Abstract
Background. Temporomandibular disorders (TMDs) and cervical spine problems are a growing public health issue, as they increase the risk of disability in people with hypermobility joint syndrome (HJS).
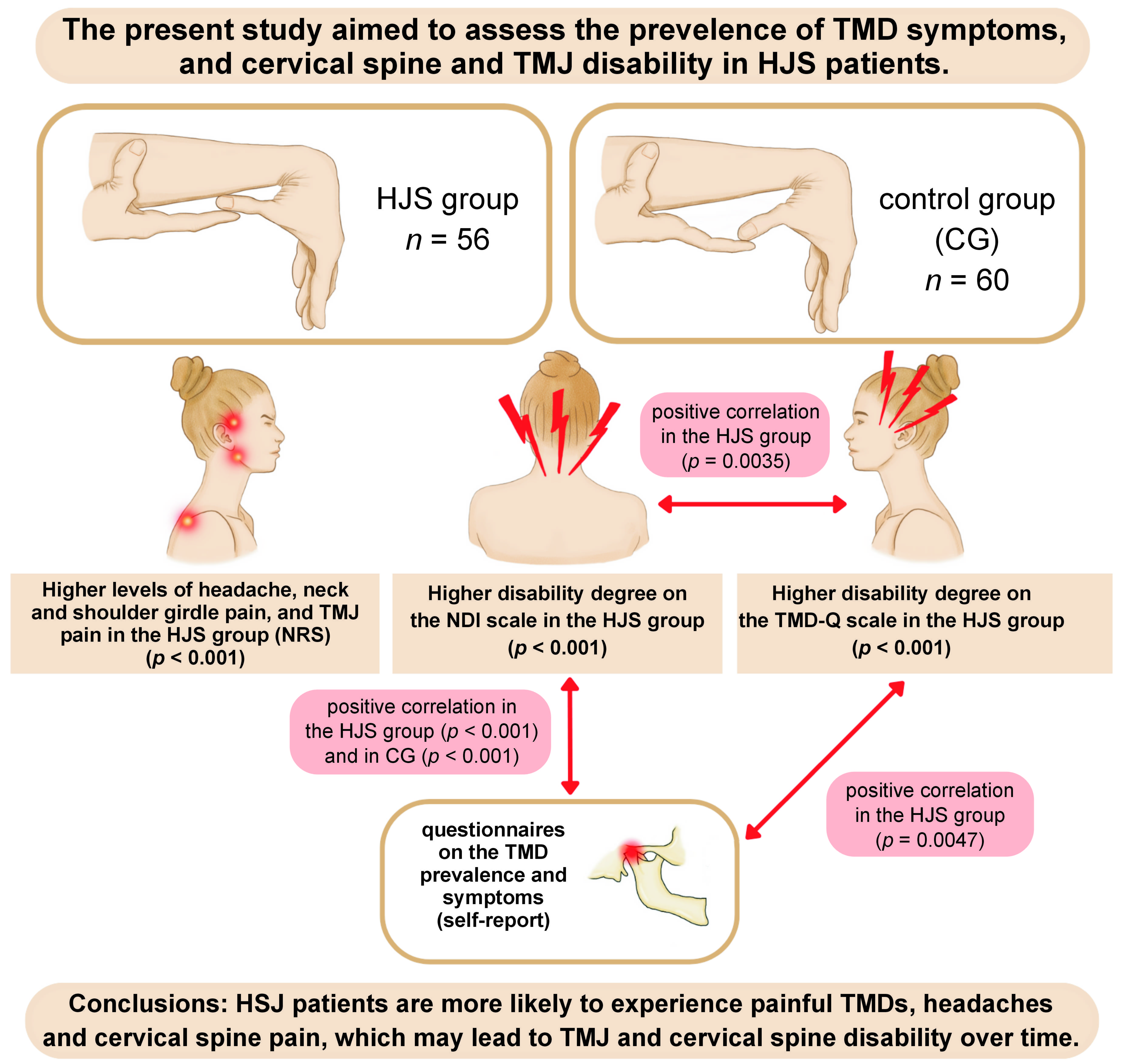
Objectives. The present study aimed to assess the prevalence of TMD symptoms, and cervical spine and TMJ disability in HJS patients.
Material and methods. A survey was conducted among physical therapy students (mean age: 21 years). The study comprised 2 stages. The 1st one was HJS assessment (the Beighton scale and the Brighton criteria). Based on the assessment, 56 HJS subjects were enrolled for the study. The control group (CG) consisted of 60 HJS-free subjects, according to the aforementioned criteria. The 2nd stage of the study involved conducting a self-administered questionnaire on the prevalence of TMD symptoms. Both the TMD disability questionnaire (TMD-Q) and the neck disability index (NDI) scores were recorded. Pain intensity was assessed using the numeric rating scale (NRS).
Results. The HJS group showed higher NRS scores (p < 0.001). Headache, neck and shoulder girdle pain, and temporomandibular joint (TMJ) pain were found to be more severe in almost each patient from the HJS group as compared to CG. Those individuals had a greater degree of disability on the TMD-Q and the NDI scales (p < 0.001). The HJS group showed significant positive correlations between the TMD-Q and NDI scores (p = 0.0035), and between the TMD-Q and TMJ symptom questionnaire scores (p = 0.0047). A significant positive correlation between the NDI and TMJ symptom questionnaire scores was found both in the HJS group (p < 0.001) and CG (p < 0.001).
Conclusions. The HJS bearers tended to obtain higher TMJ and cervical spine disability scores, at the same time reporting increased headache, neck and shoulder girdle pain, and TMJ pain intensity. Therefore TMJs should be carefully examined for possible signs of dysfunction in HJS subjects prior to dental or prosthetic treatment. According to our data, TMJ and cervical spine disability assessment should be included as a routine practice in the case of HJS patients, who should remain under the long-term care of a multidisciplinary team of doctors and therapists.
Keywords: temporomandibular disorders, cervical spine, disability, joint laxity, hypermobility syndrome
Introduction
Hypermobility joint syndrome (HJS) is classified as a generalized, hereditary connective tissue disorder with a general population prevalence of 2–57%.1 The contributing factors for HJSs vary in different individuals, and can include impaired protein synthesis and connective tissue matrix production. Disproportion in the type I and III collagen content, as well as cellular imbalance in tissue organization with regard to fibrillin – a major protein co-forming elastic fibers – is currently under thorough scientific scrutiny. Most studies aim to unravel a complete list of hereditary contributing factors for HJS development, as the exact genetic factors influencing this condition are not very well known.2
The main symptoms of HJS include, but are not limited to, the flaccidity of the joint capsules and ligaments, increased joint mobility, and numerous dysfunctions of body areas congenitally rich in connective tissue.1
Hypermobility joint syndrome is believed to be more prevalent in young women, tending to subside as one matures. The healthy aging process seems to be more important than the cessation of connective tissue abnormalities.3 The disorder significantly reduces the quality of life, as it can be associated with chronic injuries, e.g., joint dislocations and sprains, damage to the ligaments, chronic pain, and persistent fatigue, resulting over time in an impaired sensory function of musculoskeletal system tissues. Repeated trauma may lead to irreversible damage to joint surfaces, which can result in disability.4
The diagnostic criteria for HJS embrace the Beighton scale and the Brighton Criteria, both of which are widely used for joint laxity assessment.5 The Beighton scale includes 5 simple activities that measure joint mobility on a nine-point scale, where excessive joint mobility is defined by a score ≥4. Additionally, special criteria called the Brighton criteria have been developed for the diagnosis of HJS.6 Hence, the Beighton scale is used for identifying hypermobility and the possibility of symptoms such as joint pain, spine degenerative changes, joint subluxations, physique similar to that observed in Marfan syndrome, skin and/or ocular symptoms, the possibility of herniae, varicose veins, and uterine or anal prolapse. The proper fulfillment of the abovementioned criteria, according to a specific formula, constitutes evidence of HJS.6 A study by Bravo and Wolff shows that by applying the Brighton criteria, a high detection rate of HJS is achieved.7
Hypermobility joint syndrome may be considered a predisposing factor for temporomandibular disorders (TMDs).8 Some initial reports are available, connecting the prevalence of certain laxity-associated single nucleotide polymorphisms (SNPs) (COL5A1 rs12722) with intracapsular temporomandibular joint (TMJ) disorders.9 People with connective tissue disorders tend to overstretch the TMJ capsules and retrodiscal tissue ligaments. Wide-mouth opening and subconscious nocturnal and/or diurnal activities (e.g., bruxism) may lead to TMJ disc displacement and orofacial pain. According to the available data, 70% of HJS patients have been found to have TMJ articular disc displacement without reduction, which does not manifest with clicking/popping, but maximum jaw opening is limited to ≤30 mm.9, 10 As a result, inflammation (e.g., swelling, warming) may occur over time, destroying the articular surfaces, and leading to TMJ structural remodeling and degenerative lesions (osteophytes). In TMJ hypermobility, the activity of the masticatory muscles is reduced, resulting in the disruption of the chewing process, both in adolescents and adults.11
Despite reports on HJS and TMDs, there are still no clear, tangible results assessing their co-occurrence and causes, suggesting the need for further research in this area, mostly on the molecular level.12
Therefore, the present study aimed to assess the prevalence of TMD symptoms, and cervical spine and TMJ disability in HJS patients. We hypothesized that HJS patients are more prone to develop painful TMDs, which translates into the onset of disability.
Material and methods
This study took place between January 2020 and June 2022 at the Department of Rehabilitation of Musculoskeletal System, Pomeranian Medical University in Szczecin, Poland, and was based on the surveys conducted among physical therapy students (2nd to 4th year, mean age: 21 years). All respondents signed formal, written consent to participate in the study, which was approved by the Bioethics Committee (KB 0012/104/15) and supported by a grant from the Pomeranian Medical University in Szczecin, Poland (MB-329-212/16).
The inclusion criteria were as follows: students of physical therapy who were not disabled, without any known disease, aged 18–25 years. Students with known diseases, inconsistent age, and who did not provided consent to participate in the study were excluded.
Assuming an effect size of 0.5, a power of 0.95 and a significance level of 0.05, the minimal sample size, as calculated using the G*Power software (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower), was determined to be 47.
The 1st stage of the study consisted in a survey to evaluate the presence of HJS with the use of 2 standardized tools – the Beighton and Brighton scales.13, 14 The data was acquired by the same trained and calibrated physical therapist, experienced in working with HJS patients. The Beighton scale is a five-point test assessing the passive extension of the 5th finger of the hand past 90°, the passive adduction of the thumb to the inner surface of the forearm, the hyperextension of the elbow joint past 10°, the hyperextension of the knee joint past 10°, and the ability to position the hands on the floor while bending forward with the knee joints straight. Each symptom is assigned a ‘0’ or ‘1’ point. The point summary with a minimum score of 4 out of maximum 9 points resulted in the participant being included in the HJS group. The examiner demonstrated certain movement patterns to be repeated in a given time and the examinee performed this movement to the terminal part of their active range, as instructed.13
The Brighton scale is complementary to the Beighton test and the scores are integrated. The Brighton criteria were divided into major (Beighton scale: ≥4 points – either present or history – and pain lasting more than 3 months in at least 4 joints) and minor ones (Beighton scale: 1–3 points). The minor criteria comprise the following: pain lasting 3 or more months in 1–3 joints; back pain (lasting 3 or more months); spondylosis; spondylolysis/spondylolisthesis; dislocation/subluxation in more than one joint or more than once in a single joint; soft tissue rheumatism with 3 or more symptoms (epicondylitis, tenosynovitis and bursitis); Marfanoid physique; arachnodactyly; a positive Steinberg sign; carpal tunnel syndrome; skin abnormalities – striae, hyperextensibility, thinning, papillary scars; ocular manifestations – drooping eyelids, myopia, antimongoloid eyelid folds; lower limb varicose veins; hernia; rectal or vaginal/mammary prolapse; and mitral valve prolapse. The recognition of HJS is based on the presence of 2 major criteria or 1 major and 2 minor criteria or 4 minor criteria.14
Participants diagnosed with HJS based on the Brighton–Beighton scale were enrolled in the HJS study group (n = 56; 16 males and 40 females).
The control group (CG) consisted of 60 physical therapy students (18 males and 42 females) who were excluded from the study group, thus not meeting HJS thresholds according to the Beighton test and the Brighton criteria.
In the 2nd stage of the study, all participants completed standardized questionnaires about the presence of TMD symptoms and probable bruxism occurrence; the TMJ and cervical disability scores were recorded as well.15, 16 The data collected via the questionnaires was based on self-reports. The ‘paper-and-pencil’ method was used, and it took approx. 20 min to complete the questionnaires.
Thus, data acquisition was based on the following:
– a self-administered questionnaire containing specific questions about age, gender and the body mass index (BMI), and including subjective health assessment;
– 8 close-ended questions on TMD symptoms (headache, TMJ and preauricular pain, TMJ sounds, an increased activity of masticatory muscles, TMJ locking upon mouth opening, and tooth clenching and/or grinding – self-reported or partner-reported). Pain intensity was assessed using the numeric rating scale (NRS);
– TMD disability questionnaire (TMD-Q) – the subjective evaluation of TMD symptoms and TMJ functional limitations during daily activities. The TMD-Q consisted of 10 statements referring to specialized TMJ functions, such as speaking, dental care, eating, social activities, and non-specialized TMJ functions. Functional limitations were measured on a scale from 0 to 4, where 0 means no limitations, and 4 means maximum limitations. The minimum score was 0, and the maximum score was 40. The higher the score, the greater the degree of disability reported.15
– the neck disability index (NDI) – the Polish version of the NDI questionnaire (NDI-Polish version, NDI-PL) was used to evaluate cervical spine issues. It consisted of 10 questions concerning pain intensity, nursing, lifting objects, reading, headache, the ability to focus, working, driving, sleeping, and resting. Each question was graded on a scale of 0–5 points. The composite score was presented on a 0–50-point scale, where 0–4 corresponded to no disability, 5–14 was considered mild disability, 15–24 – moderate disability, 25–34 – severe disability, and 35–50 corresponded to terminal suffering and extreme disability.16
Statistical analysis
Data is presented in tables. Quantitative variables are presented as mean and standard deviation (M ±SD), and as median (Me) with the 1st and 3rd quartiles. The normality of the distribution of quantitative variables was assessed using the Shapiro–Wilk test and, alternatively, histograms and quantile–quantile (Q–Q) plots. Pearson’s χ2 test was used for intergroup comparisons of qualitative variables. For quantitative variables with a normal distribution, the t test was used, while for quantitative variables with an abnormal distribution, the Wilcoxon and Kruskal–Wallis tests were used. Kendall’s tau-b (τb) test was used for correlation analysis. The analysis was performed using the R language in the RStudio environment (http://www.rstudio.com). The statistical significance level was set at a p-value below 0.05.17
Results
A total of 82 women (70.69%) and 34 men (29.31%) participated in the study. A total of 52%, 29%, and 19% were second-, third- and fourth-year students, respectively.
The results regarding group characteristics are presented in Table 1.
There were no significant differences between the groups with regard to age and BMI. However, there was a statistically significant difference between the groups in the Beighton scale scores. Moreover, there was a statistically significant difference in the Brighton scale scores between the study group and CG, indicating HJS occurrence within the study group (p < 0.001) (Table 1).
According to the analysis of the subjective health assessment, 50.0% of respondents with HJS assessed their health as good, 44.6% as sufficient, 5.4% as bad, and 0% as very good. In CG, the responses included 50.0% as good, 41.7% as very good, 8.3% as satisfactory, and 0% as bad or very bad.
Prevalence of TMD symptoms
According to the self-assessment questionnaire on TMD symptoms, pain in the adjacent tissues, masticatory motor function disorders, headache, neck and shoulder girdle pain, and TMJ pain were significantly more frequent in the HJS group, and the pain intensity levels were greater. Based on the analysis of the NRS scores, it could be concluded that there was a higher level of headache in the HJS group as compared to CG (p < 0.001); in the HJS group, most respondents indicated a NRS level of 3 (35.7%), while in CG, no pain was reported by 68.3% of the respondents. Neck and shoulder girdle pain was also higher in the HJS group, with up to 37.5% of the respondents reporting a NRS level of 5, while in CG, the most common response was no pain (75.0%). Scrutinizing the TMJ pain intensity scores in the HJS group, 30.4% of the respondents reported pain at NRS levels 4 and 5, whereas in CG, 86.7% reported no painful TMDs. Consecutively, in the HJS group, TMJ sounds (p < 0.001), TMJ locking upon mouth opening (p < 0.001) and tooth clenching and/or grinding (p < 0.001) occurred significantly more frequently as compared to controls.
TMJ disability
According to the TMD-Q responses, the HJS group and CG differed significantly with regard to questions 1 (verbal communication; p < 0.001), 3 (normal daily activities; p < 0.001), 4 (social/recreational activities; p < 0.001), 5 (non-specialized jaw function; p < 0.001), 6 (sexual function; p < 0.001), 8 (response to treatment; p < 0.001), 9 (tinnitus/vertigo/ear sounds; p < 0.001), and 10 (dizziness; p < 0.001). In the HJS group, the respondents were more likely to pinpoint at least one of the issues above, varying in severity, as compared to non-HJS controls (Supplement, available on request from the corresponding author).
Cervical spine disability
Using the cervical spine disability scale (NDI), responses in the HJS and CG groups were significantly different for questions 1 (pain intensity; p < 0.001), 3 (object lifting; p < 0.001), 4 (reading; p < 0.001), 5 (headache; p < 0.001), 6 (focus; p < 0.001), 7 (work; p < 0.001), 9 (sleep; p < 0.001), and 10 (rest; p < 0.001). There was a statistically significant difference in the degree of cervical spine disability between the groups. In the HJS group, 73.2% had mild disability and 26.8% had moderate disability, while in CG, 83.3% had no disability and 16.7% had mild disability (Supplement, available on request from the corresponding author).
The statistical analysis of the TMD-Q, NDI and NRS scores is presented in Table 2.
People with HJS reported significantly higher pain intensity on NRS (p < 0.001). In each case, headache, neck and shoulder girdle pain and TMJ pain were significantly more intense than in CG. Furthermore, HJS individuals expressed a greater degree of disability according to the TMD-Q and NDI scales (p < 0.001).
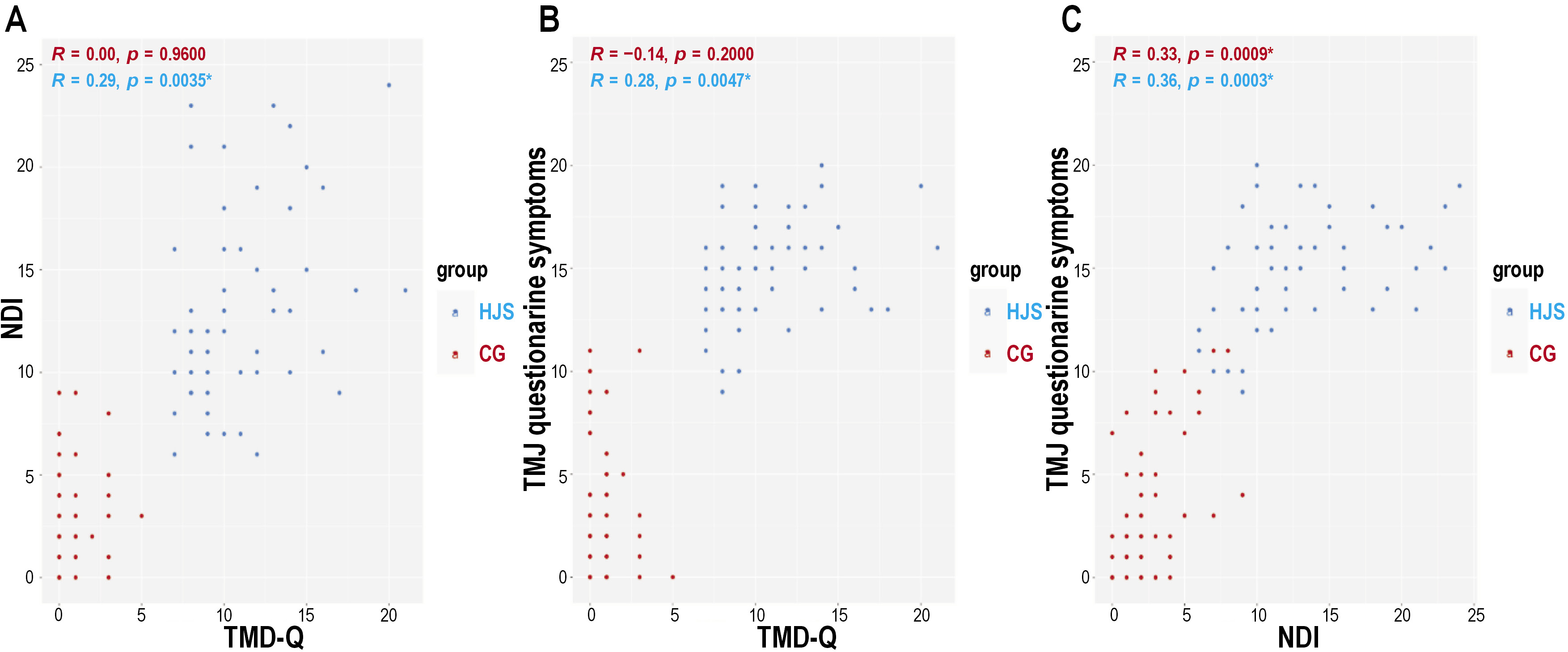
The mutually intertwining TMD-Q, NDI and the surrounding tissue issues in the HJS group and CG are presented in Figure 1.
The HJS group showed significant positive correlations between the TMD-Q and NDI scores (p = 0.0035) (Figure 1A), and between the TMD-Q and TMJ symptoms questionnaire scores (p = 0.0047) (Figure 1B). An increase in the TMD-Q scores was tied to elevated NDI scores and TMJ symptoms.
A significant positive correlation between the NDI and TMJ symptom questionnaire scores was noted in the HJS groups (p < 0.001) and CG (p < 0.001) (Figure 1C).
Discussion
According to our study data, the HJS bearers were significantly more prone to headaches, neck pain, and painful TMDs (p < 0.001). In 94.6% of the HJS respondents, headaches were reported, while 100% of them noticed significant cervical spine and TMJ pain, 80.4% reported TMJ sounds, 33.9% reported TMJ locking during jaw movements, and 66.1% noticed tooth clenching and/or grinding. A significant difference (p < 0.001) was observed in all the symptoms mentioned between the two examined groups. The present results confirm those from a study by Abbot et al., who highlighted a higher prevalence of neck pain in HJS-diagnosed study participants.18 Similarly, other papers reported that the onset of headaches, including migraines, was significantly more prevalent in the HJS group as compared to healthy controls.19, 20 Chiodelli et al. emphasized the need for a more thorough observation of TMD prevalence in HJS bearers, possibly using larger cohorts.21 Their study concluded that TMJ and preauricular pain were significantly more common in HJS patients.21 According to Kavuncu et al., up to 79.7% of TMD patients had HJS,22 with similar results obtained by Pasinato et al. (64.71%).23 Additionally, the latter group of authors described a higher percentage of myofascial pain without mouth-opening restrictions in HJS participants (81.82%) as compared to non-hypermobile controls (58.33%).23
The results of the present study show that an ample prevalence of masticatory movement disorders (tooth clenching and/or grinding) was associated with the HJS group. According to Westling and Mattiasson, sleep-related movement disorders were considered to have greater detrimental effects on hypermobile individuals than on those with no connective tissue disorders.24 Harkins and Cueva came to another valuable conclusion, namely, that HJS and masticatory parafunctions in women, when present simultaneously, are significantly associated with symptoms of intraorbital TMDs (p < 0.001).25 Therefore, in patients with HJS, a greater emphasis should be put on tooth clenching and/or grinding. The researchers concluded that bruxism in conjunction with HJS presence might cause irreversible forms of ligament disability in the masticatory motor system and TMJs more rapidly than in non-HJS subjects.25
In our study, a significant difference was found between the groups in terms of TMJ pain. Consistently, Pasinato et al. brought up that painful mouth-opening issues were statistically more common in the HJS group than in the non-HJS CG (p = 0.0279).23 Contrary to these results, no such causal relationship was found by Winocur et al. in a study on adolescent girls.26
Our data showed that the HJS patients commonly presented TMJ disability as compared to the non-HJS controls. Hence, a positive correlation between the TMD-Q score (the higher the score, the greater the disability) and the number of TMJ and surrounding tissue symptoms reported in the questionnaire was found (p = 0.0047). However, we did not find similar studies assessing TMJ disability with the use of TMD-Q in joint laxity cases, which makes our results incomparable with any other scientific data. However, given the validity of TMD-Q for assessing TMJ functions, we could cautiously draw a conclusion that HJS in patients with concomitant TMDs, i.e., pain, sounds, etc., might be considered an additional contributing factor for TMJ disability.
This study confirmed that HJS patients were more prone to the cervical spine disability onset as compared to healthy controls – these conclusions were drawn based on statistical significance. Moreover, we obtained a positive correlation between the NDI and TMJ symptoms questionnaire scores in the HJS group (p < 0.001), as well as in controls (p < 0.001). Hence, with regard to the previously mentioned considerations, the safest assumption would be that ligament laxity issues could be defined as hereditary, underlying conditions, generalized as a systemically altered quality of the connective tissue. These results suggest an impaired efficiency of the ligaments attached to cervical segments in HJS subjects. Proprioceptive dysfunction, and a greater predisposition to myofascial pain and spine trauma seem to be a contributing factor to the higher incidence and severity of cervical disability in hypermobile patients.27 Few other studies focused on biomechanical links, despite strong functional relationships between TMJs and the cervical spine. According to Kashif et al., the association of TMDs with cervical spine disability and the NDI score was clearly significant (p < 0.001).28
Lee et al. showed an increased frequency and intensity of neck pain in the HJS group as compared to those without HJS (frequency: p = 0.020; intensity: p = 0.001).29 In contrast, Keser et al. found no association between cervical spine degeneration (magnetic resonance imaging (MRI)), neck pain (the visual analog scale (VAS)) and cervical disability (NDI) in HJS bearers.30 However, one should notice that their study was conducted on a group of patients aged 20–50 years, which is a significantly different age range in comparison with most groups scrutinized by other authors.
To our best knowledge, we are the first to demonstrate that in HJS patients there is a positive correlation between the presence of TMJ disability (TMD-Q) and cervical spine disability (NDI) (p = 0.0035). These results are very promising, hereby encouraging the design and implementation of more studies on this matter in larger cohorts and diverse populations.
The results of this study allowed us to conclude that TMD assessment in HJS patients, with the subsequent implementation of appropriate therapeutic interventions, would contribute to lessening the effects of dysfunction. Standardized procedures to assess the degree of TMJ and cervical spine disability should be considered in the daily clinical work for joint laxity patients. Affected patients require comprehensive, long-term care and follow-up with a skilled, multidisciplinary team of clinicians and therapists.
The obtained data leads to the conclusion that screening for HJS seems to be of highest importance for physical therapy students, as exposure to numerous tensions and overloads is an inherent part of their future profession. The detection of HJS at an early stage should lead to the swift implementation of both preventive and therapeutic methods aimed at reducing the effects of HJS, including observing the principles of ergonomics at work or introducing individual exercises to heal the proprioception function of the joints, which, like drug therapy, should be administered as needed and according to clinical judgment.
Limitations
As our study was based on the patient’s self-reports, some participants might not have been fully eligible to understand and answer the questionnaire accurately. Additionally, no molecular tests were performed to confirm HJS, just the 2 solid and widely used questionnaires, i.e., the Beighton scale and the Brighton criteria. Although this is standard practice, it is important to note that the increasing availability of genetic tests assessing connective tissue insufficiency hereditary profiles would yield an earlier, more objective, yet highly personalized standard of care for hypermobile patients.31, 32 Another limitation was the lack of a DC/TMD (Diagnostic Criteria for Temporomandibular Disorders) diagnosis of TMDs and a standardized tool to assess the presence of TMJ dysfunction. In the future, as a follow-up of this study, more data needs to be included from larger patient cohorts in conjunction with molecular tests, a DC/TMD questionnaire and three-dimensional (3D) imaging. These may contribute to a significant increase in TMD recognition and the implementation of relevant treatment modalities in HJS patients.
Conclusions
Hypermobility joint syndrome patients are more likely to experience painful TMDs, headaches and cervical spine pain, which may lead to TMJ and cervical spine disability over time.
Ethics approval and consent to participate
All respondents signed formal, written consent to participate in the study, which was approved by the Bioethics Committee at Pomeranian Medical University in Szczecin, Poland (KB 0012/104/15).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.