Abstract
Background. Nano-silver fluoride (NSF) has been introduced to improve enamel lesions. The effective use of varnishes is important in the prevention of dental caries.
Objectives. The study aimed to compare the effect of conventional sodium fluoride varnish with the same varnish containing 1% and 2% silver nanoparticles (AgNP) on the surface microhardness of enamel.
Material and methods. The baseline surface microhardness of 40 premolar teeth was measured using a Vickers microhardness tester. After immersing the samples in a demineralizing agent for 24 h, the microhardness was measured again. In group B, a layer of conventional fluoride varnish was applied to the tooth surfaces using a microbrush with soft bristles, following the manufacturer’s instructions. Groups C and D were treated with 1% and 2% NSF varnishes, respectively, while group A received no varnish. Surface microhardness tests were conducted on all specimens, including those previously tested.
Results. The microhardness of the enamel surface increased significantly in all 3 test groups compared to the microhardness after demineralization (p < 0.05).
Conclusions. Conventional fluoride varnish and fluoride varnishes containing 1% and 2% AgNP are equally effective in remineralizing initial caries.
Keywords: dental enamel, dental caries, fluorides, silver, varnish
Introduction
Dental caries is one of the most common chronic diseases that varies in severity among the population.1 Recent studies have reported an increase in the incidence and prevalence of dental caries in deciduous and permanent teeth.2 This medical condition is a dynamic process, with intermittent demineralization and remineralization. Repeated exposure to cariogenic challenges result in the formation of incipient enamel lesions that may progress, remain unchanged or remineralize over time.3
Prevention is crucial in the context of dental caries, especially in children, given the necessity for high dexterity on the part of the dentist and the potential for general anesthesia, which can result in considerable costs.1 Additionally, treatments to arrest caries have some advantages, such as minimal discomfort, minimal caregiver education requirements, ease of application, low costs, and non-invasiveness compared to restorative procedures for carious lesions.4
Fluoride is one of the materials applied topically in dental offices to prevent lesions. It is available in different forms, including solutions, gels, varnishes, foams, and prophylactic pastes.5 Among these, dentists tend to prefer 5% fluoride varnish due to its ease of use, fast application, low risk of ingestion, and patient acceptance.6 Fluoride varnish is a standard remineralizing agent that increases the contact time between the fluoride and the tooth surface and serves as a reservoir for slow fluoride release.7, 8 Fluoride prevents dental caries by inhibiting the dissolution of the mineral content of the tooth by adsorption to the crystalline surfaces, inducing remineralization of the crystalline surfaces by forming a fluorapatite layer that is highly resistant to acid attacks, and inhibiting bacterial metabolism by inhibiting the activity of necessary enzymes.9 A 5% sodium fluoride varnish contains 226,000 ppm of fluoride and is used to inhibit dental caries.10
Several studies have evaluated the incorporation of silver nanoparticles (AgNP) into materials used in dentistry and have shown that these particles can enhance the properties of dental materials. For example, Haghgoo et al. suggested the use of nano-silver fluoride (NSF) under amalgam restorations to reduce microbial counts and prevent recurrent caries.11 In a study conducted by Nozari et al., NSF was very useful in the remineralization of the enamel of primary teeth.12 However, Akyildiz and Sönmez reported that NSF was not as effective as sodium fluoride varnish and silver diamine fluoride (SDF) on enamel carious lesions.13
Considering the abovementioned discrepancies and the importance of more effective use of varnishes in the prevention of dental caries, this study aimed to compare the effect of conventional sodium fluoride varnish and the same varnish containing 1% and 2% AgNP on the microhardness of the enamel surface.
Material and methods
This in vitro experimental study was conducted in the dental material laboratory of Shahid Beheshti University of Medical Sciences (Tehran, Iran) and the protocol was approved by the ethics committee of Shahid Beheshti University of Medical Sciences (approval No. IR.SBMU.RIDS.REC.1396.486). The study included 40 premolar teeth that had been extracted for orthodontic reasons within the previous 6 months and were free of caries and fissures. The extracted teeth were stored in deionized water containing 0.1% thymol to prevent dehydration and microbial growth until testing.
Before testing, the teeth were cleaned with fluoride-free pumice paste (Nupro Prophylaxis Paste; Dentsply Sirona, Charlotte, USA) and distilled water to remove any calculi, plaque and soft tissue remnants, using a slow-speed handpiece for 10 s. Then, paper stickers, measuring 3 × 3 mm, were cut with the use of a mm-graduated ruler and a sharp scalpel blade, and affixed to the buccal surfaces of the samples. All other areas were covered with anti-acid nail polish (MODA Cosmetics, Instanbul, Turkey) in 2 stages. The teeth were mounted in transparent self-cured acrylic resins (Acropars; Marlic Medical Ind. Co., Tehran, Iran) inside a cylindrical plastic mold, with their buccal windows exposed. Each enamel block was polished to achieve a smooth surface using 1,000- and 1,200-grit silicon carbide abrasive paper (MATADOR GmbH & Co.KG, Remscheid, Germany).
To prepare fluoride varnish containing 1% and 2% AgNP, 40-nm AgNP was added to fluoride varnish in the amount of 1% and 2%, respectively, and manually mixed in a plastic container using a mixer with a plastic head for 1 min to achieve a uniform consistency. Then, the enamel blocks were randomly assigned to 4 groups: group A (no treatment); group B (conventional fluoride varnish); group C (fluoride varnish with 1% AgNP); and group D (fluoride varnish with 2% AgNP).
The enamel blocks were immersed in 15 mL of a demineralizing solution (2.2 mM CaCl2, 2.2 mM NaH2PO4 and 0.05 M acetic acid; pH: 4–4.5 adjusted with NaOH and HCl) for 24 h to simulate dental caries. After demineralization, the surface microhardness of all samples was measured using a Vickers hardness tester (Zwick Roell, Worcester, England) with a 50-gr force applied for 10 s. The microhardness of each sample was determined at 3 points within the enamel block window. Finally, the mean value was calculated. After determining the baseline microhardness of each sample, the samples were stored in distilled water until the next stage of the study.
In the next stage, group B’s tooth surfaces were covered with a thin layer of conventional fluoride varnish using a small microbrush with soft bristles, according to the manufacturer’s instructions. In the same manner, 1% and 2% AgNP-containing fluoride varnishes were applied to the tooth surfaces in groups C and D, respectively. No treatment was administered to group A.
After the varnish dried, the samples were placed in separate containers with distilled water and incubated at 37°C for 24 h. Then, the teeth underwent a pH cycling procedure to simulate the caries process. First, the samples in each group were immersed in a demineralizing solution in separate containers for 3 h, followed by a mild rinse in distilled water. The samples were then immersed in distilled water for 30 min. Next, the samples were immersed in a remineralizing agent (15 mM CaCl2, 0.9 mM NaHPO4 and 0.15 mM KCl; pH: 6.5–7) without sodium fluoride, at 15 mL/tooth. Then, the samples were briefly rinsed in distilled water and immersed in distilled water for 30 min. The 24-hour cycles were repeated for 7 days. The demineralizing and remineralizing solutions were refreshed daily, and all procedures were carried out in an incubator at 37 ±2°C.
At the end of the 7th day, if the remnant layer of varnishes remained after washing, it was removed with a scalpel. The microhardness of the teeth in all 4 groups was reassessed by an operator who was blinded to the groups. The Vickers microhardness tester was used with a 50-gr force for 10 s at 3 points in each sample, and the mean values were calculated.
Statistical analysis
The data was analyzed using the IBM SPSS Statistics for Windows software, v. 22.0 (IBM Corp., Armonk, USA). The mean and standard deviation surface microhardness values in different study groups were calculated before treatment, after the first demineralization cycle, after the application of varnish, and after pH cycling at the end of the 7th day. The normality of data was confirmed using the Shapiro–Wilk test. Analysis of variance (ANOVA) and post hoc Tukey’s tests were used to compare the surface microhardness at different intervals in each group and between the groups. The statistical significance level was set at p < 0.05.
Results
The present study analyzed 40 extracted human premolar teeth, which were divided into different treatment groups (no treatment, conventional fluoride varnish, fluoride varnish containing 1% AgNP, and fluoride varnish containing 2% AgNP). Table 1 displays the mean and standard deviation values as well as changes in the enamel surface microhardness for each study group at different time intervals.
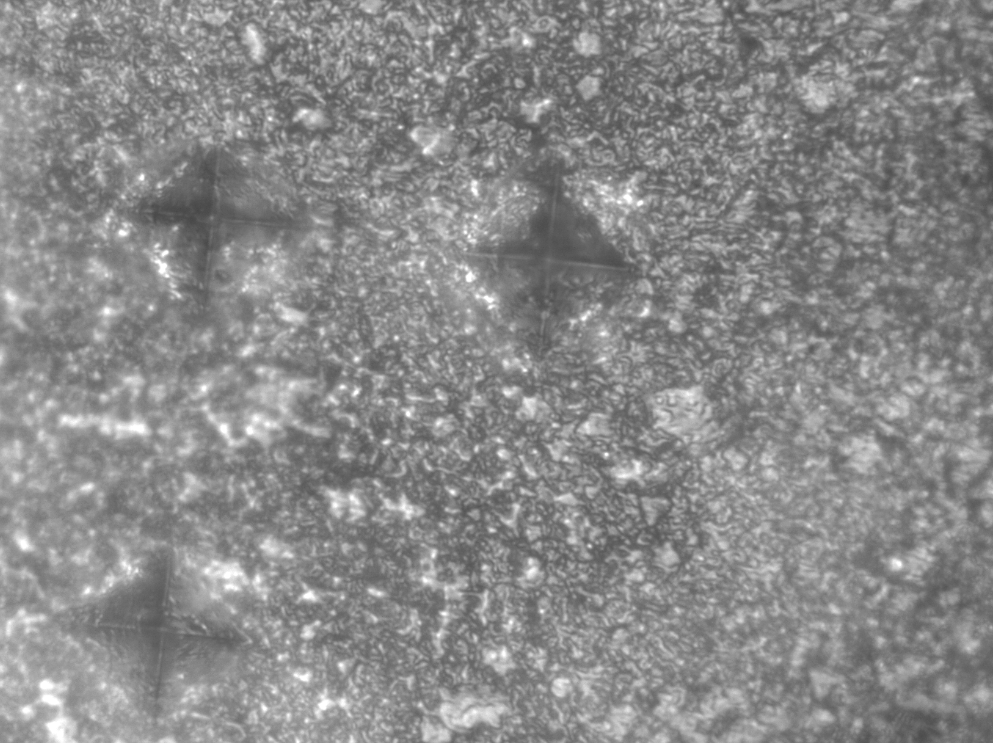
Vickers microhardness images of the selected samples before and after demineralization are shown in Figure 1 and Figure 2.
The study found significant differences in the surface microhardness between the 3 time intervals in the control group (p < 0.0001), as confirmed by ANOVA. The results of Tukey’s test demonstrated significant differences in the surface microhardness before demineralization, after demineralization and after treatment. However, there was no significant difference in the surface microhardness between 2 intervals, after demineralization and after treatment in the control group (p = 0.12). Figure 3 presents the Vickers image of a sample from the control group after the application of the study cycles.
In the conventional fluoride varnish group, significant differences in the surface microhardness were found between the 3 time intervals (p < 0.0001), as confirmed by ANOVA. The results of post hoc Tukey’s test indicated significant pairwise differences between the 3 time intervals (p < 0.0001). Figure 4 presents the Vickers image of one of the samples from this group after treatment.
In the fluoride varnish group with 1% AgNP, ANOVA showed significant differences in the surface microhardness values between the 3 time intervals (p < 0.0001). The results of post hoc Tukey’s test indicated significant pairwise differences between the 3 time intervals. Figure 5 presents the Vickers image of a sample from this group after treatment.
In the fluoride varnish group with 2% AgNP, ANOVA revealed significant differences in the enamel surface microhardness between the 3 time intervals (p < 0.0001). The results of post hoc Tukey’s test indicated significant pairwise differences between all 3 time intervals. Figure 6 presents the Vickers image of a sample from this group after treatment.
Table 2 presents pairwise comparisons of changes in the enamel surface microhardness between the study groups, along with the relevant p-values. The results of ANOVA indicated significant differences in these changes between the 4 study groups (p = 0.004), after confirming the normal distribution of data. The results of post hoc Tukey’s test revealed significant differences in these changes between the control group and all the other groups. However, no significant differences were found regarding these changes between the 3 study groups.
The evaluation of Vickers images revealed that the severity of demineralization and the porosities formed in the control group were higher than in the other groups.
Discussion
Despite recent advances in orodental care, dental caries remains a significant health problem.14, 15 Research is currently underway to develop higher quality remineralizing agents for carious lesions. One of the new materials that has been introduced for this purpose is AgNP. Therefore, this study aimed to evaluate the effect of incorporating AgNP at 1 and 2 wt% in fluoride varnish on the remineralization of enamel lesions. The enamel surface microhardness was determined in different groups to compare the effects of different varnish products on the remineralization of enamel lesions. The surface microhardness is a reliable index for evaluating fluoride effects and overall tooth mineralization.16, 17, 18
The 5% fluoride varnish is one of the most effective agents for remineralizing incipient enamel caries.14, 19, 20 In order to improve its efficacy, several agents, including calcium phosphate salts and xylitol, have been incorporated into it. Fluoride varnish used in the present study contained 5% sodium fluoride, tricalcium phosphate and xylitol at a fluoride concentration of 22,600 ppm. Rirattanapong et al. demonstrated the effectiveness of fluoride varnishes containing tricalcium phosphate in inhibiting the progression of incipient enamel lesions.21
Recently, a study evaluated the effect of adding silver compounds to fluoride for the improvement of enamel lesions. For this purpose, 2 forms of silver particles are available.22 One form is the silver ion, which is used in commercial SDF solutions and is directly applied to carious lesions. Clinical studies have shown that it is successful in inhibiting and preventing dental caries through 2 mechanisms.23 First, SDF has a direct antibacterial effect. Second, it reacts with hydroxyapatite and forms fluorapatite.24 However, a study by Mohammadi and Farahmand Far showed that although there was a decrease in the enamel surface microhardness after the use of SDF solution compared to fluoride varnish, the difference was not significant. Both were equally effective in preventing demineralization of the enamel in anterior primary teeth.10 Besides, the use of SDF has been associated with certain disadvantages, such as dark discoloration of the teeth, painful lesions that may appear within 48 h and a metallic taste.24, 25, 26
Another form of silver particles, AgNP, has recently attracted a great deal of attention.27 These nanoparticles are as effective as the SDF solution and do not cause brown discoloration of teeth. In addition, they can penetrate the bacterial matrix and disrupt some cellular processes, such as DNA transcription, especially in gram-negative bacteria.22
In the present study, 1 and 2 wt% of AgNP were added to conventional fluoride varnish to evaluate their effect on the enamel surface microhardness. The use of these nanoparticles in combination with fluoride varnish significantly increased the enamel surface microhardness after demineralization.
Based on the study results, the null hypothesis stating the lack of effect of adding AgNP to fluoride varnish on the enamel surface microhardness was rejected. All 3 fluoride varnish products were equally effective in remineralizing incipient enamel lesions.
Mares-Garcia et al. reported that remineralization with fluoride varnish containing AgNP was more effective than with fluoride varnish without AgNP for treating white spot lesions in trisomy 21 patients.28 Nozari et al. also found that the enamel surface microhardness was significantly higher after the application of NSF compared to fluoride varnish and nano-hydroxyapatite.12 However, Akyildiz and Sönmez reported that the enamel surface microhardness was higher after demineralization in the sodium fluoride varnish and SDF groups compared to the fluoride–AgNP group. They concluded that fluoride varnish with AgNP was not as effective as sodium fluoride varnish and SDF in treating enamel carious lesions.13 Additionally, in our study, there were no significant differences between fluoride varnishes with and without AgNP. Differences in the results of the studies may be attributed to differences in the structure of the evaluated enamel. For example, Nozari et al. evaluated primary anterior teeth,12 while Akyildiz and Sönmez13 and the present study evaluated permanent third molar and premolar teeth, respectively. Primary teeth have more unstructured enamel compared to permanent teeth.6 Therefore, AgNP can penetrate the enamel structure more efficiently and increase the surface microhardness by precipitating within the lesions.4, 12 It appears that the use of AgNP for the remineralization of enamel lesions in permanent teeth is less effective than in primary teeth due to the higher mineral content of permanent teeth.
We examined the effect of NSF on enamel initial caries by evaluating its impact on the surface microhardness. The treated samples exhibited higher surface microhardness values compared to the control group. This finding is consistent with the results of studies by Teixeira et al. and El-Desouky et al., who showed that NSF was more effective in increasing remineralization after pH cycling than the negative control samples.29, 30
El-Desouky et al. concluded that NSF and fluoride varnish are equally effective anticariogenic materials in limiting enamel demineralization caused by cariogenic challenges.30 This is in agreement with our results and proves both NSF and fluoride varnish effective in enhancing remineralization of enamel with no significant differences.
Similarly, Teixeira et al. found no statistically significant difference between a NSF-containing dentifrice and a sodium fluoride dentifrice in preventing demineralization. However, the study did show that sodium fluoride resulted in a lower percentage of microhardness variation.29 These results can be explained by the different pH cycling protocols used and the repeated application of dentifrice slurries before each pH cycle, which could have accentuated the effects of the materials used.
The results of the present in vitro study may be different when carried out in the presence of the dynamic interactions occurring at the tooth surface in the oral cavity, such as the presence of the biofilm and oral flora, variations in salivary components, individuals eating habits, and oral hygiene practices. In addition, the present study was limited to a period of 7 days, while demineralization and remineralization are long-term processes.
Conclusions
Based on the results of this in vitro study, conventional fluoride varnish and fluoride varnish containing 1 and 2 wt% of AgNP are equally effective in remineralizing initial caries. However, the results of this study are based solely on surface microhardness readings and need to be clinically validated.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of Shahid Beheshti University of Medical Sciences (approval No. IR.SBMU.RIDS.REC.1396.486).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.