Abstract
Background. Early colonizers adhere to the dental surface and facilitate the initial adhesion of secondary colonizers to form oral biofilms, which may cause oral infections.
Objectives. This study aimed to determine the antimicrobial, anti-adhesion and antibiofilm potency of inverted amino acids on early colonizer streptococci and their mixed species.
Material and methods. The following test strains were used: Streptococcus gordonii (American Type Culture Collection (ATCC) 35105); Streptococcus mitis (ATCC 49456); Streptococcus oralis (ATCC 10557); Streptococcus salivarius (ATCC 7073); and Streptococcus sanguinis (ATCC BAA-1455). The concentration-dependent antimicrobial potency of d-alanine (d-ala), d-arginine (d-arg), d-leucine (d-leu), d-methionine (d-met), and d-tryptophan (d-try) was determined using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method with AlamarBlue modification. The adhesion of primary colonizers in the presence of 25-mM d-amino acids (dAAs) was assessed using the colony forming unit (CFU) assay. The CFU assay was conducted on 24-h flow cell bacterial biofilm models after exposure to 25-mM inverted dAAs.
Results. No minimum inhibitory concentration (MIC) point was detected at any concentration tested. The minimum bactericidal concentration (MBC) point was not observed. The adhesion of S. mitis, S. oralis and mixed species was reduced by all tested dAAs. No adverse effects were observed on S. gordonii with any of the tested dAAs. The biofilm biomass of test strains under flow conditions was significantly reduced after a 5-min exposure to all tested dAAs at 25-mM concentration.
Conclusions. D-amino acids did not inhibit bacterial growth and did not show bactericidal or bacteriostatic effects on test strains at any concentration tested (ranging from 6.25 mM to 100 mM). However, dAAs effectively inhibit the adhesion of early colonizers, thereby preventing the formation of oral biofilm.
Keywords: biomass, methionine, biofilms, anti-infective agents
Introduction
The human oral cavity, including mucosal and tooth surfaces, provides a natural habitat for various types of microorganisms. It represents one of the body surfaces with a huge population of diverse microorganisms.1 In the oral cavity, these microorganisms attach to the oral surfaces and form three-dimensional structures, biofilms. These are surface-attached microbial communities that are enclosed in a matrix of extracellular material derived from the cells themselves and from the environment.2 The attached surface could be either biotic or abiotic.3 Oral biofilm formation is a complex and dynamic process. First, pellicles are formed on oral surfaces by adsorbing salivary proteins and glycoproteins. The surface conditioning film is known as the acquired enamel pellicle. The major components of a pellicle are salivary glycoproteins, phosphoproteins, lipids, and other host molecules. Oral bacteria generally possess more than one type of adhesion molecule, including integrins, cadherins and selectins, on their surface. They can participate in multiple interactions with host surface molecules and similar receptors on other microorganisms. The dental biofilm pioneer species modify local ecological conditions and promote further colonization by other species that may lead to diseases, such as caries and periodontal diseases.1
In the first 6 h of formation, the dental biofilm microbiota is mainly composed of early colonizers, including Streptococcus spp. (Streptococcus mitis, Streptococcus oralis, Streptococcus salivarius, Streptococcus gordonii, and Streptococcus sanguinis).4, 5 These species possess adhesins and other bacterial surface ligands with affinity to host dental-pellicle molecules.1 Biofilms are significantly different compared to their planktonic counterparts.6, 7 The community lifestyle provides a multitude of benefits to the participating organisms.8 These include a broader habitat range for growth produced by the metabolism of early colonizers. Early colonizers alter the local environment, creating an optimal environment for the attachment and growth of late colonizers.9
The oral microbiota plays an important role in both oral and systemic health.10 It contributes to host health by maintaining homeostasis within the oral cavity, resisting pathogens and modulating the immune system.10, 11 Furthermore, oral microbiota thwarts disease progression by preventing the adherence of pathogens onto specific surfaces, degrading the pathogen’s virulence factors, and hindering the pathogen’s ability to multiply.11 When the sensitive ecosystem within the oral cavity gets out of balance, either by overload or a weak immune system, the oral microbiota shows pathogenic potential.10
Although early colonizers are not identified as pathogens, they are the early contributors to the formation of pathogenic polymicrobial oral biofilms. Therefore, the most effective strategy of oral biofilm control is to prevent early colonization. This study is aimed at identifying an effective treatment option against early colonizers.
Biofilms exhibit greater resistance to antimicrobials compared to suspension-grown planktonic cells.12 Different types of strategies are used in the control and treatment of oral biofilm infections. Conventional methods of biofilm removal include mechanical removal and the use of antibacterial mouthwashes and dentifrices.13
Inverted amino acids have recently been found to disrupt biofilms by inducing the self-dispersal of cellular components and the matrix of the biofilm.14 The current study investigates the impact of 5 d-amino acids (dAAs), namely d-alanine (d-ala), d-arginine (d-arg), d-leucine (d-leu), d-methionine (d-met), and d-tryptophan (d-try) on the planktonic bacterial growth, initial adhesion, biofilm formation, and biofilm dispersion of 5 early oral colonizers (S. mitis, S. oralis, S. salivarius, S. gordonii, and S. sanguinis) and their mixed species. There is a paucity of data on the efficacy of dAAs on biofilms formed by early colonizers.
Material and methods
D-amino acids
D-alanine, d-arg, d-leu, d-met, and d-try were purchased from Sigma-Aldrich (St. Louis, USA) (purity >95%). All amino acids were prepared in 100-mM working solutions by dissolving the relative weight of dAAs in distilled water or liquid culture medium, followed by filter sterilization. All dAA solutions were freshly prepared.
Bacterial cultures and media
Stock cultures of test strains, S. gordonii (American Type Culture Collection (ATCC) 35105), S. mitis (ATCC 49456), S. oralis (ATCC 10557), S. sanguinis (ATCC BAA-1455), and S. salivarius (ATCC 7073), were obtained from the Area of Microbiology and Immunology at the Piracicaba Dental School of State University of Campinas in Brazil. The cultures were stored in a skim milk solution (Sigma-Aldrich) at −20°C. The stock cultures were thawed and subcultured on Mitis Salivarius Agar (MSA; BD Difco™, Franklin Lakes, USA), and incubated at 37°C with 5–10% CO2 for 24 h to obtain microbial colonies for the next steps. A few colonies of the above cultures were suspended and incubated for 24 h in sterile Brain-Heart Infusion (BHI) broth (Oxoid, Lenexa, USA), supplemented with 2% sucrose, to prepare a standard cell suspension, which was measured for absorbance at 550 nm (approx. 1×105 CFU/mL).
Effect of dAAs on planktonic bacterial growth
The effect of dAAs and their concentration on planktonic bacterial cells was determined using the Clinical and Laboratory Standards Institute (CLSI) M7-A10 broth microdilution method with AlamarBlue modification.15 The procedure involved preparing a two-fold dilution of 100-mM working solution of dAAs (6.0–100.0 mM) in sterile BHI broth, with 100 µL/well added to 96-well sterile flat-bottom microtiter plates. Each well was inoculated with 100 µL of standard cell suspension (final Candidal cell concentration: approx. 5×104 CFU/mL) and incubated at 37°C and 5–10% CO2 for 24 h.
After incubation, 10 µL of content from each well was inoculated on BHI agar plates and incubated at 37°C for 24 h to determine the minimum bactericidal concentration (MBC) of dAAs. Subsequently, 25 µL of 0.02% AlamarBlue solution was added to each well, and the plates were incubated for additional 2 h. The plates were observed visually for color changes to determine the minimum inhibitory concentration (MIC).
Effect of dAAs on bacterial adhesion under static conditions
Standard cell suspensions of test strains and their mixture were prepared in sterile, 2% sucrose-supplemented BHI broth with added 25-mM dAAs (d-ala, d-arg, d-leu, d-met, and d-try), followed by filter sterilization of the medium. Sterile polystyrene slips (diameter: 10 mm) were placed in the bottom of the 12-well cell culture cluster. The prepared cell suspension was inoculated into the 12-well cell culture cluster triplications, which were then incubated aerobically at 37°C for 6 h. After the incubation, the polystyrene slips were carefully washed with sterile distilled water to remove non-adherent cells and transferred to centrifuge tubes containing 5 mL of sterile normal saline (NS). The centrifuge tubes were then vortexed to detach adherent cells. A total of 100 µL of this inoculum was transferred to another Eppendorf tube containing 900 µL of sterile NS. Using this suspension, 10-fold dilutions (up to 10−8) were prepared. Twenty-five microliters of the prepared dilutions were inoculated onto BHI agar plates in triplicate. The plates were then incubated at 37°C in a CO2 incubator for 24 h, after which the number of colonies was counted.16, 17
Effect of dAAs on preformed biofilms
The flow cells were assembled and sterilized using the previously described method with a few modifications.18 The flow cells were inoculated with standard cell suspensions of test organisms and their mixture separately in the absence of 2% sucrose-supplemented sterile BHI medium. The cells were allowed to adhere to the coverslip for 6 h. After 6 h, the flow was started (0.6 rpm) and continued for another 24 h at 37°C. Then, the produced biofilms were treated with 25-mM dAA solutions for 5 min, with the flow turned off. After the treatment, the biofilms containing flow cells were washed with a flow of sterile NS, after which the biofilm density was determined by CFU assay, as previously described. The biofilm-containing coverslips were removed. Then, the biofilms were completely scraped and dissolved in 5 mL of sterile NS. The CFU assay was conducted to determine the viable biofilm biomass after dAA treatment.
Statistical analysis
The statistical analysis was carried out using the SPSS for Windows, v. 16 (SPSS Inc., Chicago, USA). A one-way analysis of variance (ANOVA) and two-way ANOVA were used to compare multiple means of more than 3 data sets. The level of significance was set at 5% (p < 0.05).
Results
Antimicrobial activity of dAAs
The minimum concentration of dAAs required to kill the bacterial population completely was defined as MBC. The presence of visible growth on BHI agar plates was observed after the incubation (Table 1).
There was no inhibition of planktonic bacterial growth on BHI agar after treatment with different concentrations of dAAs for 24 h.
No MIC point was noted (wells without color change of AlamarBlue stain) in any of the inverted AA samples.
Effect of dAAs on bacterial adhesion under static conditions
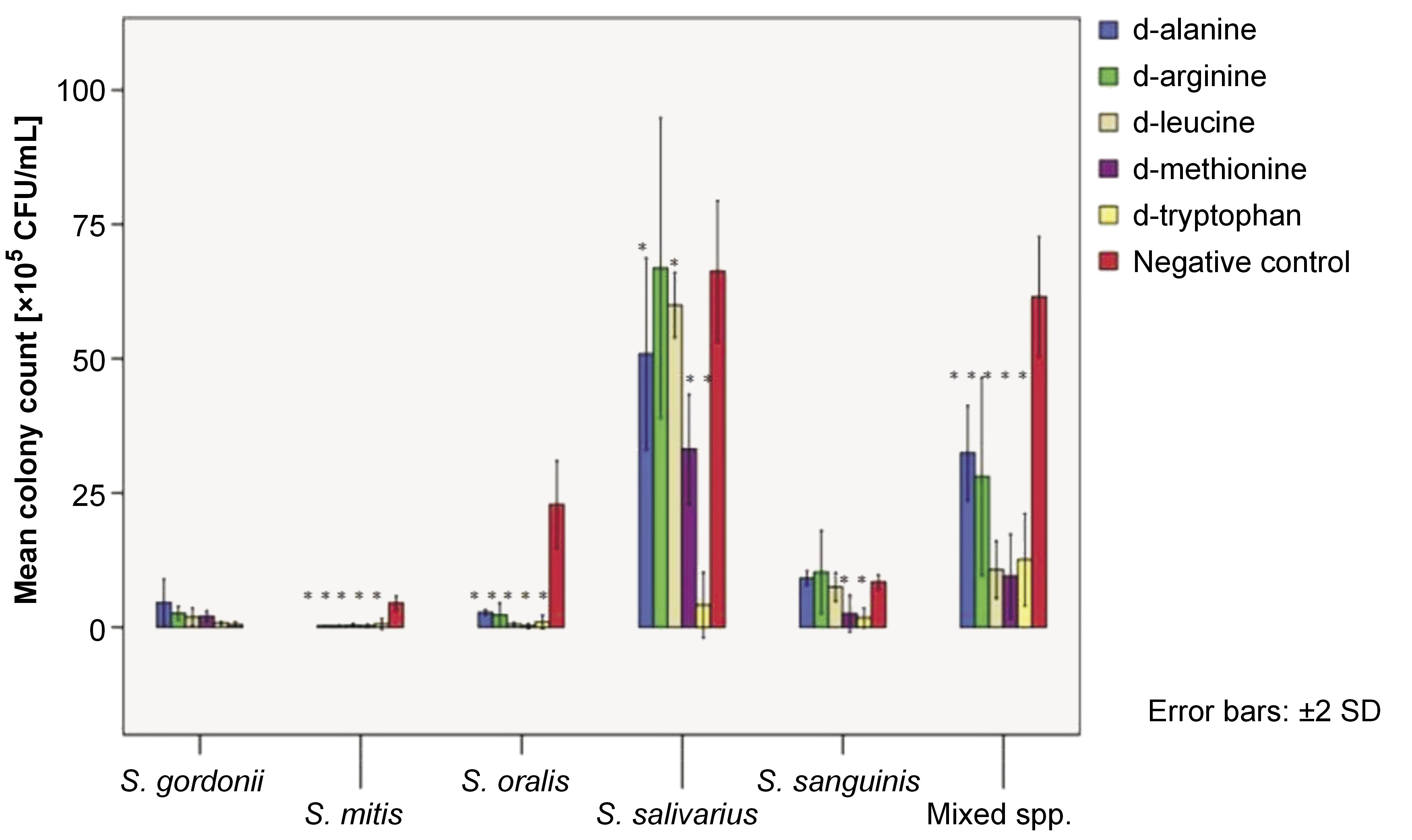
The adhered cell mass on the polystyrene surface in the presence of 25-mM dAAs was quantified using the CFU assay. Figure 1 depicts the mean colony counts of test organisms per adhered cell mass after a 6-h test period.
The adhesion of S. mitis, S. oralis and mixed species was reduced by all tested dAAs (p < 0.05). No adverse effects were observed on S. gordonii adhesion with any of the tested dAAs. Significant adhesion depletion of S. salivarius was observed with all tested dAAs, except for 25-mM d-arg. Only d-met and d-try were found to effectively reduce the adhesion of S. sanguinis onto the polystyrene surface (p < 0.05).
Effect of dAAs on preformed biofilms
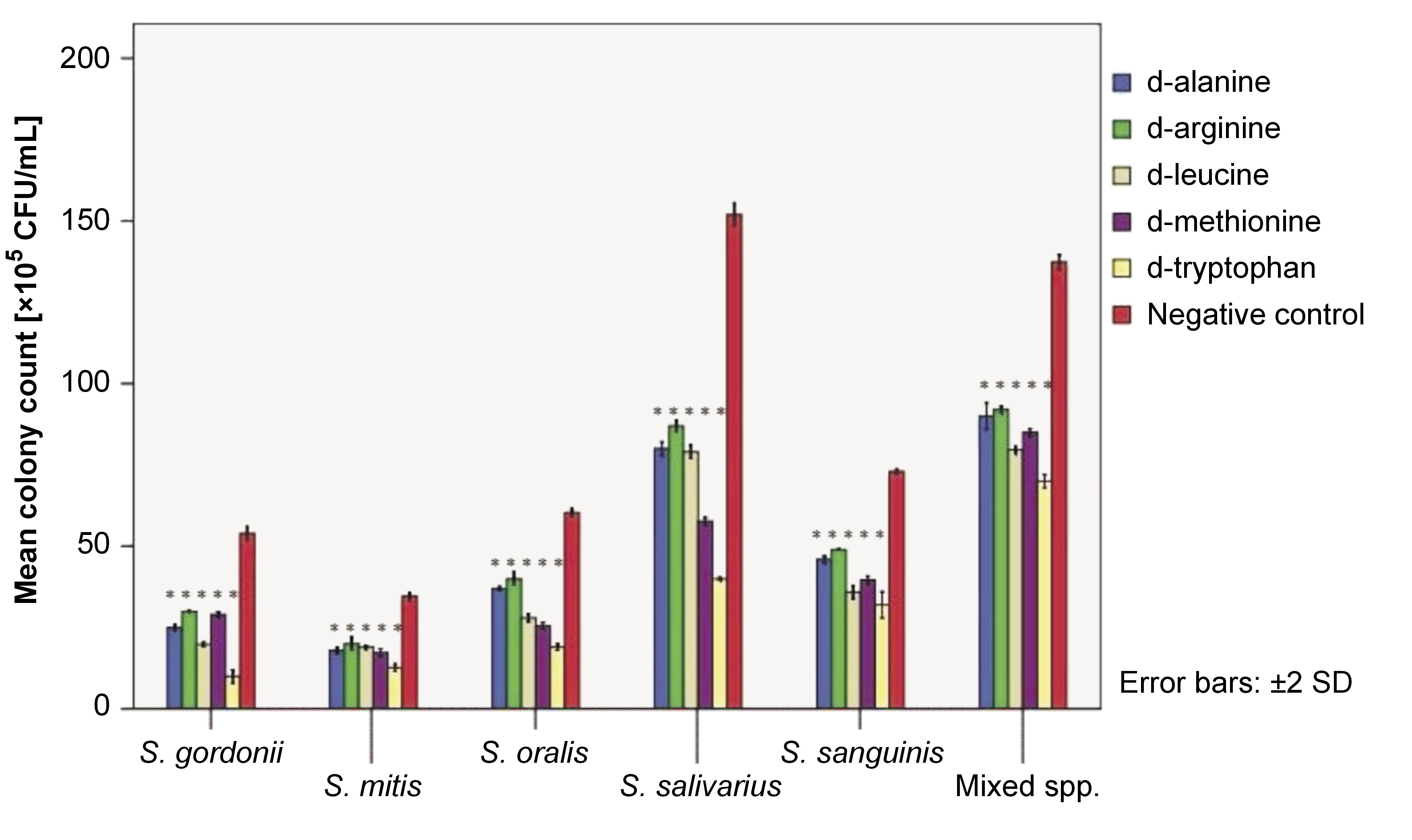
A 24-h biofilm of test strains and their mixed species was subjected to a CFU assay, followed by treatment with 25-mM dAA solutions for 5 min. The CFU assay readings were obtained (Figure 2). A 5-min treatment with 25-mM d-ala, d-arg, d-leu, d-met, and d-try resulted in a significant reduction in biofilm biomass for all test strains and their mixed species biofilms (all p-values <0.05).
Discussion
The oral cavity contains hundreds of different microorganisms, including bacteria, viruses and fungal species. Many of these microorganisms can associate with one another to form biofilms, which makes these organisms more resistant to mechanical stress and antibiotic agents.
The formation of oral biofilm is a dynamic process. Once the tooth surface has been cleaned, it is exposed to the fluid environment of the buccal cavity, which causes the adsorption of a thin layer of the acquired pellicle. This pellicle consists of saliva glycoproteins, mucins, statherin, agglutinin, etc.19 The pellicle coating alters the net surface charge and free energy of oral surfaces, thereby promoting the adhesion of initial colonizers.20 The most prevalent early colonizers of dental surfaces are Gram-positive facultative anaerobes, including Streptococcus spp. (S. mitis, S. oralis, S. salivarius, S. gordonii, S. sanguinis, etc.) and Actinomyces spp. These colonizers provide a basement surface for further progression of the oral biofilm. Once the early colonizers have attached to the dental surface, the biofilm develops through continued growth and subsequent adsorption of late colonizer species via coaggregation.19 Since the transition from a healthy commensal flora towards a pathogenic flora is mediated by the progression from the initial colonizing streptococci and bacilli to the secondary colonization of late colonizers, effective control of oral biofilm formation relies on the removal of early colonizers from oral surfaces.21 With the increased knowledge of oral biofilms, efforts to develop cost-effective, non-toxic and successful anti-biofilm strategies led to the identification of some possible biofilm control strategies. These include increasing the accessibility of antimicrobial agents to the biofilm by weakening the microbial biofilm structure, so that the action of the antimicrobial agent is more effective.22
Few reports point to the effects of inverted AAs on microbial biofilms. In 2010, Kolodkin-Gal et al. reported that dAAs prevent biofilm formation of Staphylococcus aureus, Bacillus subtilis and Pseudomonas aeruginosa by disassembling its components.23 Another in vitro study conducted by Zilm et al. revealed the anti-biofilm effect of dAAs on Enterococcus faecalis biofilms in the presence of routine antimicrobials.22
The current study is among a select few which evaluate the effects of biofilm dispersal of inverted AAs (i.e., d-ala, d-arg, d-leu, d-met, and d-try) and their mixtures along with their antimicrobial and anti-adhesive properties using 5 early colonizing Streptococcus spp. (S. mitis, S. oralis, S. salivarius, S. gordonii, and S. sanguinis). Previous studies have shown that dAAs do not cause any damage to the cell wall or any other cellular component. Rather, they only lead to modifications in the synthesis of peptidoglycan, a cell wall component. D-amino acids alter the chemical composition, strength and concentration of peptidoglycan by incorporating into the polymers via regulating enzymes related to peptidoglycan synthesis and modification. Since the effect of dAAs is only limited to cell wall components and it does not cause any damage to cytoplasmic compartments, the tested dAAs did not show any bactericidal or bacteriostatic effects on the test strains.24
However, the adhesion of S. mitis and S. oralis to the polystyrene surface was significantly reduced by all tested dAAs. The adhesion of S. salivarius was affected by all tested dAAs, with the exception of d-arg. The adhesion of S. sanguinis was not affected by d-ala, d-arg or d-leu. Furthermore, no dAA had a negative effect on the adhesion of S. gordonii. Interestingly, the adhesion of mixed species co-cultures was significantly diminished by all tested inverted AAs at a concentration of 25 mM. Although the adhesion of some pure cultures was not significantly reduced, this finding is of great importance, given that the oral microbial biofilms are polymicrobial in nature.
On the other hand, the cell density of mature 24-h biofilms of early colonizers was quantified after 5 min of exposure to 25-mM solution of dAAs using the CFU assay. The CFU assay demonstrated a significant reduction in the viable biofilm cell mass for treated biofilms, indicating the ability of the inverted AAs to disassemble the biomass. The mechanism of action of dAAs in inhibiting or disassembling biofilms remains unclear. Some reports suggested that dAAs interfere with cellular protein synthesis, replace d-ala in the cell wall, or act as signaling molecules that enable bacterial cells to adapt to the changing surroundings.23, 24
Conclusions
In conclusion, the current study showed that dAAs have the potential to be used as a means of preventing the formation of oral biofilm by inhibiting the adhesion of early colonizers. Further toxicology studies and experiments are recommended to determine the effect of dAAs on mature biofilm matrix before the use of dAAs as oral rinses or oral care products.
Ethics approval and consent to participate
Not applicable.
Data availability
All data generated and/or analyzed during this study is included in this published article.
Consent for publication
Not applicable.