw closing muscles, making spasms less likely to occur.81 When a clear cause has been identified, such as vascular compression of trigeminal nerve motor roots, surgical procedures such as microvascular decompression can be beneficial for patients.86, 87
Differential diagnosis of involuntary movements
Characteristic clinical findings for the differential diagnosis of movement disorders of the stomatognathic system are summarized in Table 1.
Oromandibular dystonia (OMD)
Among the 6 subtypes of OMD (Figure 1), tongue dystonia, jaw opening dystonia, and lip dystonia are rarely diagnosed as bruxism. These patients are often suspected to have psychiatric disorders and are referred to psychiatrists. Jaw closing, deviation, and protrusion dystonia are often diagnosed as awake bruxism if associated with jaw closure, clenching, and grinding. Jaw closing dystonia accounts for more than 60% of OMD cases and is the most frequent subtype.1, 6, 7, 8 According to the current definition of bruxism,9 approx. 70% of OMD patients may be diagnosed with bruxism. Among the aforementioned characteristic clinical features of OMD, task specificity and sensory tricks are less likely to occur over long periods after OMD onset. In other words, symptoms may occur only during conversations in the early stages of OMD onset, but abnormal muscle contractions gradually persist during eating and waking hours.1, 7 Stereotypy and morning benefits are often relatively preserved. Although OMD symptoms subside during sleep, sleep bruxism can coexist with other movement disorders in some patients. Careful differential diagnosis between OMD and awake bruxism is essential for proper treatment.
Functional stomatognathic movement disorders (FSMDs)
Most patients with FSMDs are suspected to have psychiatric disorders and are often referred to psychiatrists. Common patterns observed in patients with FSMDs include jaw deviation, jaw closing, lip pulling, and tongue movement.66 When a patient exhibits jaw closing, they may be diagnosed with awake bruxism. According to the latest definition, approx. half of FSMD patients may be diagnosed with awake bruxism.11 Functional movement disorder presents with various clinical features, including inconsistent or incongruous symptoms, spreading to multiple sites, and paroxysmal symptoms.66 The FSMD patients should be carefully examined and, if suspected, referred to a psychiatrist or a specialist in involuntary movements.
Hemimasticatory spasm (HMS)
The differential diagnosis of HMS from bruxism is problematic. All patients diagnosed with HMS by the author had previously received a bruxism diagnosis from dentists or oral surgeons.81 Involuntary movements mostly disappear during sleep; however, HMS symptoms persist, and associated pain can occasionally awaken patients. Patients are often suspected of having bruxism and seek dental care. Despite being considered a rare disorder, nearly all patients with HMS are initially diagnosed and treated for bruxism by dental professionals.81 The distinguishing features of HMS include unilateral symptoms (98.3%), morphea or scleroderma, and unilateral facial atrophy (Table 1). A notable finding in HMS is the absence of a silent period on EMG (Figure 3B). When HMS is suspected, EMG should be performed for differential diagnosis, and referral to a neurosurgeon is recommended for a comprehensive evaluation to identify potential vascular compression of the trigeminal nerve motor root. Vascular decompression surgery can provide complete relief from symptoms.86, 87
Proposals for future studies
Numerous dental clinicians and researchers have been interested in bruxism for more than half a century, and many related studies have been published. A PubMed search revealed nearly 5,000 articles on bruxism and approx. 800 review articles. Additionally, the number of published papers has increased rapidly in recent years. Many excellent papers have been published that took a multifaceted approach and studied bruxism in terms of arterial hypertension,89 genetic basis,90 metabolic and hormonal disturbances,91 sleep structure,92 serotonin pathway,93 the relationship between temporomandibular disorders,94 and rhythmic masticatory muscle activity clusters,95 but many focusing on sleep bruxism. Studies on awake bruxism, although increasing in number, are lacking. Despite researchers working on bruxism for a long time, there have been no significant developments in treatment methods, and various clinical issues remain inconclusive. One reason for this may be that the definition or diagnostic criteria for bruxism are unclear, and other involuntary movements may be diagnosed as bruxism. According to the current definition, all cases of jaw closing dystonia and HMS, as well as some cases of FSMDs or oral dyskinesia, can be diagnosed as bruxism.11 Furthermore, because bruxism may be diagnosed if there is bracing or thrusting, even without tooth contact,11 several other movement disorders can also be classified as bruxism. Involuntary movements other than HMS and palatal tremors disappear during sleep. Therefore, involuntary movements are less frequently diagnosed as sleep bruxism and more frequently as awake bruxism. In the current definition, various jaw muscle activities without tooth contact are included under the umbrella term “bruxism”.20 With this definition, because there is no cut-off point, muscle activity after the end of sleep apnea96, 97 is also considered a potential protective factor, and masticatory muscle activities are considered not only as disorders but also as behaviors.11 Several healthy individuals without clinical problems can be diagnosed with bruxism. The author believes that the diagnostic criteria need to be stricter, and exclusion criteria should be added to rule out other involuntary movements. Otherwise, it may be necessary to distinguish between primary and secondary bruxism and redefine both terms. Conti et al. concludes that the absence of an adequate definition of bruxism, the non-distinction between circadian manifestations, and the use of various measurement techniques found in several studies preclude any solid and convincing conclusions on the existence of “secondary” bruxism.98 If the experimental group in a study included patients with bruxism with other involuntary movements or healthy individuals whose etiology was completely different from that of primary bruxism, the probability of not obtaining statistically significant results would increase. It has been postulated that a stricter definition of bruxism would provide more reliable results.
The Standardized Tool for the Assessment of Bruxism (STAB) was developed to provide a multidimensional evaluation of bruxism status, comorbid conditions, etiology, and consequences.99, 100 The STAB contains only one questionnaire on orofacial motor disorders in Axis B with the following question: “Have you been diagnosed with or do you suffer from possible signs of one of the following conditions?” Examples of orofacial motor disorders include orofacial dyskinesia, OMD, Parkinson’s disease, Tourette syndrome, hemifacial spasms, and tardive dyskinesia. Patients with Rett syndrome, FSMDs, or HMS were excluded. Hemifacial spasms are often caused by compression of the facial nerve by blood vessels, leading to involuntary contraction of the orbicularis oculi and/or facial muscles. Hemifacial spasms are rarely associated with bruxism. As mentioned above, movement disorders of the stomatognathic system are unlikely to be correctly diagnosed, and an examiner must diagnose orofacial motor disorders that the questionnaire may miss. Experts in bruxism may make such a diagnosis; however, this is difficult for most dental clinicians. In addition, the bruxism screener (BruxScreen), developed for large-scale epidemiological research projects and general dental practice, does not include questions regarding movement disorders.101 Therefore, other involuntary movements that cause mouth closure, clenching, and grinding may also be classified as bruxism. Furthermore, a common rating scale is required to objectively evaluate bruxism symptoms and changes after treatment. The latest definition of bruxism includes no cut-off point, and masticatory muscle activities are considered not only as disorders but also as behaviors; therefore, a rating scale may be considered unnecessary. Rating scales specific to each type of dystonia, such as cervical dystonia and blepharospasm, have been developed. A rating scale for OMD has also been developed, and its reliability and validity have been verified and applied clinically.6, 55
Involuntary movements of the stomatognathic system are considered a blind spot in medicine and dentistry. Several patients with these conditions spend years transferring between medical and dental departments. Most dental professionals focus only on bruxism and have very little knowledge of, and may be indifferent to, the other involuntary movements discussed in this review. Conversely, many medical professionals, particularly neurologists specializing in involuntary movements, are not interested in the involuntary movements of the oral cavity. Problems arise when medical professionals administer botulinum toxin to the masticatory muscles, especially the lateral pterygoid muscle.1 Several patients with involuntary movement from the United States and Europe visit our outpatient clinic for treatment. Although some of the world’s most famous experts on involuntary movements can diagnose movement disorders in the oral region, they often cannot identify which masticatory muscles are involved or administer botulinum toxin injections. Therefore, several patients visit our department at their own expense for airfare, accommodation, and treatment. The diagnosis and treatment of involuntary movements in the stomatognathic system require cooperation between medical professionals (neurologists, neurosurgeons, and otorhinolaryngologists) and dental professionals (dentists, oral surgeons, temporomandibular joint specialists, and prosthodontists). Dental and medical professionals should take an interest in movement disorders of the stomatognathic system. In the 1990s, Lavigne et al. applied polysomnography, a sleep medicine method, to sleep bruxism studies, resulting in dramatic advances in the study of sleep bruxism.29, 30 Although research and clinical management of awake bruxism seem to require knowledge and experience regarding movement disorders, the international expert panel currently working mainly on the definition of bruxism does not seem to include movement disorder experts. Clinicians are often unaware that they are diagnosing and treating patients with a variety of involuntary movements as bruxism. When a patient ceases treatment with a clinician, the clinician may assume that the symptoms have subsided. However, in several cases, they often seek care and visit a neurologist or neurosurgeon because treatments for bruxism often prove ineffective. Nevertheless, even neurologists and neurosurgeons, unless they specialize in involuntary movements, may encounter challenges in accurately diagnosing and effectively treating these patients with involuntary movements in the oral region. The author hopes that a multidisciplinary team approach will be possible in many hospitals in various countries, where medical and dental professionals are interested in involuntary movements in the oral region and can collaborate to diagnose and treat them.102, 103 The author believes that this would benefit wandering patients with movement disorders in the stomatognathic system and patients who are not properly diagnosed and treated. It could also lead to rapid advancements in clinical practice and research on involuntary movements in the oral region.
Conclusions
Each movement disorder has its characteristic clinical features: OMD – task specificity, sensory tricks and the morning benefit; FSMDs – inconsistent and incongruous symptoms, spreading to multiple sites and the lack of sensory tricks; and HMS –the paroxysmal contraction of unilateral jaw closing muscles, the persistence of symptoms during sleep and the loss of a silent period. A careful differential diagnosis is essential for adequate and effective treatment of involuntary movements. Refining the definition of bruxism may be necessary to prevent involuntary movements from being diagnosed as bruxism. The movement disorders of the stomatognathic system should be diagnosed and treated using a multidisciplinary approach.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.