Abstract
The correct obturation of the root canal system achieved by means of a core and a cement is essential for the success of endodontic treatment. There are several root canal cements (RCCs) on the market; however, because of their excellent characteristics, epoxy resin-based sealers (ERBSs) have been widely used. The main aim of this review was to analyze and integrate the available information on different ERBSs. An electronic search was performed in the PubMed and Scopus databases, using “epoxy resin” AND “root canal treatment”, and “epoxy resin” AND “endodontics” as search terms. In general, ERBSs have good flow properties, film thickness, solubility, dimensional stability, sealing capacity, and radiopacity. They are also able to adhere to dentin while exhibiting low toxicity and some antibacterial effects. However, their main disadvantage is the lack of bioactivity and biomineralization capability. A large number of ERBSs are available on the market, and AH Plus keeps being the gold standard RCC. Yet, information on many of them is limited or non-existent, which could be due to the fact that some of them are relatively new. The latter emphasizes the need for relevant research on the physicochemical and biological properties of some ERBSs, with the aim of supporting their clinical use with sufficient evidence via prospective and long-term studies.
Keywords: root canal sealants, root canal filling materials, AH Plus, epoxy resin-based root canal sealer
Introduction
Three-dimensional obturation of the root space is essential for the long-term success of endodontic treatment. There are various materials and techniques available for obturation of the root space, with most techniques using a central core material and root canal cement (RCC). Regardless of the central core, the use of RCC is essential for hermetic sealing and fluid tightness.1 Currently, there are several types of endodontic sealers available on the market with different compositions, the most common being RCCs consisting of zinc oxide eugenol, calcium hydroxide (Ca(OH)2), glass ionomers, silicone sealers, calcium silicates, methacrylate resins, and epoxy resins,2, 3, 4 even though they do not comply with all the requirements described by Grossman.5 Epoxy resin-based sealers (ERBSs) can be considered the RCC of choice6, 7 for obturation of the root canal system because of their adequate physicochemical properties.7, 8 Most recent studies deal with ERBSs on a general basis2, 9 or approach their properties separately,6, 7, 10, 11, 12, 13, 14, 15, 16 but notably, the present study analyzes, discusses, and integrates the properties of several of these types of RCCs available on the international market, and is the first one to approach their formulation-behavior relationship. This review aimed to analyze and integrate the available data on the different ERBSs, compiling information on the physical, chemical, and biological properties, formulations, and other areas of clinical interest of these RCCs.
Methods
In November 2020, a preliminary search was carried out for literature reviews related to the physicochemical properties of ERBS, and no studies were found that presented an extensive and updated overview of these sealers. In April 2021, an electronic literature search was performed utilizing the PubMed and Scopus databases and the search terms “epoxy resin” AND “root canal treatment”, as well as “epoxy resin” AND “endodontics”, to find studies that contained these search terms that had been published within the last 10 years. A second search was performed in August 2022, to analyze the information pertaining to the ERBS formulation components.
Only original works published in English were included. A total of 604 and 264 manuscripts were found in PubMed and Scopus, respectively. The search was limited to clinical trials, in vitro studies, literature reviews, systematic reviews, and textbook chapters. Interim reports, abstracts only, letters, brief communications, studies that did not focus on ERBSs, and duplicated works were excluded. Additionally, agar diffusion studies and sealability studies, including linear and volumetric dye penetration assessment methodologies, autoradiographic detection of isotope penetration, radionuclide detection, culture techniques to detect bacterial penetration, salivary penetration models, fluid filtration techniques, fluorometry, intracanal reservoir techniques, and electrochemical techniques were also excluded because such studies have not been considered useful since reliable and reproducible evaluation methods related to clinical outcomes are required.17 Subsequently, the titles and abstracts of relevant articles were reviewed and a manual search of the references of each selected article was performed to complement the electronic search. Finally, 91 articles and 6 textbook chapters were considered relevant and included in this review.
Since we only searched two electronic databases, this decision could have limited the results with regard to the inclusion of relevant literature in our review, e.g., grey literature was excluded during the literature search stage. Additionally, being an integrative literature review, the present study has inherent limitations, i.e., the complexity of using diverse selected studies, which apply different methods, has the potential to contribute bias and might, therefore, complicate data evaluation and analysis. However, at the same time, this type of review is the broadest of its kind and has the potential to resolve the complexities brought about by varied perspectives.18
General characteristics
and formulations of ERBSs
Epoxy resin was patented by P. Casta, a Swiss chemist from DeTrey (Zurich, Switzerland), in 1938.9 ERBSs were introduced into endodontics by Schroeder in 1950, with the market launch of AH 26® (Dentsply Maillefer).19 Due to its release of formaldehyde, which causes cytotoxicity in periapical tissues, this sealer has been modified to what is now marketed as AH Plus® (Dentsply Sirona).10, 20 This RCC has been extensively evaluated and compared to other alternatives and, based on its physicochemical properties and biological response, is currently considered the gold standard (Figure 1).21, 22, 23, 24 However, there are other commercially available ERBSs, with different compositions, according to the manufacturer, and are included in Table 1. Based on our performed search, there is no review that integrates information on the characteristics as well as the physicochemical and biological properties of these types of sealers. A compilation of the information on ERBS physical, chemical, and biological properties with highlights of clinical interest is presented in detail below in different sections.
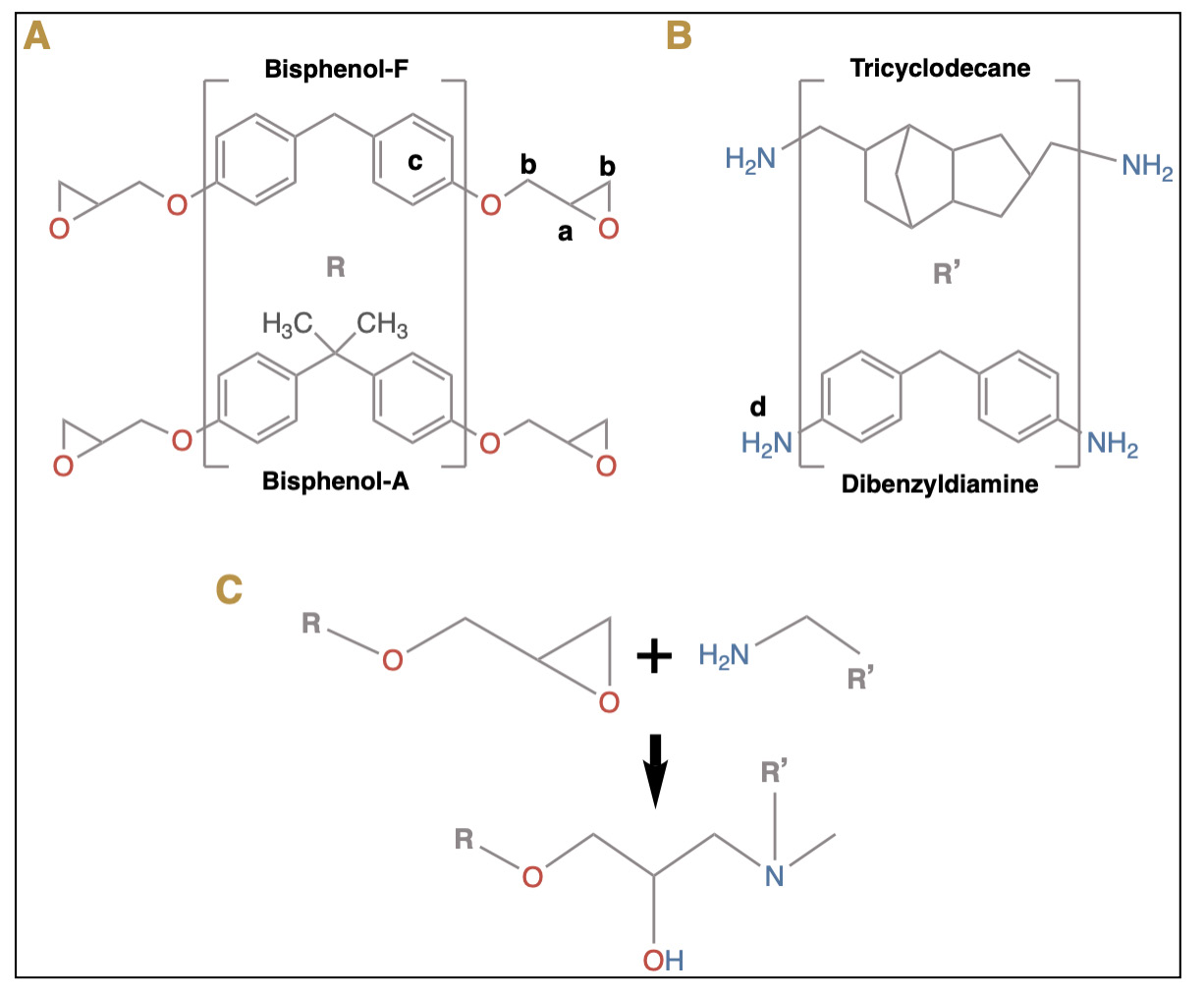
With regard to Table 1, it must be emphasized that all of the listed ERBSs shall finally form an epoxy resin; however, one should take into consideration that the structure of a material results in the formation of its properties, and the latter determines the behavior of the material. In this regard, most commercially available epoxy resins are based on diglycidyl ethers of bisphenol-A, bisphenol-F, or other phenolic compounds,25 which react with curing agents. However, almost none of the ERBSs have the same formulation; thus, in the next paragraphs of this section, we will discuss some relevant compounds in the formulations described in Table 1, alongside various other compounds in subsequent sections.
As for the curing agents listed in Table 1 (which are aliphatic and aromatic amines), epoxy resins may be cured with any of them, because they have a labile hydrogen atom or hydroxyl group that reacts with the epoxy rings and initiates the polymerization process, even at room temperature. However, aliphatic amines are strong skin irritants, while aromatic amines impart higher temperature stabilities.25 AH-26, Sealer 26, Sealer Plus, and Acroseal contain methenamine (hexamethylenetetramine), which releases formaldehyde during the polymerization process, being an inherent disadvantage of these sealers due to its toxicity26, although the released quantity is considered negligible.27 Poly(aminobenzoate) is contained in Adseal, MM-Seal, Sicura Seal, and SimpliSeal and is effective in applications where long working times, substrate wettability, and lower heat-build are required,28 thereby improving the performance in these ERBS. On the other hand, methenamine (which decomposes into formaldehyde and ammonia in an acidic environment) is curiously also used as a food preservative or fraudulently in dairy products.29 Additionally, formaldehyde is a natural by-product of amino acid metabolism in almost all cells, with the endogenous level known to be 3–12 ng/g in tissues30 and 2.5 ppm in plasma.31
On the other hand, Sealer Plus has silicone and siloxanes (the –Si–O–Si–O– backbone of silicones is referred to as siloxane) added to its composition. In this regard, this backbone confers silicones with a very high thermal stability25 and results in the enhancement of flexibility, toughness, durability, and chemical and weather resistance.32 These could be the reasons for their addition to this sealer. AH Plus, Thermaseal Plus, Topseal, Sealer Plus, and 2Seal have silicone oil (polydimethylsiloxane) in their composition, which is hydrophobic in nature, resistant to bacterial degradation, has an extremely low surface tension, and adsorbs strongly to solid surfaces.33 Additionally, it possesses high heat resistance and lubricant properties.34 All these properties would certainly favor the clinical behavior of these sealers.
It has been stated that the most important additive in an adhesive composition is the filler (improving thermal stability, bond strength, and flow properties).25 However, ERBS manufacturers do not specify it in their formulations. In this regard, some sealers (AH Plus, Thermaseal Plus, Topseal, Sealer Plus, 2Seal, and Obturys) contain silica – also defined as silicon dioxide35 – in their formulations. This is an inorganic filler that has exhibited pivotal effects in relation to reducing shrinkage during curing as well as conferring thixotropic properties and improving the bond strength of epoxy adhesives25; it also stimulates osteogenesis by inducing biomineralization.36 Finally, titanium dioxide is a filler in AH26 and Sealer 26 and is widely used to produce a white color in numerous products.37
Acroseal is the only ERBS that contains (three) plant-derived ingredients, i.e., hydrogenated rosin, Venice turpentine, and enoxolone. Hydrogenated rosin (colophony) and Venice turpentine are diterpenic resins with very similar compositions (complex mixtures of resinous acids)38 that enable cross-linking and polymerization in the polymer matrix.39 Both of them possess excellent adhesive properties and are often included as additives in adhesive formulations to increase adhesion, brightness, and toughness.38 Recently, rosin has attracted attention in the formulation of biobased epoxy resins from renewable resources.40 Furthermore, enoxolone (glycyrrhetinic acid) is a bioactive triterpenoid compound of licorice (Glycyrrhiza glabra) that exhibits anti-inflammatory, antioxidant, and anti-nociceptive properties,41 which are ideal for an endodontic sealer formulation.
Oxyranes – also known as epoxides42 – are described in Obturys formulation. They represent one of the new monomers that have been developed to substitute bisphenol A-containing BisGMA dental composites.43 Oxyranes are cyclic ether compounds that are more hydrophobic than methacrylates, and they polymerize via cationic ring-opening processes, which reduces polymerization shrinkage stress.42 Although the latter would benefit the clinical behavior of the ERBS, there are no published studies that have evaluated such issues.
Perma Evolution contains poly(hexamethylenebiguanide)-hydrochloride, which is considered an antibacterial agent44 and could improve the antibacterial properties of this sealer. However, there are no published studies that have evaluated this property. Other ERBS-containing antibacterial agents are described below in the antibacterial section, and those containing Ca(OH)2 are described in the biocompatibility and bioactivity sections.
On the other hand, Sicura seal and SimpliSeal contain ethylene glycol salicylate, also known as 2-hydroxyethyl salicylate, which is formed from the condensation of the carboxyl group of salicylic acid with one of the hydroxyl groups of ethylene glycol.45, 46 Salicylates are very often used in the formulation of topical anti-inflammatory products for the treatment of mild to moderate pain.45, 46 Additionally, derivatives of salicylate resins are used to obtain resins/polymers47 and it has been shown that the flow ability of some sealers is influenced by the type of salicylate resin and its particle size.46
Finally, regarding the ERBS compositions listed in Table 1, it must be taken into consideration that specific processes and/or ingredients for formulating sealers are proprietary of the manufacturer. Moreover, many manufacturers do not provide any information about the composition ratio of these sealers.46 These issues inherently limit an ample discussion on the above-mentioned structure-properties relationship.
Physicochemical properties
The physicochemical properties of ERBSs are described in the sections that follow, and a condensed table of information regarding these properties is presented in Table 2, including their flow, film thickness, solubility, setting time, dimensional change, and radiopacity.
Flow
According to the American National Standards Institute and American Dental Association (ANSI/ADA) No. 57 and The International Organization for Standardization (ISO) 6876, RCCs should have a minimum flow rate of 17 mm.48, 49 Available evidence shows that the sealers AH Plus,11, 12, 20, 21, 50, 51, 52, 53, 54, 55, 56, 57, 58 ThermaSeal Plus,50 Acroseal,11 Adseal,11, 20, 56, 59 EasySeal,53 EZ-Fill Xpress,52 MM-Seal,58 Pherma Evolution,12 Radic Sealer,20 Sealer Plus,21, 51 and SimpliSeal52 meet the established requirements. On the other hand, 1 study evaluated Dia-Proseal and AH Plus (Table 2),59 which fell short of achieving the required values; this difference may be due to the methodology used since the authors mention that more precise evaluation techniques (rheometer) should be used.59
The activation of sealer cements with sonic and ultrasonic protocols has shown an increase in flow values of AH Plus and Adseal, which attained the highest values after ultrasonic activation while still complying with ANSI/ADA No. 57 and ISO 6876 standardizations. The heat generated during this process reduced the viscosity of the sealers, increasing their flow and improving their rheological and mechanical properties, especially their cohesive strength.63 On the other hand, the manufacturer of EZ Fill Xpress recommends that it be warmed using a heated spatula to improve its fluidity.64 However, high flow may result in apical extrusion, possibly leading to periapical tissue injury due to RCC cytotoxicity50 and subsequent postoperative pain.65
Film thickness
ANSI/ADA No. 57 and ISO 6876 suggest that this thickness should not exceed 50 µm.48, 49 Resin-based sealers have shown greater adhesion to dentin in thicker layers. On the contrary, in thin layers, there is greater penetration of the RCC into the dentinal tubules. In this regard, the resin matrix of the cement penetrates the dentinal tubules, while the filling particles do not, due to their larger size, thus leaving a layer enriched with particles but without resin in the canal wall, resulting in a lower adhesion strength.13, 14 These findings suggest that the “ideal” thin film for this type of RCC needs to be reconsidered.14
The sealers AH Plus,11, 52, 53 Easy Seal,53 EZ-Fill Xpress,52 and SimpliSeal52 meet standardizations. On the other hand, 1 study reported values of 85 ±8 µm for the film thickness of AH Plus.55 Acrosel and Adseal obtained values higher than 50 µm (Table 2). Although the different studies comply with the standardized methodology, the available information does not specify the causes of the variations in the results.
Water solubility
Solubility indicates the mass loss of the material when immersed in water. RCCs must have a low solubility.60 The solubility, according to ANSI/ADA No. 57 and ISO 6876, must be less than 3%.48, 49 Conventional methodologies for assessing solubility have some limitations, so micro-computed tomography (micro-CT) imaging methods are currently being used to complement the tests performed by ANSI/ADA No. 57 and ISO 6876.57
The difference in material weight before and after immersion in water may not represent the solubility of all RCCs, as some of these materials may absorb water, even though they exhibit solubility.57 A soluble RCC can degrade and leach chemicals over time, creating voids within the material or at its interface with surrounding tissues/materials.54 These voids could serve as pathways for microorganisms to transverse the root canal into the periapical tissues, while the leaching of chemicals can irritate periapical tissues.53, 54
ERBSs have low solubility,11, 55 which may be due to the strong cross-linking of these RCCs.55, 58 This characteristic is desirable if the stability of the material in the intraradicular space is taken into account but may not be the best property when the material is extruded. The fate of the RCC will depend on its solubility in tissue fluids and its susceptibility to phagocytosis.66, 67 According to a solubility evaluation of AH Plus and Obturys, values of 0.0% and 0.2% at 24 h, respectively, were obtained.60 The solubility studies of AH Plus,21, 51, 53, 54, 55, 56, 57, 58, 59, 60 Topseal,61 Acroseal,11, 61 Adseal,11, 56, 59 AH-26,61 Dia-Proseal,59 EasySeal,53 MM-Seal,58 Obturys,60 Sealer 26,51 Sealer Plus,21 and 2Seal61 meet the standardizations (Table 2).
Setting time
This time should not exceed more than 10% of that indicated by the manufacturer49; however, a sufficiently long time is required to allow the placement and adjustment of the sealing material, which provides a clinical advantage.68 On the other hand, a slow setting time may cause tissue irritation and affect solubility, leading to seal failure,54 and is therefore considered a critical clinical issue.57 The setting time of AH Plus can be affected by the portion of the tube from which the paste is dispensed, i.e., the initial, intermediate, or final segment.15, 55 Thus, it is more fluid at the beginning than at the end, since it is not uniform and its consistency changes along the tube; there is incomplete miscibility between the components, which certainly alters the monomer–catalyst ratios.15 The setting times obtained by different authors are detailed in Table 2. Their high values are probably due to the occurrence of slow polymerization between the amines in the epoxy resin, where the conversion of monomers into polymers occurs gradually.55, 58
One study evaluated how sonic and ultrasonic activation influences the setting times. AH Plus increased its time from 7.71 ±0.02 to 8.63 ±0.24 and 16.52 ±0.12 h, respectively, as these procedures can raise the temperature inside the root canals by up to 2°C. The ultrasonic devices may generate radicals in the organic portion (catalysts) due to increases in temperatures and pressures, generating a slow polymerization reaction.56 On the contrary, Adseal showed the opposite behavior, decreasing the setting time from 4.02 ±0.16 to 2.60 ±0.19 h with sonic and to 2.36 ±0.12 h with ultrasonic activation, which may be related to the different percentages and types of polymerizing agents present in the compositions of these sealers.11, 56
Dimensional change after setting
ANSI/ADA No. 57 standardizations recommend that this value should range from −1% (linear shrinkage) to +0.1% (expansion).48 ERBSs are considered “shrinkage-free” during the setting reaction11; however, their expansion is still possible because they are capable of absorbing water.55 AH Plus,53, 56, 59 Adseal,56, 59 Dia-Proseal,59 and Easy Seal53 did not meet the standard (Table 2). These studies showed increases in dimensional changes, which could be explained by water absorption. However, Adseal showed higher values, owing to its property of high hygroscopicity, which distinguishes it from other cement and could contribute to improving the sealing capacity.59 Another possible explanation for the latter result is the relatively high values of the standard deviation in this study, suggesting measurement inconsistencies. Additionally, the different methodologies used in different studies are prone to errors, as air bubbles may be present in the freshly mixed sealer materials, thus changing their density.53
The existence of voids is of clinical relevance because shrinkage of sealers of as low as 1% can result in voids and spaces that are sufficiently large enough for the penetration of bacteria and their harmful products.69, 70 In a study that evaluated the single cone technique in root canals via micro-CT and nano-CT, AH Plus demonstrated a significantly higher void fraction in terms of internal, external, and combined voids compared to Total BC and Sure Seal, which are calcium silicate-based sealers (CSBSs).69
Radiopacity
ANSI/ADA No. 57 and ISO 6876 standardizations require a radiopacity greater than 3 mm/Al.48, 49 The sealers AH Plus,21, 51, 54, 55, 56, 57, 58, 59, 62 Acroseal,11 Adseal,11, 56, 59 Dia-Proseal,59 MM-Seal,58 and Sealer Plus21, 51 meet the standardizations (Table 2). AH Plus and Sealer Plus have the same radiopacifying agents, namely calcium tungstate, zirconium oxide, and iron oxide,55, 58 while Adseal has bismuth subcarbonate and zirconium oxide, and Acroseal contains only bismuth subcarbonate.11 It has been reported that there is a deposit of radiopacifying agents at the lower end of the tube, while the upper portion may present a lower content,11, 55 which could be due to the above-mentioned incomplete miscibility between the organic and inorganic components contributing to segregation between both phases.
On the other hand, the radiopacity test shows variations in the behavior of the sealers in relation to the activation protocols of AH Plus and Adseal. As regards sonic activation, the variation in radiopacity may be related to greater or lesser exposure to the inorganic compounds present, which can occur randomly and are due to the hydrodynamic movement caused by the sound waves. Application of the ultrasonic protocol increased the radiopacity of AH Plus and reduced that of Adseal, which may be due to the induced changes in the crystal structures of the radiopacifying agents. The cavitation phenomenon, which induces the implosion of air bubbles and causes a local increase in temperature and pressure conditions, in combination with microflows generated by cavitation oscillations, would cause dispersion effects and agglomerate fragmentation in the inorganic components present in the sealers.56
Effects of heat application
Obturation techniques with high temperatures and/or long durations are associated with earlier polymerization, resulting in changes in the chemical structure of epoxy monomers, amine hardeners, and calcium tungstate fillers. These changes are temperature- and time-dependent, and the latter would have a greater impact.63
For AH Plus, it has been reported that heat treatment had an adverse effect on physical properties, such as setting time, which was reduced to 12.9 ±0.7 min when the temperature was raised from 37°C to 140°C for 10 min.71 This reduction may be associated with a change in the setting reaction.72 The flow rate was raised to 25.6 ±0.7 mm when the temperature was raised from 25°C to 140°C.71
In one study, temperatures of 37°C or 100°C for 1 min were used on AH Plus, resulting in a reduction in setting time and an increase in film thickness.73 This ERBS showed a decrease in its N–H groups when heated at 100°C for 1 min, whereby the reduction of polyamines (dibenzyl diamine, aminoadamantane, and tricyclodecane) affected the polymerization process, with changes in the physical and mechanical properties of the material.73 However, the overheating of AH Plus was performed using temperatures above those applied in clinical conditions.72
Adhesion to dentine
The chemical adhesion of epoxy resins to the tooth structure is produced by covalent bonds between the open epoxy groups and the exposed amino groups in the collagen network of the dentin. This is one of the reasons for the good dislodgment resistance of ERBSs.74, 75, 76 Mechanical bonding is provided by the penetration of the cement into the dentin tubules (tags), and its characteristics depend on the physical properties of the RCCs.1
Unlike methacrylate resins, epoxy resins have a lower tag frequency. This may be due to the hydrophilic characteristics of the methacrylate resins as well as their slow chemical reaction, which promotes the reduction of shrinkage stress and allows the sealer to flow more freely, reaching deeper into the dentinal tubules and thus forming a greater number of tags. However, the micromechanical retention of sealers through the penetration of the tags into the tubules is not the most important factor affecting adhesion.77 The higher bond strength of AH Plus, in contrast to its low tag formation, could be explained by the higher prevalence of cohesive failures for this RCC16 in contrast to methacrylate resins that presented mixed or adhesive failures with dentin.77
Factors that can influence bonding strength
Dentin wettability, the use of antimicrobial irrigants and chelating agents
Adhesion can be affected by the condition and degree of wettability of the dentin,78 due to the hydrophobic nature of cements.79 Residual moisture could adversely affect the conversion of the epoxy resin monomer, leading to incomplete polymerization of the resin and decreased bond strength to dentin.78, 79 The use of sodium hypochlorite (NaOCl) may affect the adhesion of ERBSs if it is used as a final irrigant.80, 81 Traces of this strong oxidizing agent or its oxidative by-products, such as hypochlorous acid and hypochlorite ions, would also compromise the bond strength of the sealer to root dentin and its sealing capacity.80 Another logical reason for this is that oxygen bubbles, which form after the use of NaOCl, impede the penetration of the sealer into the fine openings of the dentin tubules.80
Evidence shows that the final irrigation with EDTA 17%, SmearClear, and QMiX promoted proper smear layer removal, which ensured the adequate bond strength of AH Plus.82
Laser
Laser application is another type of treatment of the dentin surface that can influence the bond strength of the RCC.83 A study on the effect of chemical treatments and the use of lasers on the bond strength revealed that citric acid had a higher average bond strength compared to the Er:YAG laser for RealSeal, AH Plus, and EndoREZ sealers, but not Acroseal.84 On the contrary, EDTA activation with Nd:YAG (1,064 nm) and diode (980 nm) lasers resulted in better bond strength of the ERBSs at the level of all root canal thirds compared with EDTA alone and EDTA with ultrasonic agitation. The application of these wavelengths, together with EDTA activation, could increase the permeability of the root dentin.85
Filling techniques
The highest values of bond strength have been observed using the lateral condensation technique (LCT) and Tagger’s hybrid technique (THT).86 Similar results were obtained in another study wherein the strengths of the bonds to human dentin using AH Plus/gutta-percha (GP), Sealer 26/GP, Epiphany SE/Resilon, and Epiphany SE/GP root canal filling materials, when LCT or THT were used, were evaluated by means of push-out tests. The highest push-out forces were obtained when the canals were obturated using LCT with AH Plus and GP, followed by Sealer 26 and GP.87 On the other hand, the lowest bond strengths were found with the continuous wave condensation technique, which could be explained by the presence of a thin cement layer, although the micro-CT images showed better results regarding the filling quality.86
Considering the need for heat to obtain a positive result in thermoplasticized GP techniques, a systematic review compared these techniques to cold lateral condensation, using micro-CT to evaluate the quality of root canal filling.88 Although it was evidenced that neither technique could completely obturate the root canal, thermoplasticized techniques did have significantly fewer voids in most studies, which is clinically desirable. It is relevant to point out that six out of the nine included studies used ERBSs.88
Retreatment
Once the sealer penetrates the dentin tubules, its removal during retreatment is physically impossible89; therefore, no filling material can be completely removed.90, 91 Several studies have evaluated the retreatability of CSBSs compared to AH Plus, showing that the former achieved better results with less RCC residues and shorter retreatment times.90, 91 On the other hand, obturation with BC Sealer and a single GP master cone may result in blockage of the apical foramen and a loss of permeability in some cases, which is not the case for AH Plus obturation. The inability to regain working length and/or permeability may compromise retreatment by preventing adequate cleaning and shaping of the apical canal space, which may harbor bacteria. There is also evidence of retreatability for AH Plus and EndoSequence BC sealer, as they showed similar characteristics during retreatment procedures.89
The use of GP solvents like xylene and Endosolv E has been evaluated demonstrating a negative effect on the bond strength of AH Plus to the root canal. These solvents can change the chemical composition of the dentin surface because they are oil-based, making it difficult to remove them completely from the root canal. This waxy film may interfere with the development of resin–dentin bonds.92
Biological properties
Biocompatibility (cytotoxicity)
RCCs have demonstrated severe inflammation, but over time, most sealers lose their irritant components and become relatively inert.22, 93 In cases wherein RCCs are extruded, they may be solubilized in periradicular tissue fluids, phagocytized, or become encapsulated by fibrous connective tissue.66 In a study, only 15% of cases with AH Plus extrusion have shown complete clearance of the material over periods of even 10 years.66
The cytotoxicity of an ERBS seems to be directly related to its component epoxy resin and to the type of polymerization promoted by the amines, with the waste products of this reaction being toxic to cells.4 It has been suggested that ERBSs containing bisphenol A diglycidyl ether can produce cytotoxicity upon release since it is a mutagenic component of these materials.10, 93 These cements could release small amounts of formaldehyde, which could explain their short-term toxicity.4, 22, 93 AH Plus also has a greater release of calcitonin gene-related peptide compared to EndoSequence, which indicates a greater potential for causing pain and neurogenic inflammation.89
In the case of SimpliSeal, its calcium oxide and calcium phosphate components could contribute to its improved biocompatibility. On the other hand, although Sealer Plus has a similar composition to AH Plus, the addition of Ca(OH)2 in its composition improved its histological results, leading to mild inflammation at 7 days.22
As for Sicura Seal, bisphenol A diglycidyl ether is not included in its composition; however, exudates or polymerization and/or degradation products may cause increased cytotoxicity.93 The cytotoxicity of AH-26 occurs mainly in the first hours after polymerization since this sealer contains hexamethylenetetramine, which decomposes into ammonia and formaldehyde, which have shown significant cytotoxic effects.10
Antimicrobial effects
RCCs seem to have some degree of antimicrobial activity due to their composition. This effect is time-dependent, and it is unknown whether it can prevent reinfection of the root canal system in the long term.94 In this regard, the development of RCCs that have long-term antibacterial properties has been suggested to prevent potential reinfection.94, 95, 96 In recent years, there have been attempts to modify RCCs with antimicrobial nanoparticles, antibiotics, and antiseptics to endow them with such properties, but with minimal or no impact on their physicochemical properties. However, studies used different methodologies to evaluate these effects which precludes the possibility of direct comparisons.94
The incorporation of a small percentage of quaternary ammonium polyethylenimine (QPEI) nanoparticles into AH Plus95, 96 and an experimental ERBS97 have exhibited a strong antibacterial effect on species such as E. faecalis found in dentinal tubules.95, 96, 97 In addition, it has been proven that adequate physical properties are maintained in the experimental cement with added QPEI.97 The use of quaternary ammonium-based compounds and functionalized nanoparticles seems promising as an approach for conferring bacterial inhibition. Nevertheless, the safety of nanoparticles for human body systems and tissues must first be confirmed before proceeding with their clinical use.94
Bioactivity/Biomineralization
A bioactive material has the ability to create a hydroxyapatite (HA) layer when it is in contact with calcium- and phosphate-rich tissue fluid.98 The pH level, along with the release of calcium ions, are closely involved in this process.21 Sealers with calcium oxide or Ca(OH)2 included in their composition have the ability to dissociate into calcium and hydroxyl ions, which could lead to an increase in the local pH and the formation of mineralized tissues.21 The release of hydroxyl ions, or even the release of calcium ions, depends on the material’s area of contact with tissue fluids and its chemical characteristics (hydrophilic or hydrophobic), the presence of calcium-containing substances, the setting time, and the solubility.21, 99
Based on these biological events, and with the goal of promoting biochemical conditions that accelerate tissue recovery,100 nanostructured fillers of synthesized bioactive glass (BAG), HA, fluoride substituted hydroxyapatite (FHA),7 and magnesium hydroxide,101 among others, have been incorporated into AH Plus. ERBSs such as Acroseal,11 Sealer Plus,21 Sealer 26,11 Dia-Proseal,59 and Obtuseal102 have Ca(OH)2 within their composition. However, due to some of the physicochemical properties that each of them possesses, they are not able to release sufficient hydroxyl ions or calcium to promote mineralization. Thus, 1 study analyzed the results of Sealer Plus, in which it was determined that its extremely short setting time in conjunction with its low solubility precludes the release of hydroxyl ions21; meanwhile, Acroseal showed the longest setting time, but its calcium release was lower compared to Sealapex due to the presence of its insoluble epoxy base, so it did not demonstrate bioactivity either.99
BAG and HA nanostructured fillers represent a promising approach, as they improve the in vitro capacity of ERBSs for apatite formation, while FHA particles do not improve apatite layer formation.7 As for magnesium hydroxide, it has been found to adequately stimulate bone mineralization, and it has been mentioned that it would be an ideal additive to achieve bioactivity in cements such as AH Plus, as it causes greater osteoblastic differentiation compared to calcium ions.101
ERBSs vs. CSBSs
Recently, CSBSs have been introduced in the market as a new class of RCCs.103 Their biological properties, such as high alkalinity sealing capacity, antibacterial properties, as well as bioactive induction of periapical healing and hard tissue formation,23 as well as their fine particle structure and ability to set in wet environments,103 have been highlighted as their main advantages over conventional sealers.24 Considering these properties, a recent study suggested that GuttaFlow® Bioseal could even represent a promising material for root-end filling as it showed progressive healing, better tissue organization, and a reduction in the inflammatory response.104
We are facing a paradigm shift in obturation approaches, in which the objective is no longer only to provide a hermetic seal against bacteria and the reinfection of the root canal but, rather, to establish a more biological concept of obturation, in which CSBSs could become the most important sealers in coming years.23 However, the number of formulations available on the market, the lack of relevant information on CSBSs in the literature, their high solubility compared to ERBSs,6 as well as the unavailability of long-term clinical studies105 prevents the recommendation and positioning of these RCCs as the gold standard in the field of root canal obturation.
Finally, if we consider that bioactivity and biomineralization are the desired properties in an RCC, perhaps the time has come for sound analysis, e.g., a position statement on this issue and a modification of the requirement list of an ideal sealer as originally proposed by Grossman.5 In fact, some authors have already listed the capacity to be bioactive as an ideal criterion.9
Highlights of clinical interest
Shake sealer cements before use.
Discard the initial portion of the dispensing tube, as it may alter the flow, the setting time and radiopacity.
The ultrasonic activation of ERBSs can help to seal anatomical complexities. Take care of sealer extrusion.
ERBS have low solubility, so they are more stable, thus showing fewer spaces and voids, which could affect long-term clinical results.
ERBS can be used in controlled-heat obturation techniques with minimal changes in their chemical structure.
These sealers can be used with LCT and THT, obtaining higher bond strength values and, with the continuous wave condensation technique, show better results in terms of filling quality.
According to present evidence, when using the single cone technique, ERBS may not be a good option, owing to their higher void fraction, as opposed to CSBSs.
The use of ERBS is highly compatible with irrigation protocols that use chelating agents as the final irrigant, prior to root canal drying.
The use of oily solvents should be avoided during retreatment.
Extrusion should be avoided, as it may cause some degree of short-term cytotoxicity.
Conclusions
Despite the large amount of commercially available options for endodontic obturation, the “ideal” material has not yet been identified. This has led to the development of several obturation materials and experimental sealers incorporating nanoparticles and conferring them favorable physicochemical properties, such as increased antibacterial efficacy and bioactivity, which may lead to a concept transformation from a purely preventative cement into a biologically active one.
In general, the ERBSs have good flow properties, film thickness, solubility, dimensional stability, sealing capacity, and radiopacity. They are also able to adhere to dentin while exhibiting low toxicity and some antibacterial effects. However, their main disadvantage is their lack of bioactivity and biomineralization capability. AH Plus sealer, which has been extensively studied, is still considered the gold standard and has become the most important representative of a considerable number of sealer formulations based on epoxy resins, some of which, at present, even lack scientific evidence. The latter emphasizes the need for relevant research on the physicochemical and biological properties of some ERBSs, with the aim of supporting their clinical use with sufficient evidence via prospective and long-term studies. Finally, clinicians and researchers should consider formulation components of the different ERBSs to understand the characteristics and properties of these types of RCCs.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.