Abstract
Background. The probability of a positive outcome of root canal therapy is substantially higher if the infection is eradicated successfully before the obturation of the root canal system. Irrigation is an essential aspect of root canal debridement, as it enables more thorough cleaning than is possible with root canal instrumentation alone. To overcome the side effects of chemical irrigants, there has been a search for herbal medicines as substitutes.
Objectives. The aim of the present study was to explore the antimicrobial efficacy of white tea-mediated silver nanoparticles (AgNPs) formulated as an intracanal irrigant against Enterococcus faecalis, and to compare it with the efficacy of chlorhexidine and sodium hypochlorite irrigants.
Material and methods. The experimental groups were as follows: group I – white tea-mediated AgNPs; group II – 2% chlorhexidine; and group III – 2.5% sodium hypochlorite. The characterization of AgNPs was performed using ultraviolet-visible (UV-Vis) spectroscopy and transmission electron microscopy (TEM) analysis. Enterococcus faecalis was inoculated onto Mueller–Hinton agar plates. The disks impregnated with irrigants were placed on the inoculated plates and incubated aerobically at 37°C for 24 h. Then, the growth inhibition zones were measured. Statistical analysis was performed using the one-way analysis of variance (ANOVA) and the post hoc tests.
Results. A concentration of 50 µL of white tea-mediated AgNPs exhibited the greatest zone of inhibition (32 ±2 mm), followed by 2% chlorhexidine (25 ±1 mm) and 2.5% sodium hypochlorite (23 ±3 mm).
Conclusions. White tea-mediated AgNPs showed promising results in the elimination of E. faecalis, being superior to chlorhexidine and sodium hypochlorite irrigants.
Keywords: herbal, silver nanoparticles, root canal therapy, white tea, irrigant
Introduction
The success of endodontic treatment relies on the thorough disinfection of the root canal system. The role of microorganisms in the pathogenesis of pulp-periapical lesions is well-established in the literature.1 Enterococcus faecalis, in particular, has gained special attention, as it is one of the dominant microbes isolated from failed root canals. It is more resistant to endodontic therapy due to its ability to penetrate dentinal tubules and its resistance to intracanal medicaments.2, 3
Studies have shown that necrotic tissues, the microbial by-products remaining within dentinal tubules, canal ramifications, and resorption pits that are resistant to mechanical instruments result in persistent periradicular inflammation.4 Therefore, chemo-mechanical preparation, which involves various instrumentation techniques, irrigation protocols and intracanal medicaments, is proposed to prevent the further entry of microorganisms from an infected root canal and to create conditions conducive to tissue healing.4, 5
Intracanal irrigation complements the instrumentation technique by expediting the removal of necrotic tissues and microbes from the root canal. Various studies have reported that portions of root canal walls remain undebrided during mechanical instrumentation.6, 7, 8 Hence, irrigating agents with strong antimicrobial activity are essential adjuncts to instrument preparation, thereby aiding in the removal of pulp remnants and residual microorganisms from intricate root canals.5 Chlorhexidine and sodium hypochlorite have been utilized globally for irrigating both permanent and primary root canals. Recently, there has been increased interest in herbal agents to replace chemical irrigants due to their limitations, such as tissue toxicity, allergic reactions, the discoloration of clothes, and unpleasant odor and taste. Several herbal irrigants with antimicrobial and therapeutic effects have been suggested for use as endodontic irrigants.9, 10, 11
White tea, which is extracted in an unfermented form from the leaves of the tea plant Camellia sinensis, possesses strong antioxidant properties in addition to its medicinal properties due to high levels of polyphenols.12 Nanoparticles (NPs) have also been studied in the field of endodontics to reduce E. faecalis adherence to dentine and to reinforce root canal disinfection.13
The purpose of the current study was to explore the antimicrobial efficacy of white tea-mediated silver nanoparticles (AgNPs) formulated as an intracanal irrigant against E. faecalis, and to compare it with the efficacy of chlorhexidine and sodium hypochlorite irrigants.
Material and methods
The approval for the current in vitro study was obtained from the Ethics Committee at the Saveetha Institute of Medical and Technical Sciences (SIMATS), Chennai, India (SRB/SDC/PEDO-1803/20/05). The experimental groups were as follows: group I – white tea-mediated AgNPs; group II – 2% chlorhexidine; and group III – 2.5% sodium hypochlorite.
Preparation of the white tea extract and the incorporation of AgNPs
Fresh white tea leaves (Ketley Gold Marketing, Assam, India) in the amount of 10 g were boiled for 15 min in 50 mL of distilled water. The obtained plant extract was filtered through sterile filter paper. In another container, 0.0169 g of silver nitrate (AgNO3) powder was added to 50 mL of distilled water and stirred continuously to obtain a AgNO3 solution. The plant extract and the AgNO3 solution were thoroughly mixed, using an electronic magnetic stirrer for 8 h. The mixture was then placed in test tubes and centrifuged for 15 min at 8,000 rpm.
UV-Vis spectroscopy and TEM analysis
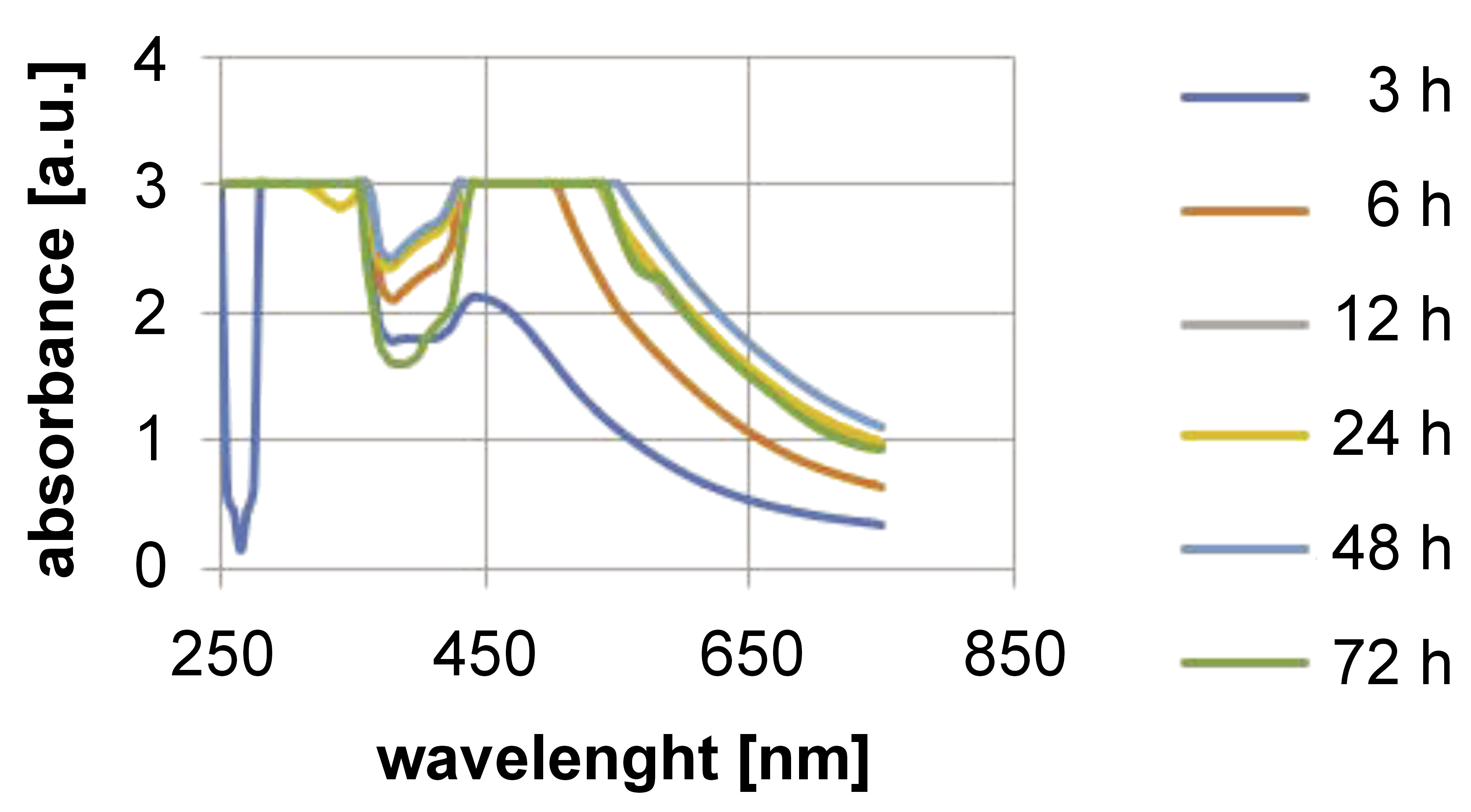
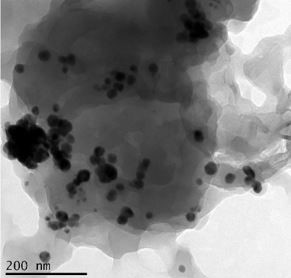
The formation of AgNPs through the reduction of AgNO3 was monitored at different time intervals up to 72 h, between 250 and 700 nm. A transmission electron microscope (TEM) was used to examine the surface morphology.
Isolation of microorganisms
A total of 100 µL of E. faecalis suspension taken from the prepared cultures was inoculated onto culture plates, 130 mm in diameter, with the previously set layers of Mueller–Hinton agar.
Antimicrobial activity
Using a sterile cork borer (HiMedia Laboratories, Mumbai, India), 3 wells (approx. 0.5 mm in diameter) were cut into the Mueller–Hinton agar plates. Using a micropipette, approx. 25 µL, 50 µL and 100 µL of the white tea-mediated AgNP solution was transferred into the wells, and then impregnated in sterilized 3-millimeter blank disks. The disks were placed on the Mueller–Hinton agar plates with gentle pressure against the agar surface to ensure uniform contact. The plates were incubated at 37°C for 24 h, and the antimicrobial efficacy was then evaluated.
To compare antimicrobial activity among the groups included in the study, the concentration of white tea-mediated AgNPs which exhibited the greatest zone of inhibition (group I) was further compared with 2% chlorhexidine (Asep-RC; Anabond Stedman Pharma Research, Chennai, India) (group II) and 2.5% sodium hypochlorite (Prime Dental Products, Thane, India) (group III) in Petri dishes containing 3 wells of a 0.5-millimeter diameter, using the same method as described earlier.
For the antimicrobial activity assessment, the diameter of the inhibition zone around the disks was measured using a ruler to the nearest whole millimeter. To ensure reliability, all experiments were replicated 3 times, and the average of the 3 measurements was recorded as the final value.
Statistical analysis
The recorded data was analyzed using the IBM SPSS Statistics for Windows, v. 23.0 (IBM Corp., Armonk, USA). Statistical significance was determined with the one-way analysis of variance (ANOVA) and the post hoc tests, with a p-value set at 0.05.
Results
Incorporation of AgNPs into the white tea extract
The addition of the white tea extract to the AgNO3 solution initially resulted in a dark brown color, which then faded to lighter brown after continued magnetic stirring.
UV-Vis spectroscopy and TEM analysis
The UV-Vis spectroscopy results are shown in Figure 1. The formation of AgNPs was observed between 250 and 700 nm. White tea-mediated AgNPs exhibited a peak between 420 and 460 nm, confirming their synthesis. The TEM analysis revealed that AgNPs were uniformly distributed, spherical in shape, and had a particle size ranging from 30 nm to 35 nm (Figure 2).
Antimicrobial activity
The antimicrobial activity of white tea-mediated AgNPs at 3 different concentrations was assessed by measuring the zone of inhibition against E. faecalis in a disk diffusion test (Table 1). The 50 µL concentration of white tea-mediated AgNPs showed the largest zone of inhibition. Comparative tests with 2% chlorhexidine and 2.5% sodium hypochlorite indicated that white tea-mediated AgNPs exhibited a larger zone of inhibition than both chlorhexidine and sodium hypochlorite irrigants (Table 2).
Discussion
The main goal of any treatment is to provide the optimal benefit to the patient with minimal harm and discomfort. In the case of primary teeth, thorough mechanical preparation is not desirable due to the presence of thin dentinal walls. Consequently, root canal irrigants play a significant role in the debridement of the root canal system.4, 14 Sodium hypochlorite, chlorhexidine gluconate, ethylenediaminetetraacetic acid (EDTA), citric acid, hydrogen peroxide, and other root canal irrigating solutions have been suggested for use in clinical practice for primary teeth.15 Chemical irrigants, however, cannot be used too frequently in primary teeth due to their lower safety profile. To address the side effects of chemical irrigants, there has been a search for herbal alternatives. With the rise of antibiotic-resistant strains, it has become essential to explore herbal medicines with strong antimicrobial properties to improve the outcome of biomechanical procedures.16, 17
Enterococcus faecalis was chosen as the test organism, as this facultative Gram-positive bacterium is commonly associated with secondary infections, with a prevalence rate of 24–77%, and it rapidly colonizes dentinal tubules.2, 18
The agar diffusion test was used in this study, as it allows the direct comparison of test materials against microorganisms, indicating whether the material has the potential to kill bacteria in the local environment. It is also one of the most widely used methods for determining the antimicrobial activity of endodontic irrigants.19, 20
Sodium hypochlorite, with concentrations ranging from 0.5% to 6%, has been recommended for root canal irrigation. It is inexpensive, readily available, has a long shelf life, and excellent tissue-dissolving and antibacterial properties.21 The chlorine (Cl) in sodium hypochlorite exerts an antimicrobial effect by inhibiting bacterial enzymes, leading to the irreversible oxidation of crucial bacterial enzyme sulfhydryl groups. The strong pH of sodium hypochlorite contributes to its antimicrobial properties by disrupting cytoplasmic membrane integrity, altering cellular metabolism and causing the degradation of phospholipids.22 However, when sodium hypochlorite is accidentally injected past the root apex, it can cause tissue reactions, such as pain, swelling, necrosis, and hemorrhage.10
Chlorhexidine gluconate is a cationic bisbiguanide that acts by adsorbing onto the cell walls of microorganisms and inducing the leakage of intracellular components.23 Chlorhexidine has a bacteriostatic effect at low concentrations, allowing the leaching of small-molecular-weight compounds from microorganisms. At higher concentrations, chlorhexidine has bactericidal activity due to cytoplasmic precipitation and/or coagulation, likely mediated by protein cross-linking.24 Clinical trials have reported severe reactions, including postoperative pain, following the extrusion of the chlorhexidine irrigant into periapical tissues.25
A mild form of tea, white tea, is produced from the young leaves of the tea plant C. sinensis. It retains high catechin concentrations found in fresh tea leaves.12 White tea is processed the least, so it contains the most polyphenols, which are not oxidized or lost during processing. It has more potent antioxidants than green tea, and has been shown to be even more beneficial. The high content of epigallocatechin gallate is primarily responsible for its strong antioxidant properties.26, 27
Silver nanoparticles bind to and penetrate the cell walls of both Gram-positive and Gram-negative bacteria, disrupting cell function by releasing Ag ions. As a result, they are used for fighting drug-resistant microorganisms and inhibiting biofilm formation.13 In dental practice, AgNPs are incorporated into the bonding agents and restorative materials to prevent biofilm formation and reduce caries. Nanoparticles are used in endodontics to minimize E. faecalis adherence to dentine, remove biofilm and improve root canal disinfection.28, 29 This study aimed to evaluate the efficacy of white tea and AgNPs in combination against E. faecalis.
The activity of AgNPs in the prepared white tea solution was confirmed by visual observation, UV-Vis spectroscopy, and TEM analysis. Free electrons in metal NPs produce a surface plasmon resonance (SPR) absorption band due to the mutual vibration of electrons in resonance with light waves.28 Peaks in UV-Vis spectroscopy confirmed the SPR characteristics of AgNPs in the prepared white tea solution. The particle size was confirmed by TEM micrographs as 30–35 nm. According to Shrestha et al., NP characteristics, such as contact time, concentration, particle size, and surface charge, affect their antimicrobial activity.30 Afkhami et al. reported that irrigation with 100 ppm AgNPs had the same antimicrobial potency as 2.5% sodium hypochlorite irrigation.31
In the present study, the zones of inhibition of bacterial growth achieved with white tea-mediated AgNPs were greater than those obtained with other irrigants for E. faecalis. This indicates that this herbal irrigant has higher efficacy against E. faecalis as compared to sodium hypochlorite and chlorhexidine. This result contrasts with the findings of Jose et al.9 and Saxena et al.,10 who found that sodium hypochlorite was more effective against E. faecalis than the herbal irrigants tested. Sodium hypochlorite showed a mean inhibition zone of 23 mm for E. faecalis, which is consistent with the analysis by Jose et al.9 Other researchers have reported similar findings regarding the use of chlorhexidine as an endodontic irrigant.24, 25 It has been proven that 2% chlorhexidine effectively removes the smear layer in 15 s and enriches the dentinal surface with chlorhexidine digluconate.32 Baca et al. reported 100% biofilm inhibition when using 2% chlorhexidine against E. faecalis.33 According to Garcia et al., 0.12% chlorhexidine has a significant inhibitory effect on the activity of dentinal proteolytic enzymes.34 In terms of inhibition zone, 2% chlorhexidine was found to be comparable to sodium hypochlorite.
According to the findings of this study, white tea-mediated AgNPs had the greatest antibacterial potency and the optimal inhibition ability. Its biocompatible antioxidant nature renders it an effective endodontic irrigant.
A major limitation of this in vitro study is that the results cannot be fully transferred to clinical scenarios, since the efficacy of extracts may be affected by additional factors, such as staining, substantivity, as well as action against other caries-causing microorganisms, which were not evaluated.
Advanced methods, such as the ETEST®, which combines the disk diffusion and agar dilution methods, or bioautography techniques, could be used in future research to explore these natural extracts further in the search for new antimicrobial irrigants.
Most previous research has focused on evaluating herbal irrigants in vitro; thus, detailed in vivo research is needed to assess the long-term stability of AgNPs, the susceptibility and toxicity of such irrigants, as well as their capacity to act against various other microorganisms.
Conclusions
Within the limitations of the present study, white tea-mediated AgNPs showed promising results in the elimination of E. faecalis, demonstrating superior efficacy in comparison with chlorhexidine and sodium hypochlorite irrigants. Further in vitro and clinical studies are required to evaluate their efficacy, biocompatibility and safety before they can be conclusively recommended as a root canal irrigant.
Ethics approval and consent to participate
The approval for the current in vitro study was obtained from the Ethics Committee at the Saveetha Institute of Medical and Technical Sciences (SIMATS), Chennai, India (SRB/SDC/PEDO-1803/20/05).
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.