Abstract
Background. Type 2 diabetes mellitus (DM) is a known systemic risk factor for periodontitis. An increased expression of CD44 has been suggested in type 2 diabetics and periodontitis patients.
Objectives. The present study aimed to assess the expression of CD44 antigen in patients with chronic periodontitis (CP) and type 2 DM in a South Indian urban population. Additionally, the relationships between the expression of CD44 antigen in gingival tissues, periodontal clinical parameters, and the random blood sugar (RBS) and glycated hemoglobin (HbA1c) levels were assessed.
Material and methods. A total of 63 subjects were divided into 3 groups: systemically and periodontally healthy controls (group H); CP patients, otherwise healthy (group CP); and CP patients with type 2 DM (group CP+DM). Periodontal parameters were recorded for all groups, and additionally the RBS and HbA1c levels for group CP+DM. Gingival tissue samples were obtained and subjected to immunohistochemical analysis for CD44.
Results. The expression of CD44 was significantly higher in the diseased groups. Epithelial CD44 expression was significantly stronger in group CP+DM as compared to groups CP and H (p < 0.001), whereas connective tissue CD44 expression was similar in groups CP and CP+DM (p = 0.657). Furthermore, an inverse relationship was observed between blood glucose parameters and CD44 expression in the epithelium and connective tissue.
Conclusions. The expression of CD44 increased with the severity of periodontal disease. Additionally, glycemic control in patients with CP and type 2 DM had an impact on CD44 expression. Our findings indicate a possible destructive role of CD44 in the pathogenesis of periodontal diseases in individuals with type 2 DM.
Keywords: diabetes mellitus, hyaluronan receptors, immunohistochemistry/methods, periodontitis, etiology
Introduction
Diabetes mellitus (DM) is a chronic non-communicable metabolic disorder afflicting the global population, characterized by abnormal insulin secretion and activity.1 The American Diabetic Association (ADA) recognizes chronic periodontitis (CP) as the 6th complication of type 2 DM, along with 5 others, namely retinopathy, neuropathy, nephropathy, macrovascular disease, and altered wound healing.2, 3 These complications are direct consequences of hyperglycemia.4 The hyperglycemic state activates innate immunity, which leads to an increased release of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis factor alpha (TNF-α).4, 5 Chronic hyperglycemia facilitates anaerobic infection in tissues by inhibiting the pathways of inflammation resolution, with the inflammation increasing insulin resistance by dysregulating glycemic mechanisms. Hyperglycemia can diminish the synthesis of extracellular matrix (ECM) components and connective tissue remodeling by fibroblasts and osteoblasts. The interaction between advanced glycation-end products (AGEs) and their receptors (RAGEs) leads to the formation of free radicals. This alters the relationship between connective tissue cells and their matrix, as well as capillary integrity.6, 7
CD44 is a type 1 transmembrane glycoprotein expressed in various cell types, including epithelial, endothelial, lymphoid, and myeloid cells, and fibroblasts. The molecule is responsible for a wide array of functions, including the cell–cell and cell–matrix interactions during inflammatory tissue remodeling and regeneration.8, 9 The glycosaminoglycan hyaluronan (HA) is a key ligand that binds to CD44. The receptor–ligand interaction leads to enhanced immunological events, thus playing a significant role in chronic inflammatory diseases.10 CD44 is known to be strongly expressed in the epithelium and connective tissue of patients with type 1 and 2 DM and CP.11
India has the second largest diabetic population after China. It has been reported that the advancement of pre-diabetes to diabetes is more rapid in urban Indian populations.12 Given that type 2 DM is a recognized systemic risk factor for CP and periodontitis is a recognized complication of uncontrolled type 2 DM, we hypothesized that the dysregulation of CD44 expression in individuals with type 2 DM plays a substantial role in this bidirectional pathogenesis involving periodontitis patients.13, 14 As such, this study aimed to evaluate the expression of CD44 antigen in patients with CP and type 2 DM in a South Indian urban population. Additionally, we assessed the relationships between the expression of CD44 antigen in gingival tissues, periodontal clinical parameters, and the random blood sugar (RBS) and glycated hemoglobin (HbA1c) levels. The null hypothesis stated that there are no differences in CD44 antigen expression between individuals diagnosed with CP and those with CP and type 2 DM.
Material and methods
This cross-sectional study was conducted at the Department of Periodontics, SRM Dental College, Chennai, India, between October 2011 and January 2013. The study was approved by the Institutional Scientific and Ethical Review Board before commencement (No. SRMU/M&HS/SRMDC/2010-13/M.D.S-PG student/504). All subjects received the explanation of the study purpose and procedures, and provided written informed consent to participate. The study was conducted prior to the release of the new periodontal disease classification in 2018.15 Hence, we continue using terms such as CP throughout this paper. However, according to the new classification, we would have referred to Stage 3 or 4 and Grade B.15
Study population
A total of 63 subjects aged 20–70 years (mean age: 43.1 years) were recruited for the study based on the selection criteria. The study participants were divided into 3 groups: group H – systemically and periodontally healthy control subjects (7 males and 14 females; mean age: 30.90 ±8.98 years); group CP – patients with CP who were otherwise healthy (6 males and 15 females; mean age: 43.90 ±10.22 years); and group CP+DM – patients with CP and type 2 DM (10 males and 11 females; mean age: 54.33 ±10.91 years).
Inclusion criteria
Group H included subjects with no clinical evidence of gingival inflammation, the sulcus depth ≤3 mm and no clinical attachment loss (CAL). Group CP included patients with a diagnosis of CP based on the American Academy of Periodontology (AAP) 1999 Classification of Periodontal Diseases and Conditions16; they had at least 20 teeth, with the probing pocket depth (PPD) ≥4mm, CAL ≥ 3mm and bleeding on probing (BOP). Group CP+DM recruited patients with self-reported DM with a minimum duration of 6 months. Additionally, the RBS and HbA1c levels were assessed to include patients with HbA1c ≥ 6% of total hemoglobin.
Exclusion criteria
Patients with known systemic diseases other than DM, smokers, those with a history of periodontal treatment in the last 6 months, those using antibiotics or anti-inflammatory drugs in the past 6 months, and pregnant and lactating women were excluded. In addition, patients with normal HbA1c levels were excluded from group CP+DM.
Clinical protocol
Full-mouth clinical parameters, including the plaque index (PI), the gingival index (GI), PPD, and CAL, were recorded. A single calibrated examiner recorded PPD and CAL using a UNC 15 periodontal probe. Following the examination, the patients from group CP+DM were evaluated for their blood sugar levels, and the eligible participants had gingival samples collected at the subsequent appointment.
Gingival sample collection
In group H, gingival samples were collected during surgical crown lengthening procedures and tooth extraction for orthodontic purposes. In groups CP and CP+DM, the samples were collected from areas of Grade 3 tooth mobility, resulting from severe periodontitis and indicated for extraction.
Gingival biopsies were performed with blade No. 15 under adequate local anesthesia (2% lignocaine with 1:80,000 adrenaline), using an internal bevel incision. The samples were washed with a sterile saline solution and immediately fixed in 10% neutral buffered formalin (NBF). The biopsy specimens were embedded in paraffin blocks. The blocks were sectioned to a thickness of 4 µm, mounted on poly-L-lysine-coated glass slides, incubated at 37°C for 1 day, and further incubated at 58°C for 1 h before deparaffinization.
The paraffin-embedded sections were deparaffinized, rehydrated and treated with 3% hydrogen peroxide at 37°C for 30 min to block endogenous peroxidase activity. Then, they were rinsed with phosphate-buffered saline (PBS) for 5 min and treated in a microwave oven with Tris-EDTA buffer (pH 6.0) for 10 min for antigen retrieval.17 The slides were cooled to room temperature for 20 min, rinsed with distilled water for 5 min and washed twice with PBS (pH 7.6) for 5 min. Next, they were placed in 3% hydrogen peroxide for 15 min and washed with PBS for 5 min. To prevent non-specific reactions with other tissue antigens, the slides were treated with Power Block™, which contains casein and proprietary additives, in PBS with sodium azide 15 M for 15 min. The sections were then incubated with the CD44 primary antibody at room temperature for 1 h. After rinsing 3 times with PBS for 5 min, the sections were incubated with the biotinylated secondary antibody at room temperature for 30 min. The specific reaction for each antibody was visualized using 3,3’-diaminobenzidine. The sections were then counterstained with Mayer’s hematoxylin, air-dried, cleared in xylene, and mounted on slides with the use of the dibutylphthalate polystyrene xylene (DPX) medium.18
The immunodetection of CD44 was performed with the use of BioGenex Super Sensitive™ Detection Systems (BioGenex, Chennai, India). The immunohistochemistry (IHC) procedure was conducted in the Oral Pathology Laboratory, SRM Dental College, Chennai, India.
Immunohistochemistry image analysis
The mounted sections were observed under a light microscope (Olympus BX51; Olympus, Tokyo, Japan) to evaluate immunostaining in epithelial and connective tissue cells. Positivity was determined based on the brown stain of diaminobenzidine. The quantitative estimation involved counting the number of CD44-positive cells in more representative areas under light microscopy at ×400 magnification, using the Image-Pro Plus software, v. 6.3 (Media Cybernetics, Inc., Rockville, USA).18
Statistical analysis
Statistical analysis employed SPSS Statistics for Windows, v. 17.0 (SPSS Inc., Chicago, USA). The normality of data distribution was assessed using the Kolmogorov–Smirnov test. Since the values were normally distributed, parametric tests were used for further analysis. The parameters in this study were expressed as mean ± standard deviation (M ±SD). A p-value ≤0.05 was considered statistically significant. To compare continuous variables between the groups, the one-way analysis of variance (ANOVA) was used, with Tukey’s post hoc test for multiple comparisons. The Pearson correlation coefficient was used to determine correlations between variables.
Results
Demographics and clinical characteristics
The study involved 63 adults aged 20–70 years. Table 1 presents the demographic data and clinical characteristics of the participants. Periodontal parameters and age were higher in periodontitis patients with DM (group CP+DM) as compared to patients with periodontitis alone (group CP).
CD44 expression
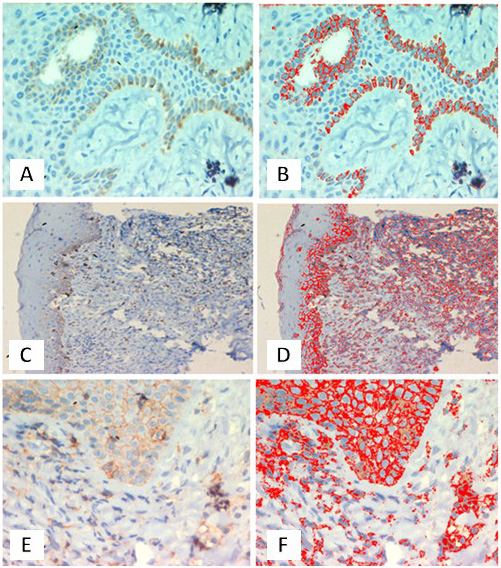
All groups exhibited CD44 immunopositivity (Table 2). Figure 1 shows representative IHC images of CD44 expression. Positive staining for CD44 was observed in the basal layer of the epithelium. Strong cytoplasmic positivity was evident in basal and parabasal cells of healthy controls (group H) (Figure 1A,B). In patients with CP but without DM (group CP), strong positive staining for CD44 was observed in the basal layer of the epithelium and the stromal cells of the connective tissue, specifically in lymphocytes (Figure 1C,D). In patients with both CP and DM (group CP+DM), stronger positive staining for CD44 was observed in most cells of the epithelial layer, suggestive of hyperplasia. Dense positivity was also noted in many cells of the connective tissue, specifically in lymphocytes and endothelial cells (Figure 1E,F).
Although all groups showed immunopositivity, the diseased groups exhibited a higher CD44 expression (Table 2). There was a significantly stronger epithelial CD44 expression in group CP+DM as compared to group CP (p < 0.001), whereas the expression of CD44 in the connective tissue was similar in groups CP and CP+DM (p = 0.657) (Table 3).
The relationships between CD44 expression and periodontal parameters are presented in Table 4. There were statistically significant positive correlations between clinical parameters (PI, GI, PPD, and CAL) and CD44 expression in the epithelium and connective tissue (p < 0.001). We further observed a statistically significant inverse relationship between connective tissue CD44 expression and blood glucose parameters (RBS and HbA1c) (p < 0.001). Epithelial CD44 expression did not show any significant correlation with the RBS and HbA1c levels, although a negative correlation was observed.
Discussion
This cross-sectional investigation analyzed the expression of CD44 in the gingival tissue samples obtained from CP patients with and without DM, from a South Indian urban population. There is mounting evidence for the rising prevalence of diabetes in both urban and rural populations of India.19 The largest national study of diabetes, funded by the Indian government, involved 15 states and reported the spread of a diabetes epidemic among those with a lower socioeconomic status within urban populations.12, 19
The current study used an immunohistochemical technique to assess CD44 expression. The findings showed strong CD44 immunopositivity in periodontal disease groups. There was a significant increase in the expression of CD44 in epithelial cells of CP patients with type 2 DM as compared to CP patients without DM and healthy controls. Furthermore, the expression of CD44 in the stromal connective tissue was higher in the diseased groups as compared to healthy controls. However, there was no significant difference between the diseased groups with and without type 2 DM, although an inverse relationship was observed between CD44 expression and the blood glucose levels.
Our findings are in agreement with previous reports on CD44 expression in gingival tissues of CP patients with type 2 DM.11 Elevated levels of soluble CD44 have also been reported in the saliva of smokers with periodontitis.20, 21 Another report indicated elevated CD44 in peri-implantitis as compared to peri-implant mucositis, suggesting a strong role of CD44 in the inflammatory response.22 The current study observed positive staining for CD44 in the basal layer of the epithelium, with strong cytoplasmic positivity evident in basal and parabasal cells of healthy controls, which is in agreement with a previous report.23 Furthermore, an increased expression of CD44 in the epithelium was noted in group CP+DM as compared to group CP, which could be due to the release of pro-inflammatory mediators, such as TNF-α, in response to AGEs. A previous in vitro study demonstrated an increased CD44v6 expression in human gingival epithelial cell lines when they were stimulated with pro-inflammatory mediators, such as TNF-α and interferon gamma (IFN-γ).24 The current findings of an increased CD44 epithelial expression contradict a previous report that did not find any difference in epithelial CD44 expression,11 though this could be attributed to the different populations investigated and methods used.
The current study found an increase in connective tissue CD44 expression among CP patients, indicating that CD44 is involved in the recruitment of inflammatory cells at the site of periodontal inflammation, as reported in a previous study.25 Additionally, HA is a key ligand for CD44 in ECM. The CD44–HA complex can mediate adhesive interactions between lymphocytes and gingival fibroblasts, and such interactions may provide intracellular cell activation signals that regulate proliferative T cell responses and stimulate the production of pro-inflammatory cytokines. Furthermore, CD44 is involved in healing by mediating the interactions between platelets and the subendothelial tissue; it has been suggested that platelets express CD44 on their surface to facilitate HA binding in the subendothelial tissue during wound healing.26 The current findings support a possible role for CD44 in the wound healing of tissues affected by inflammatory destruction. A thorough understanding of the role of the CD44–HA complex may help in effective periodontitis management.27
Type 2 DM is a metabolic condition known to cause endoplasmic reticulum stress, which further induces the deposition of HA. Furthermore, HA plays a role in retaining inflammatory cells at the site of inflammation via CD44 receptors, which may explain an increased expression of CD44 receptors in type 2 DM patients and excessive inflammatory tissue destruction.28, 29 The present study observed an inverse relationship between the blood glucose levels and CD44 antigen expression among CP patients with type 2 DM, which could be explained by well-controlled diabetes.
Animal experiments have shown that in some autoimmune diseases, such as rheumatoid arthritis and type 1 DM, blocking CD44 receptors can affect downstream adaptive and innate immune interactions, impeding further accumulation of inflammatory cells and worsening of the condition.30 Our findings suggest that dense CD44 positivity in connective tissue lymphocytes can be attributed to the activated CD44 participation in lymphocyte trafficking to the inflammatory sites, and may indicate impending episodes of hyper-inflammatory or autoimmune-type responses.8 Based on this premise, it can be speculated that there is an autoimmune-type response in periodontal tissues of diabetic patients due to the excessive expression of these receptors, which may be a potential therapeutic target.31
The strengths of this study include reporting the immunolocalization of CD44 in gingival tissues by using IHC, and having adequate power in comparison with previous studies. The findings contribute to a better understanding of the pathogenesis of CP in type 2 DM patients in a South Indian urban population with a high prevalence of DM.
Limitations of the study include the lack of assessment of the principal CD44 ligand, HA, and other CD44 isoforms, which could have provided a better insight into the exact mechanistic links in these patients.
The 2018 classification of periodontal diseases categorizes periodontitis based on staging and grading.15 The new framework has provided us with the ability to introduce potential validated biomarkers for improved case definition.32 In this context, it would be interesting to see whether CD44 could be considered a validated biomarker in the future.
India is undergoing a significant shift from communicable diseases to non-communicable diseases, such as DM, with the trend of increasing prevalence demonstrated by the results of several studies conducted in Chennai. The development of diagnostic and prognostic biomarkers for DM in a dental setting will strengthen the ability of the healthcare system to prevent complications associated with the disease.32 Understanding diabetic patients of an Asian Indian phenotype can promote advancement in preventive and therapeutic strategies in medical and dental settings.
Conclusions
To date, the role of CD44 in the pathogenesis of periodontitis and type 2 DM remains unclear. The current study rejected the null hypothesis, suggesting the possibility of a shift in the role of CD44 from protective to destructive in the host response of CP patients with DM as compared to those with CP alone. CD44 could essentially serve as a diagnostic biomarker and a therapeutic target in the future. However, longitudinal studies with a robust design are necessary in this area. This study adds to the existing evidence and suggests that blocking CD44 with CD44 antagonists might prove beneficial as a novel therapeutic strategy in the management of periodontal diseases, especially in patients with DM.
Ethics approval and consent to participate
The study was approved by the Institutional Scientific and Ethical Review Board before commencement (No. SRMU/M&HS/SRMDC/2010-13/M.D.S-PG student/504). All subjects provided written informed consent to participate in the study.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.