Abstract
The systematic review aimed to compare and evaluate the effect of resin-based sealers and bioceramic sealers on postoperative pain after endodontic treatment. Two reviewers independently conducted electronic search in PubMed, the Web of Science, ScienceDirect, the Wiley Online Library, SpringerLink, Google Scholar, and the Cochrane Library, employing a complete dual-review process to ensure the inclusion of all relevant studies in the review. The search was carried out until November 2021. After selecting eligible studies, the risk of bias assessment was carried out using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2). A total of 1,931 studies were identified from the electronic search, and finally 10 studies were included after full-text assessment. In all our included studies, the visual analog scale (VAS) was used for recording pain scores. Most of the studies recorded pain intensity starting from 6 h to 7 days. The results showed that there was no significant difference between resin-based sealers and bioceramic sealers in terms of incidence or intensity of postoperative pain at any point in time.
Keywords: postoperative pain, root canal filling materials, root canal obturation, root canal sealers, endodontic pain
Introduction
Despite significant improvements in endodontics, the pain experienced after treatment is inadvertent, but often a significant emotional concern for both patients and endodontists.
Patients after endodontic treatment experience pain ranging from 1.9% to 82.9%. Different endodontic treatment procedures are known to be associated with postoperative pain, such as calculation of working length with the apex locator connected to every other file, frequency of visits, instrumentation technique used, and also depends on the type of root canal filling materials used.1
Bacteria present on the outer surface of the tooth roots are thought to maintain periapical radiolucency and apical periodontitis.2 Since the biofilm within the apical part of the canal can be difficult to detect and capture, some authors advised foraminal enlargement (FE).3, 4, 5 This additional procedural step is shown to promote periapical healing in animal models.6, 7 However, there is disagreement about the needed extent of enlargement. An ideally prepared root canal should have a progressively tapering conical shape, which preserves the apical foramen and the original canal curvature without transportation. It has been shown that root canal preparation with engine-driven NiTi endodontic instruments results in significantly less canal transportation and fewer preparation errors without significantly compromising the tooth structure. The thickness of the remaining dentine following intra-radicular procedures may be the most important iatrogenic factor that correlates to the incoming fracture resistance of the root. Currently, available NiTi file systems have the best shaping ability, cleaning ability, and three-dimensional efficiency while at the same time preserving dentine structure and reducing the impact on tooth strength. Despite the advanced flexibility of NiTi alloy compared with stainless steel, fracture of NiTi endodontic instruments remains a problem in clinical practice. Providing NiTi files are used judiciously, the fracture incidence appears to be comparable.8, 9, 10
Adequate root canal filling further plays an important role in endodontic treatment because it prevents bacterial infection through reduced coronal leakage, closes the apex to fluid leakage in the periapical tissues, and reduces the microbial load in the root canal, thus arresting the disease progression.11, 12, 13
The sealers within the canal system disrupt periodontal tissue through the apical foramina, lateral canals, or leaching and interfere with the healing ability of the periodontal tissues. Thus, local inflammation caused by these materials eventually leads to postoperative pain. The severity of these inflammatory reactions depends on a variety of factors, including the composition of the sealers.14, 15
It has been disclosed in various studies, that bioceramic materials improve the effectuality of endodontic treatment. Bioceramic sealers usually contain particles of zirconia, alumina, bioactive glass, calcium silicates, hydroxyapatite, and soluble calcium phosphates. This structure inside the sealer makes it resistant to leaks and makes it compatible with the biological environment. Bioceramic materials release biologically active substances that stimulate intratubular biomineralization in pre-osteoblasts and also promote odontoblastic differentiation, thereby enhancing the effectiveness of endodontic treatment.16, 17, 18
Resin-based sealers have improved physical properties, but on the other hand, their cytotoxic effects should be of concern, which requires the need to establish a better root-filling material. To overcome this, bioceramic sealers have recently been established with less cytotoxic compounds compared to resin-based sealers. It has also been suggested that there is better root integrity after root canal filling using bioceramic sealers. The solubility of these sealers remains a critical aspect of their properties.19, 20, 21, 22
The three-dimensional canal system is usually obturated with gutta-percha and endodontic sealers. These materials are designed for use within the three-dimensional canal system, but sometimes, they interact closely with the periapical tissues, leading to inflammation and irritation of sensory nerve cells.
Endodontic sealer extrusion is a very common condition, but in very small amounts, it is usually well tolerated by periapical tissues. However, if the filling material is accidentally forced out to nearby neurovascular structures, nerve damage and subsequently altered sensation may occur. It is important to discuss that all root canal filling sealers are generally neurotoxic to some degree. Also, sealer extrusion is linked to future complications, such as nerve damage that may trigger symptoms of pain and cause flare-ups.23, 24, 25
To avoid problems with sealer extrusion, it is imperative to select sealers with better physicochemical properties and lower toxicities. AH Plus is a bisphenol epoxy resin that has been reported to cause an increase in postoperative pain after unintended sealer extrusion. However, patients showed less severe pain sensitivity with calcium silicate-based sealers compared to AH Plus sealers.26
However, this topic should be an area of interest to be discussed in additional literature and post-endodontic pain assessments after the release of calcium silicate-based material out of the apical foramen have yet to be identified.
There is a growing trend among clinicians who use calcium silicate-based sealers over resin-based sealers, but there is no literature evidence to prove its effectiveness in reducing pain after non-surgical root canal treatments. Therefore, the aim was to systematically review the scientific evidence regarding the influence of epoxy-resin and calcium silicate sealers on the incidence of postoperative pain after root canal treatment.
Methodology
Study design
The protocol for this study was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and the PICO strategy.27
Research question and outcome
The focused question (PICO) was developed as follows: Does obturation with recently launched calcium silicate-based sealers (intervention) positively affect the postoperative pain (outcome) more than the epoxy resin-based sealers (comparison) in patients undergoing non-surgical root canal treatment (P)?
The primary outcome of this systematic review was to compare and evaluate the intensity of postoperative pain after obturation using calcium silicate-based sealers or epoxy resin-based sealers.
Selection criteria
Inclusion criteria
The following types of studies were considered:
1. Healthy patients (>15 years old) undergoing non-surgical root canal treatment, without restrictions concerning sex, the type of endodontic diagnosis or the type of tooth treated.
2. Randomized controlled trial (RCT) studies, controlled clinical trials (CCTs), and prospective and retrospective cohort studies.
3. Studies that used any resin-based sealers.
4. Studies that used any calcium silicate-based sealers.
5. Studies that included patients requiring root canal retreatment.
6. Studies that used the visual analog scale (VAS) scale.
7. Studies that were in English.
8. Studies that had documented follow-up time.
Exclusion criteria
The following types of studies were not taken into consideration:
1. Studies that were not RCTs, such as in-vitro studies, case reports, case series, and reviews.
2. Animal studies.
3. Studies with patients taking non-steroidal anti-inflammatory drugs (NSAIDs) that might interfere with the assessment of pain after endodontic treatment.
4. Patients with periapical lesions.
5. Studies with incomplete data regarding methods or those which used any method other than VAS for measuring postoperative pain outcomes.
Search strategy in the databases
PubMed, the Web of Science, ScienceDirect, the Wiley Online Library, SpringerLink, Google Scholar, and the Cochrane Library were used for the electronic search. The research was carried out until November 2021 with search alerts as a self-updating tool. Moreover, any relevant articles obtained from the cross-referencing of the screened articles were also included.
The following MeSH terms and synonyms were used for the initial search: (“pain, postoperative” OR “postoperative pain” OR “post obturation pain”) AND (“root canal obturation” OR “endodontic obturation” OR “root canal sealer” OR “root canal sealant” OR “root canal filling materials”). A manual search of the endodontic journals in the journal section of our college library was also carried out.
Study selection
Duplicate studies were manually removed by the two reviewers and considered only once. Then, the studies were individually screened for their eligibility by the two reviewers, who analyzed the titles and abstracts of the studies that were retrieved. In case of any disagreement, a third reviewer was consulted. Then, the full texts of the selected studies were read, and the studies that met the inclusion criteria were retrieved by the same reviewers.
Data extraction from the eligible studies
After selecting the eligible articles, two independent reviewers extracted the following data: (a) author, year of publication and country; (b) study design; (c) sample size; (d) age (mean in years); (e) diagnosis of the disease condition; (f) type of teeth; (g) number of visits; (h) instrumentation; (i) obturation technique; (j) obturation material; (k) postoperative pain assessment time; and (l) postoperative pain assessment scale. The variation in the opinion between the authors over data extraction was resolved through discussion. In many selected studies, multiple treatment groups were present; in such cases, the data conforming to PICO was selected. In case of any obscure or missing data, the respective corresponding authors were contacted through mail (up to two times over 4 weeks). For the standardization of this review, studies that used VAS scores from 0–100 mm were converted to 0–10 cm.
Risk of bias assessment
Two reviewers independently assessed the quality of selected studies using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2). This tool assessed 5 important domains (bias from the randomization process, bias due to deviation from the intended intervention, bias due to the missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported results), and all these domains were rated as a high, unclear/some concerns or low risk of bias. The study was judged to be at an overall high risk of bias if at least one domain had this result. A rating of some concerns was decided if multiple domains substantially lowered confidence in the result or some concerns were raised in at least one domain. Studies found not to be at high risk of bias for any domain or judged to be at an overall low risk of bias for all the domains were identified as low-risk.28
Results
Search details
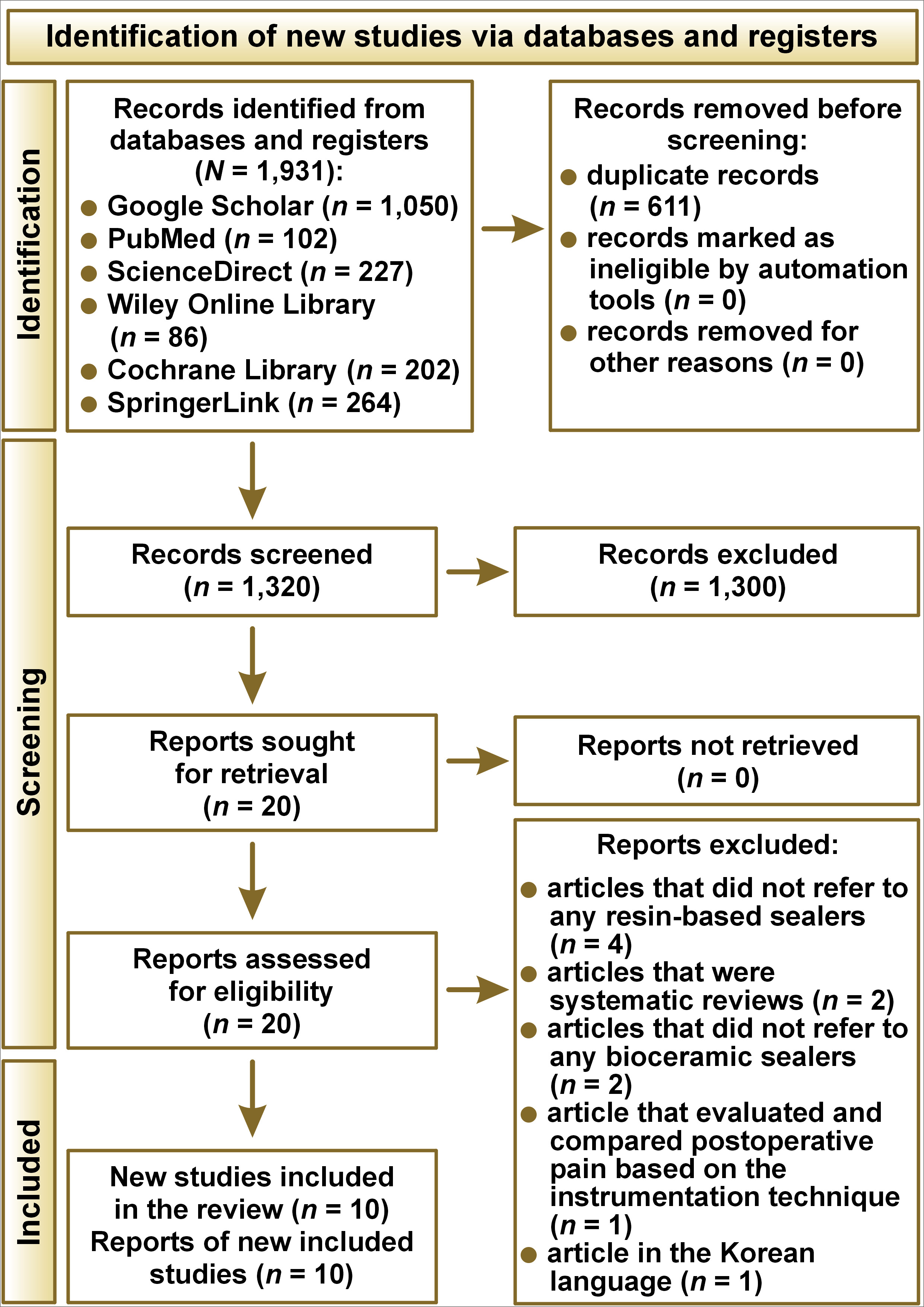
The electronic search was conducted, and a total of 1,931 studies were identified. After removing the duplicates, 1,320 studies were screened for their title and abstracts. Twenty studies were selected for the full-text assessment process, and finally, 10 studies were eligible to be included in this analysis.1, 11, 12, 14, 16, 17, 18, 19, 26, 29 The detailed depiction of search details is given in Figure 1.
Study design and characteristics
A well-detailed presentation of the data extracted from the included studies is given in Table 1
All the studies were published between 2018 and 2021. Nine studies were parallel design RCTs, and one study was a split-mouth controlled trial.16 All the studies were done with ethical approval, but only 2 studies were reported according to the guidelines of CONSORT (Consolidated Standards of Reporting Trials).8, 11 The total number of patients analyzed in the studies included 842 patients. A female predominance was noticed.
The mean age was reported to range from 27.16 to 49.04 years. Among the 10 studies, 4 studies included teeth with necrosed pulp,11, 14, 19, 29 2 studies included teeth that were asymptomatic with irreversible pulpitis,17, 27 1 study included teeth that required retreatment,13 2 studies included both vital and non-vital teeth,1, 18 and 1 study included vital and non-vital teeth, and teeth that needed retreatment.12
The resin-based sealer AH Plus was used as a control group in all included studies for comparison with calcium silicate-based bioceramic sealers. Regarding the instrumentation, 3 studies used Reciproc VDW,1, 17, 19 2 studies used WaveOne Gold,11, 26 1 study used ProTaper Next,18 1 study used ProTaper Gold,14 1 study used both ProTaper Next and WaveOne Gold,29 1 study used ProTaper Universal retreatment files with ProTaper Gold,16 and 1 study mentioned only nickel-titanium rotary files.12
The obturation techniques used varied between the studies – 2 studies used the single-cone technique,17, 19 2 studies used the warm vertical compaction technique,16, 26 2 studies used the lateral compaction technique,14, 29 1 study used the continuous-wave compaction technique (AH Plus sealer) and the single-cone technique (Endoseal MTA),18 1 study used the single-cone and vertical compaction technique,11 1 study filled root canals using the HEROfill Soft-Core obturator system,1 and 1 study used the system B technique for root canal obturation.12
All the 10 studies used VAS (0–10 cm) for the assessment of postoperative pain after endodontic treatment. The pain scores were measured, ranging from 6 h to 1 week. Studies that used scales other than VAS were excluded.
Three studies assessed the incidence of pain within 6 h and 12 hours after the endodontic procedure,1, 17, 26 whereas all 10 eligible studies assessed pain 24 h after the endodontic treatment. Eight studies assessed the pain incidence 7 days after the procedure.11, 12, 14, 16, 17, 18, 19, 29 The highest VAS scores were recorded at 6 h and 12 h after the procedure, followed by 24 h. The pain scores were greatly reduced by 48 h, and there were no significant differences in pain intensity between the groups 72 h after the procedure.
Risk of bias assessment
The risk of bias assessment of the 10 studies presented a low risk of bias in 7 studies,1, 12, 14, 16, 19, 26, 29 whereas 3 studies were judged to raise some concerns.11, 17, 18 The main shortcomings were related to bias due to deviations from the intended intervention. Two studies did not provide sufficient information about the blinding process.17, 18 One study raised some concerns over the randomization process.18 The information on the selection of the reported results was insufficient in 1 study.11 The risk of bias in the 10 studies is summarized in Table 2.
Discussion
This systematic review aimed to assess the occurrence of postoperative pain after root canal treatment performed with two different root canal sealers namely resin-based sealers and calcium silicate-based bioceramic sealers. The occurrence of postoperative pain after endodontic treatment is not attributed to a specific factor and is associated with several other factors, including age, gender, pulpal, and periradicular status, type of teeth treated, pre-operative pain conditions, and the procedure carried out during root canal treatment such as the instrumentation technique used, irrigation protocol, and obturation techniques followed.29
There have been various compositions of root canal sealers developed over the years. The formula of the root canal sealers determines the chemical reactions and properties of the materials. The activation of a local inflammatory response in the periapical tissues is due to the release of chemical mediators, mainly reactive oxygen species (ROS). In vivo studies have shown that oxidative stress is specifically produced by ROS and it has been shown to be linked with inflammatory pain. Whereas, in vitro studies proved that the production of ROS is raised by four to seven times in dental pulpal cells that had been treated with root canal sealers.30, 31
The systematic review also included studies that reported sealer extrusion. Whereas, the results of the eligible studies showed no significant association between sealer extrusion and the occurrence of postoperative pain, irrespective of the type of sealer used.1, 14, 19 Nevertheless, it is justified by the reason that the sealer extrusion was only 1–2 mm in all the cases included in the studies, and none of the cases presented extrusions close to anatomical structures. Therefore, if the vital anatomical structures are not involved, sealer extrusions in small amounts do not give rise to any postoperative complications.1, 14, 19, 32
The calcium silicate-based materials are more biocompatible than the resin-based sealer AH Plus. This is justified by the fact that the cytotoxicity of AH Plus is associated with the release of the component amine and epoxy resin. Further studies showed that there is a release of formaldehyde (3.9 ppm) in small amounts that is attributed to the cytotoxicity of the sealer immediately after mixing. Filter diffusion test and MTT assays revealed little cytotoxic effects even after 24 h of mixing. However, the studies that were conducted clinically provide a contrary result compared to the previously observed in vitro analyses.33, 34, 35
Previous studies showed that bioceramic sealers showed increased flowability than the resin-based sealers and a higher extrusion rate (59.4%) than the AH Plus sealers (28.1%). Conversely, a recent study presented that obturation with AH Plus contributed to significantly more extrusion beyond the apical foramen (62.1%) than bioceramic sealers (47.4%). The studies presented low rates of flare-ups and moderate postoperative pain in the first 48 h. However, further clinical studies are required regarding the same.12, 19, 36
In some of the included studies, it was reported that the presence of pre-operative pain positively influenced the occurrence and incidence of postoperative pain. Seven of the eligible studies included had asymptomatic patients in their study to rule out pre-operative pain, which is an important predisposing factor to the occurrence of postoperative pain. All the included studies in this systematic review showed no statistically significant differences in the incidence of postoperative pain among the studied root canal sealers at various time points. Also, there is no possible correlation between gender or age and postoperative pain at any time point. The most severe pain post endodontic treatment occurred up to a period of 24 h, and this short duration of pain after endodontic treatment is related to the production of ROS due to leakage of unpolymerized components in the root canal sealer during their setting process in the first 24 h. The setting time of AH Plus and bioceramic sealers was found to be about seven hours and four hours, respectively.37, 38, 39
Some of the studies reported the usage of rescue medications when the discomfort was too great for the patient. The results of the eligible studies showed a small proportion of patients requiring anti-inflammatory drugs during the first 24 h of their postoperative period, regardless of the sealer group to which they were allotted.
Pain is produced by both physiological and psychological components. The perception of pain is subjective, and pieces of the literature suggest the use of a rating scale as it is a reliable and effective method for recording pain intensity by the patient. In all our included studies, VAS was used for recording pain scores. For the standardization of analysis, VAS was converted to 0–10 cm. Most of the studies recorded pain intensity starting from 6 h to 7 days.40, 41
The study also presented some limitations that should be addressed. The analysis of pain intensity is only by subjective perception and as it is a distinctive experience for every patient, the results may not represent the entire population group. Further studies are required to assess postoperative pain and complications after sealer extrusions and its influence on oral health-related quality of life (OHRQoL). On the other hand, this is the first systematic review that assesses postoperative pain caused by root canal obturation using resin-based and calcium silicate-based sealers by including a larger number of articles and participants in the quantitative analysis and also assessing the postoperative pain associated with the extrusion of the studied sealers, thereby improving the body of evidence.
Conclusions
Within the limitations of this study, there was no significant difference in the incidence of postoperative pain after root canal treatment using bioceramic sealers compared to resin-based root canal sealers. Also, the results of the eligible studies showed no significant association between sealer extrusion and the occurrence of postoperative pain, irrespective of the type of sealer used. Further studies are required to justify the results obtained in this study, to increase the accuracy, and to determine the causes of postoperative pain after endodontic treatment in several pulpal and periodontal conditions.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.