Abstract
Background. The clinical and radiographic efficacy of bone grafts and biomaterials, such as platelet-rich plasma and platelet-rich fibrin (PRF), for reconstructing lost periodontal structures has been well documented. However, there is limited data regarding the presence of demineralized freeze-dried bone allograft (DFDBA) in an environment with abundant growth factors provided by platelet concentrates.
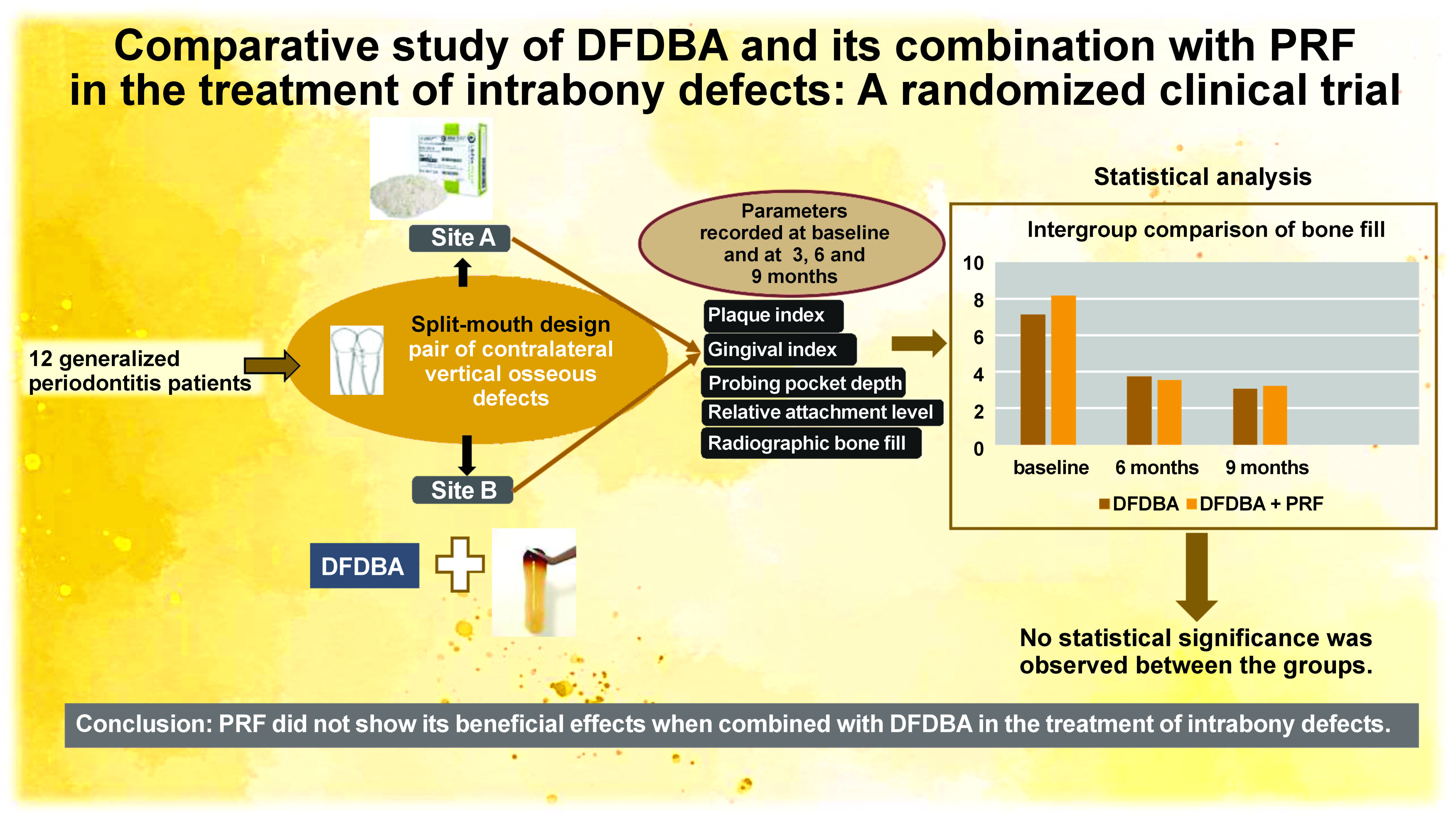
Objectives. The aim of the study was to compare the clinical and radiographic effectiveness of DFDBA with PRF versus DFDBA alone in the treatment of intrabony defects.
Material and methods. Twenty-four intrabony defects in contralateral sites were randomly assigned to either the DFDBA group or the DFDBA combined with PRF group. Clinical parameters, including the plaque index (PI), the gingival index (GI), probing pocket depth (PPD), relative attachment level (RAL), and radiographic bone fill (RBF), were measured at baseline, and at 6 and 9 months. Paired and unpaired t-tests were used for intra- and intergroup comparisons.
Results. Both the PI and the GI showed statistically significant improvements from baseline to 9 months. However, the intergroup comparisons did not reveal any significant differences (p < 0.05) between the groups with regard to clinical and radiographic measurements from baseline to 9 months.
Conclusions. Platelet-rich fibrin in combination with DFDBA did not show any additional benefit in terms of reconstructive output in the treatment of intrabony defects compared to the use of DFDBA alone.
Keywords: plaque index, gingival index, probing pocket depth, relative attachment level, radiographic bone fill
Introduction
Periodontal disease is inflammatory and polymicrobial in nature. It causes irreversible destruction of the tooth-supporting tissues.1 Conventional periodontal therapy aims to resolve inflammation and increase the clinical attachment level through repair.2 Over the past 5 decades, various modalities of treatment have been examined to increase the rate of reconstruction and meet the functional needs of the patient.3 Autogenous bone grafts have been considered the gold standard for the treatment of intrabony defects. However, these grafts require an additional surgical site and have limitations regarding the quantity of bone that can be harvested in multiple and deep osseous defects.4 The biologic principles that support the use of autologous and heterologous grafts include not only osteoconductivity and osteoinductivity but also their capacity for space provision and stabilization of blood clots. Demineralized freeze-dried bone allograft (DFDBA) has well-defined properties and has been demonstrated to induce the formation of new bone. The property of osteoinduction is provided by bone morphogenetic proteins (BMPs), which are exposed on the surface of the graft as a result of demineralization. The major BMPs involved in osteoinduction are 2, 4 and 7.5 Platelet concentrates are considered primers of the coagulation cascade and a bioactive molecular pool. Recent data suggests that the use of these materials as adjuncts has unique and augmented effects on the potential of various reconstructive protocols.6, 7 However, data pertaining to the combination of these materials is not established. Therefore, this study aimed to compare the effectiveness of DFDBA in combination with platelet-rich fibrin (PRF) versus DFDBA alone in the treatment of intrabony defects.
Material and methods
Sample size estimation
A sample size of 24 was obtained using G*Power software v. 3.1 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower). The study achieved 80% power with an effect size of 0.25 and an α value of 5%.
Study design
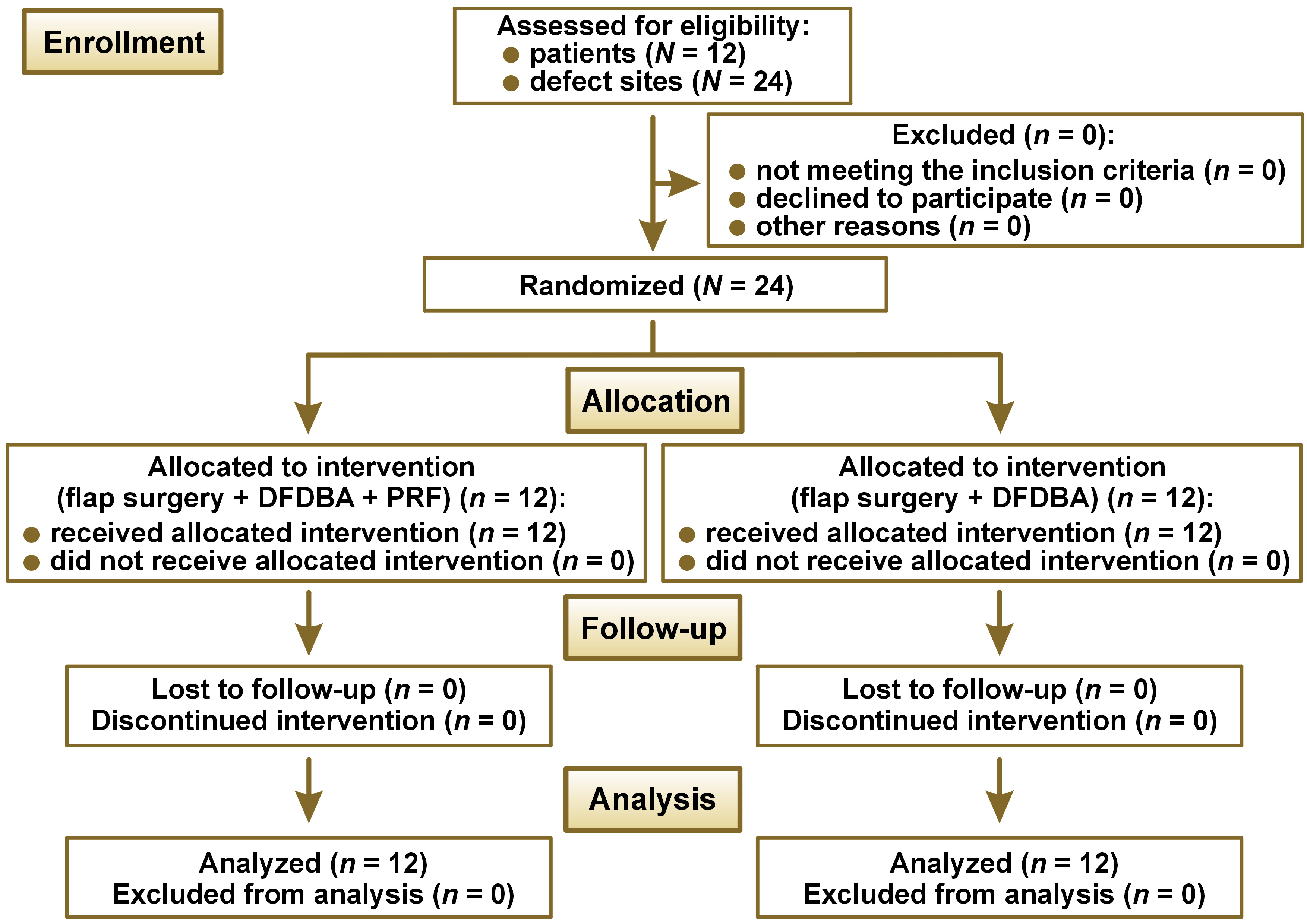
The study population consisted of subjects who had been referred to the Department of Periodontics (Sree Sai Dental College and Research Institute, Srikakulam, India) for the treatment of periodontitis. The patients were recruited from January 2021 to March 2022. This prospective, single-blind, split-mouth randomized clinical trial included 12 systemically healthy patients, each with at least a pair of contralateral vertical osseous defects (Figure 1). The defects were categorized as two-wall or three-wall defects, according to the classification by Goldman and Cohen.8 The diagnosis of these defects was based on transgingival probing, which was re-confirmed following surgical exposure. The study was approved by the Sree Sai Dental College & Research Institute Ethical Committee (approval No. SSDC&RI/IRB/IEC/2021-2021/409/8/1) and adhered to the Consolidated Standards of Reporting Trials (CONSORT) 2010 guidelines. The study was conducted in accordance with the Declaration of Helsinki, as modified in 2008, and is registered with the Clinical Trials Registry-India (reference No. REF/2021/11/049254). Each participant provided written informed consent after being informed of the benefits and risks associated with the surgical procedure.
Inclusion criteria
Patients aged between 25 and 55 years, diagnosed with stage III periodontitis (grade A) with a generalized extent and distribution, a minimum of 2 teeth with probing pocket depth (PPD) >6 mm, and angular defects on intraoral periapical radiographs were included in the study.
Exclusion criteria
Patients with systemic diseases who underwent active periodontal therapy or had a drug history within the previous 6 months, smokers, and pregnant or lactating women were excluded from the study.
Measurement of clinical parameters
The plaque index (PI) and the gingival index (GI) were measured according to Silness & Löe and Löe & Silness,9 respectively. Probing pocket depth and relative attachment level (RAL) were recorded at baseline, and at 3, 6 and 9 months postoperatively using a University of North Carolina (UNC)-15 probe (Hu-Friedy Manufacturing, Co., LLC, Chicago, USA). Customized acrylic stents were fabricated from alginate impressions for accurate measurements. Probing pocket depth with the stent was measured from the crest of the gingival margin to the base of the pocket, while RAL was measured from the point marked at the tip of the stent extending along the buccal tooth surface to the gingival margin. Calibration was conducted by a different examiner who was not involved in the surgical procedure. Before measuring the subjects involved in the study, 10 periodontitis patients were selected at random, and the equipment was calibrated twice with a 24-h interval between each calibration. Only values that were reproduced with consistent results within a margin of ±1 mm and at a confidence level of 90% were accepted.10
Measurement of radiographic parameters
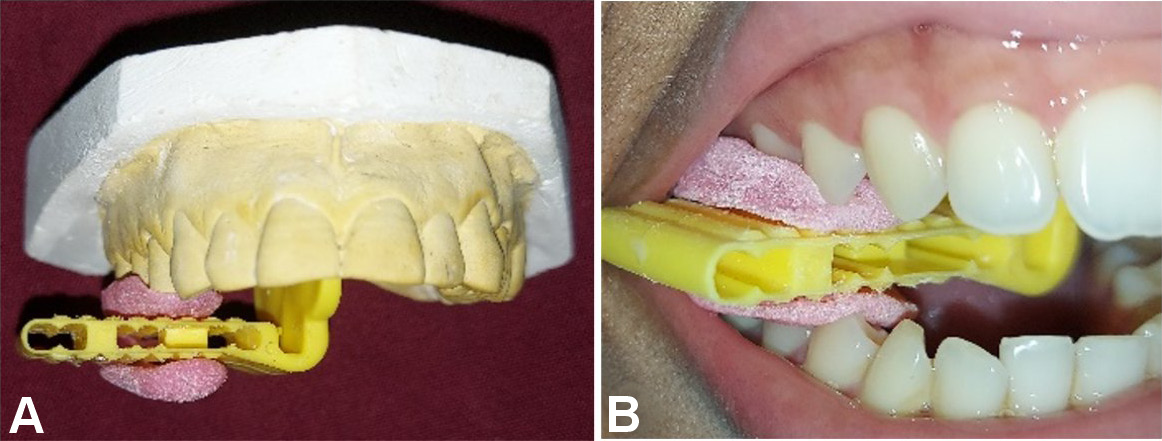
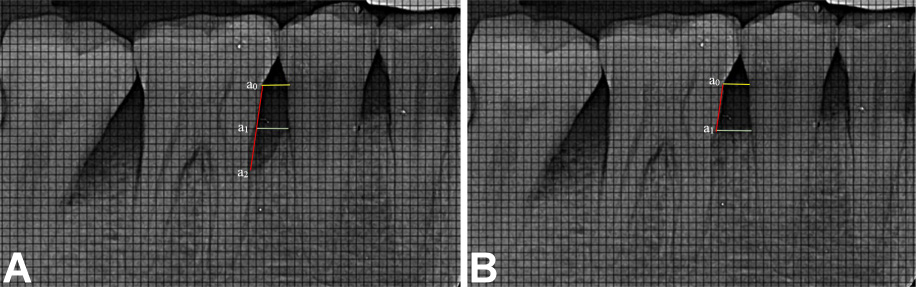
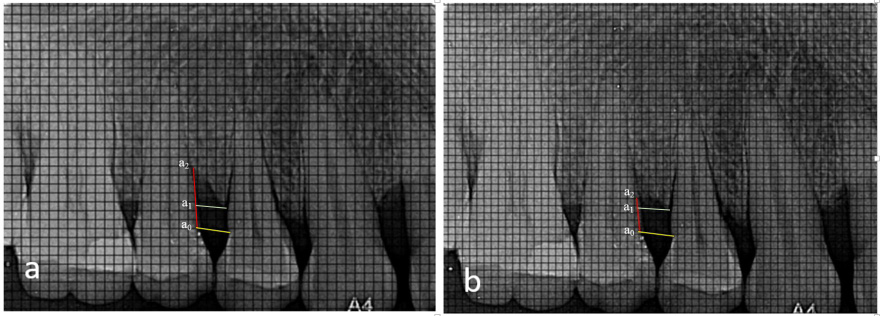
Radiographic bone fill (RBF) was recorded at baseline, and at 6 and 9 months. The models were fabricated from alginate impressions, on which customized X-ray positioning stents were prepared. L-shaped self-curing acrylic resin bite blocks were fabricated on the occlusal surface in the premolar–molar region to retain the plastic film holder using the long cone paralleling technique. The radiographic stent was then evaluated in the patient’s mouth for adequate retention and stability (Figure 2). Dentsply Rinn XCP-DS FIT (Dentsply Sirona, Charlotte, USA) with a posterior aiming ring and a radiographic grid of 1 mm × 1 mm was used for assessment. The exposure parameters were maintained at 75 kVp, 6 mA and 0.8 s. The same voltage was used for taking the radiographs, and the contrast of the radiograph was set to a similar numerical value in SOPRO Imaging software (http://www.soprotechnicalsupport.com) at baseline and follow-up. Geometric errors were reduced by stent fabrication and the use of the long cone extension technique for both baseline and follow-up radiographs. The amount of bone formed was assessed by comparing the baseline and 9-month radiographs and drawing 2 horizontal lines at the cementoenamel junction (CEJ) and at the alveolar crest, respectively, using the adjacent tooth as a reference (Figure 3, Figure 4). Radiographic bone fill was measured from the CEJ to the base of the defect using SOPRO Imaging software.
Pre-surgical preparation
The patients were provided with educational and motivational support. Full-mouth supra- and subgingival scaling and root planing procedures were performed under local anesthesia, and oral hygiene instructions were given. The full-mouth periodontal status was reassessed before surgery. Twenty-four intrabony defects were randomized into 2 groups by the clinician using a flip coin method. Site A (n = 12) consisted of open flap debridement + DFDBA, and Site B (n = 12) consisted of open flap debridement + PRF + DFDBA. Demineralized freeze-dried bone allograft with particle sizes ranging from 250 µm to 500 µm was obtained from the Tata Memorial Hospital Tissue Bank, Mumbai, India.
PRF preparation
Platelet-rich fibrin was prepared according to the protocol developed by Dohan et al.11 Immediately before the surgical procedure, 10 mL of blood was drawn from the subject’s antecubital vein. The blood sample was collected in sterile glass test tubes without any anticoagulant and immediately centrifuged in a standardized centrifugation machine (Medico Plus laboratory centrifuge; REMI ELEKTROTECHNIK LTD, Maharashtra, India) at approx. 400 g and 3,000 rpm for 10 min. The centrifuged blood mass formed a structured fibrin clot in the center of the tube, situated between the red blood cell layer at the bottom and the acellular plasma at the top. The fibrin clot (PRF) could be easily removed from the tube and shaped as desired, and was used immediately after collection.
Surgical procedure
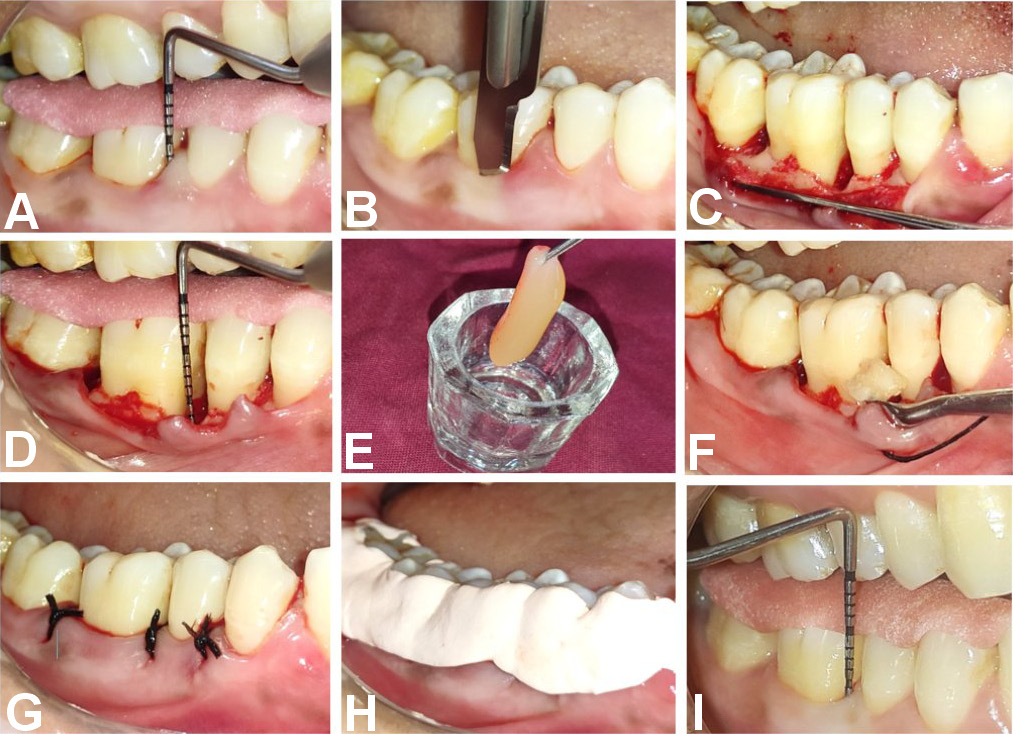
After administering local anesthesia buccally and lingually, sulcular and interdental incisions were made using a No. 15 B.P. blade, resulting in the reflection of a full-thickness mucoperiosteal flap. Meticulous debridement of the defect was performed using Gracey Curettes. Platelet-rich fibrin and DFDBA were placed in a sterile dappen dish. The combined treatments were placed and condensed at the defect in Site B, whereas the graft alone was placed in Site A, filling it to an appropriate level. The flaps were then sutured with simple interrupted silk sutures, resulting in primary tension-free wound closure. The surgical site was covered with COE-PAK™ periodontal dressing on the buccal and lingual aspects (Figure 5).
Post-operative care
Patients were instructed to avoid brushing around the surgical site for the first 3 weeks post-surgery. During this period, they were advised to rinse the mouth with a solution of 10 mL of 0.2% chlorhexidine twice daily. A combination of systemic antibiotics (amoxicillin 500 mg, 3 times a day) and analgesics (aceclofenac 100 mg + paracetamol 325 mg, 2 times a day) was prescribed for the 5-day post-surgical period. A soft diet was recommended for a period of 10 days. Patients were also instructed to use minimal pressure when brushing and to use a soft nylon bristle brush for the subsequent 2–3 weeks. Recall appointments were scheduled for pack and suture removal 7 days after surgery. Patients were instructed to report any postoperative discomfort.
Statistical analysis
In the present study, data was analyzed using the IBM SPSS Statistics for Windows software, v. 25.0 (IBM Corp., Armonk, USA). Descriptive statistics and paired t-tests were performed for intragroup comparisons, and independent t-tests were performed for intergroup comparisons at various time points. The statistician was blinded to the intervention, as both the test and control groups were masked.
Results
All patients attended the scheduled follow-up visits, and post-operative healing was satisfactory. No postoperative complications were reported. The previously described measurements were recorded accordingly.
Of the 12 patients, 8 (66.7%) were male and 4 (33.3%) were female (Table 1). The mean age of female patients was 42.25 ±8.23 years, while that of male patients was 38.75 ±5.57 years. The overall mean age of the participants was 39.91 ±6.88 years.
A statistically significant reduction in mean values was observed for both the PI and the GI from baseline to 9 months (p < 0.01), indicating good oral hygiene maintenance among the participants of the study (Table 2).
In intra- and intergroup comparisons, both groups showed a reduction in mean PPD from baseline to 9 months, but this did not reach statistical significance (p = 0.780).
At Sites A and B, there was a statistically significant reduction in mean RAL from baseline to 9 months (p < 0.01). However, in the intergroup comparison, no statistically significant differences were observed between the groups at different time points.
For RBF, both groups showed a significant reduction from baseline to 9 months (p = 0.002). However, there were no statistically significant differences between the groups (Table 3).
Discussion
In this study, DFDBA was used in the control group as it was already established as an effective regenerative material for osseous defects. However, it did not provide any benefit in ridge augmentation due to its rapid resorption rate compared to other grafts, such as FDBA and xenografts. Demineralized freeze-dried bone allograft is a graded material for periodontal regeneration, especially in the treatment of infrabony defects, because of its osteoinductivity.12, 13 This property is attributed to the presence of BMPs.14, 15, 16 The material has passed a series of compliance tests and had its antigenic property removed to ensure its safety for usage.
Platelet concentrates are considered accelerators of soft and hard tissue healing.17 The quality of the fibrin scaffold in PRF is influenced by a number of factors, including the speed and duration of centrifugation, temperature and blood hematocrit. In this study, PRF was prepared according to the protocol by Dohan et al.11 Although autografts are the gold standard for treating osseous defects, a study by Mathur et al. found that PRF had comparable efficacy in the treatment of intrabony defects when compared to autogenous bone in the context of three-wall defects.18 Platelet-rich fibrin demonstrated significantly better results when used alone or in combination with bone grafts. However, the extent of these benefits remained uncertain due to the considerable heterogeneity across the studies and the limited sample size, indicating a low degree of confidence and certainty in the treatment effects.19, 20, 21, 22
Our study assessed the combined efficacy of DFDBA and PRF compared to DFDBA alone. The study used a split-mouth design, which eliminates selection bias and reduces confounding factors, including environmental, local and systemic influences, as well as prognostic factors, thereby decreasing the heterogeneity.2 The choice between using a single graft or a combination of grafts and other regenerative materials mainly depends on the selected case and defect morphology. This study evaluated the efficacy of PRF in combination with DFDBA in the treatment of three-wall and two-wall defects, in comparison to DFDBA alone. However, only 2 cases involved two-wall defects, with the remaining cases classified as three-wall defects.
In the present study, plaque control measures were strictly followed, and the patients were highly motivated due to the digital education component. The oral hygiene status was evaluated at the recall visits, and supportive periodontal therapy was provided. Due to the patient’s meticulous oral hygiene regimen, there was a decrease in both the PI and GI scores. A reduction in the mean PPD values was observed in both groups at follow-up. These findings are consistent with those reported by Shah et al. and Chadwick et al.23, 24
Although there were statistically significant differences in RBF from baseline to 9 months in both the DFDBA and DFDBA + PRF groups, no significant differences were observed between the groups. These results are in contrast with studies by Bansal and Bharti and Agarwal et al.25, 26 The 2 main aims of reconstructing a defect are space provision and coinciding of the bone graft resorption time with that of new bone formation. The dimensional stability of PRF has a faster resorption rate and is compromised over time. The proportions of the graft and PRF cannot be controlled when placed in combination, and the graft resorption time does not coincide with PRF. Hence, this may explain the lack of added benefits.
A significant clinical attachment gain was observed in the test group, as evidenced by the active migration of the gingival margin in conjunction with the use of PRF. However, the intergroup comparison did not reach statistically significant results. These findings are in accordance with a study by Nitesh and Anushree.27 The success and size of attained PRF are contingent upon the timing of blood withdrawal and placement for centrifugation. The optimal timeframe for blood withdrawal is 15 s, with an interval of 60 to 90 s between the drawing of blood and centrifugation recommended to prevent microscopic structural changes.27
A limited number of clinical trials have utilized conebeam computed tomography (CBCT) for accurate measurement of intrabony defects. However, this study attempted to standardize the 2D images by fabricating a special template to increase the accuracy of linear value measurement. Surgical re-entry was not performed for histological evaluations due to ethical considerations. Moreover, the present study did not include a parameter for wound healing, which would have provided valuable insights.28
Within the limits of the present study, it can be stated that the use of PRF in conjunction with DFDBA has shown comparable efficacy to DFDBA alone in the treatment of periodontal intrabony defects. Further evaluation through histomorphometric or histologic analysis would have been beneficial for a more comprehensive understanding of the bone regeneration process. With regard to RBF, both treatments showed similar results despite the placement of the graft in a pool of growth factors. Further randomized controlled clinical trials with larger sample sizes and longer follow-up periods are necessary to confirm the beneficial role of PRF in combination with DFDBA in periodontal treatment modalities.
Conclusions
The present study demonstrated that PRF, when combined with DFDBA, had comparable efficacy to the control group. No additional benefit of PRF was observed. Further randomized clinical trials with larger sample sizes are required to demonstrate the economic benefits and regenerative outcomes of this approach.
Ethics approval and consent to participate
The study was approved by the Sree Sai Dental College & Research Institute Ethical Committee (approval No. SSDC&RI/IRB/IEC/2021-2021/409/8/1) and adhered to the Consolidated Standards of Reporting Trials (CONSORT) 2010 guidelines. Each participant provided written informed consent after being informed of the benefits and risks associated with the surgical procedure.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.