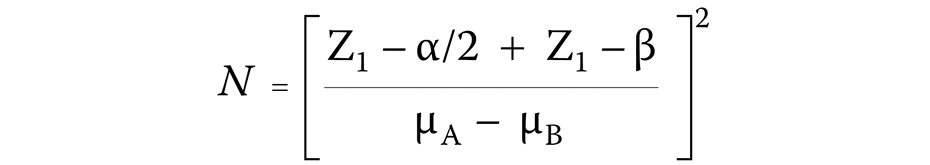Abstract
Background. Chemical plaque control with mouthwashes as an adjunct to mechanical plaque control with a toothbrush and dental floss has been considered an effective method for controlling gingivitis. The anti-inflammatory effects of chemical plaque control benefit the oral tissues by reducing inflammation and bleeding.
Objectives. The aim of the present study was to evaluate and compare the clinical efficacy of probiotic, Aloe vera, povidine-iodine, and chlorhexidine (CHX) mouthwashes in treating gingivitis patients by assessing changes in their clinical parameters.
Material and methods. This prospective study was conducted on 40 patients from our outpatient department, divided into 4 groups of 10 patients each: probiotic mouthwash group (group 1); herbal (Aloe vera) mouthwash group (group 2); povidone-iodine mouthwash group (group 3); and CHX mouthwash group (group 4). All participants were provided with the same type of manual toothbrush, the Pepsodent® toothpaste and a respective mouthwash for twice-daily use until the end of a 28-day observation period. Clinical parameters, such as the marginal plaque index (MPI) and bleeding on interdental brushing (BOIB), were recorded at baseline, and on the 14th and 28th day of the study period.
Results. All groups showed a significant decrease in the MPI and BOIB scores. The results were similar in patients who used a probiotic mouthwash and those who used a CHX mouthwash. A comparable change in the mean scores was observed among the herbal and povidone-iodine groups from baseline to day 28.
Conclusions. In the treatment of chronic gingivitis patients, a probiotic mouthwash was nearly as effective as CHX in reducing the plaque and bleeding scores. It showed better results in all clinical parameters than herbal and povidone-iodine mouthwashes. Using a mouthwash along with routine tooth brushing can help in treating gingivitis and slow the progression of the periodontal disease.
Keywords: chlorhexidine, gingival inflammation, probiotic mouthwash, Aloe vera mouthwash
Introduction
Dental caries and periodontal diseases are the most prevalent oral diseases worldwide. Periodontal diseases are inflammatory in nature and exist in 2 forms – reversible gingivitis and irreversible periodontitis. Dental plaque with various microorganisms makes up the prime factor in the initiation and progression of periodontal diseases, leading to severe destruction of the tooth-supporting structures.1 Hence, maintaining plaque control is an essential part of routine oral hygiene, as dental diseases in their initial phase are primarily halted through regular and precise plaque removal.2
Various plaque control measures are applied in routine oral hygiene. Mechanical plaque control is considered the first line of periodontal therapy, accompanied by oral hygiene instruction.3 The mechanical removal of plaque with a toothbrush and dental floss has been considered an effective method for controlling gingivitis. Nevertheless, achieving adequate brushing time, efficient cleaning of all tooth surfaces and regular oral hygiene can be challenging due to variations in oral health practices among individuals. This accounts for the high prevalence of gingivitis. Therefore, adjunctive chemical plaque control methods, such as using mouthwashes and probiotics, have been suggested as additional therapeutic strategies.2
Choosing the best mouthwash is often difficult for both patients and practitioners, given the availability of several products with various active ingredients. Chlorhexidine (CHX) is the most potent anti-plaque agent, but it has several downsides.4 The need for a safe and effective alternative to a CHX mouthwash has led to the development of a number of oral care products that are low-cost, readily available and free from significant adverse effects. When used in mouthwashes, natural herbs, povidone-iodine and probiotics have demonstrated significant benefits, similar to CHX.5
The use of herbs for dental care is prevalent in indigenous systems of medicine. Herbs such as Terminalia chebula, Aloe vera, Azadirachta indica, Piper betle, and Ocimum sanctum have antibacterial, ulcer-healing, anti-plaque, and anti-halitosis properties. The Aloe vera extract helps reduce plaque formation owing to its anti-inflammatory, antioxidant, antibacterial, antiviral, and antifungal properties, and thus can be regarded as oral hygiene aid in managing periodontal diseases.6
Povidone-iodine is an iodophor that has a broad-spectrum antimicrobial effect on bacteria, viruses, fungi, and protozoa. It delivers free iodine to the bacterial cell membrane, which reduces plaque formation, and eventually the severity of gingivitis and radiation-induced oral mucositis.7
Probiotics have been identified as a potential area of research in periodontal care. Various studies have demonstrated that probiotics can shift the balance of the oral microbiota toward beneficial species, thereby reducing gingivitis.4 The World Health Organization (WHO) defines probiotics as “live microorganisms which, when administered in adequate amounts in food or as dietary supplements, confer a health benefit on the host.”4 Probiotics repopulate healthy bacteria, which can help destroy pathogenic organisms and prevent the disease. Replacing pathogenic bacteria with beneficial ones has gained acceptance in recent years due to the growing global problem of antibiotic resistance. Oral probiotics have caused a paradigm shift in the field of periodontal healthcare, offering an alternative approach to reducing the prevalence of oral microbiome-mediated diseases like gingivitis.
The purpose of this randomized controlled clinical study was to evaluate the effects of probiotic, herbal (Aloe vera) and povidone-iodine mouthwashes in the treatment of chronic periodontitis (CP) patients, in comparison with the gold standard, a CHX mouthwash.
Material and methods
Trial design
This study was designed as a four-pronged randomized controlled trial (RCT) with a 1:1:1:1 allocation ratio. It was conducted at the Department of Periodontology, Sibar Institute of Dental Sciences, Takkellapadu, India, between February 2021 and April 2021. The study was approved by the institutional research ethics committee (ethical clearance No. Pr.2115/IEC/SIBAR(UG)2021), and was conducted in compliance with the ethical standards established by the World Medical Association (WMA) in the Declaration of Helsinki. Each patient was given a detailed verbal and written description of the study, and provided signed consent to participate in it.
Sample size calculation
The sample size was calculated using the following formula (Equation 1):
where:
N – sample size;
Z1 – Z-value;
α – level of significance;
β – level of power; and
μA − μB – mean difference between the samples.
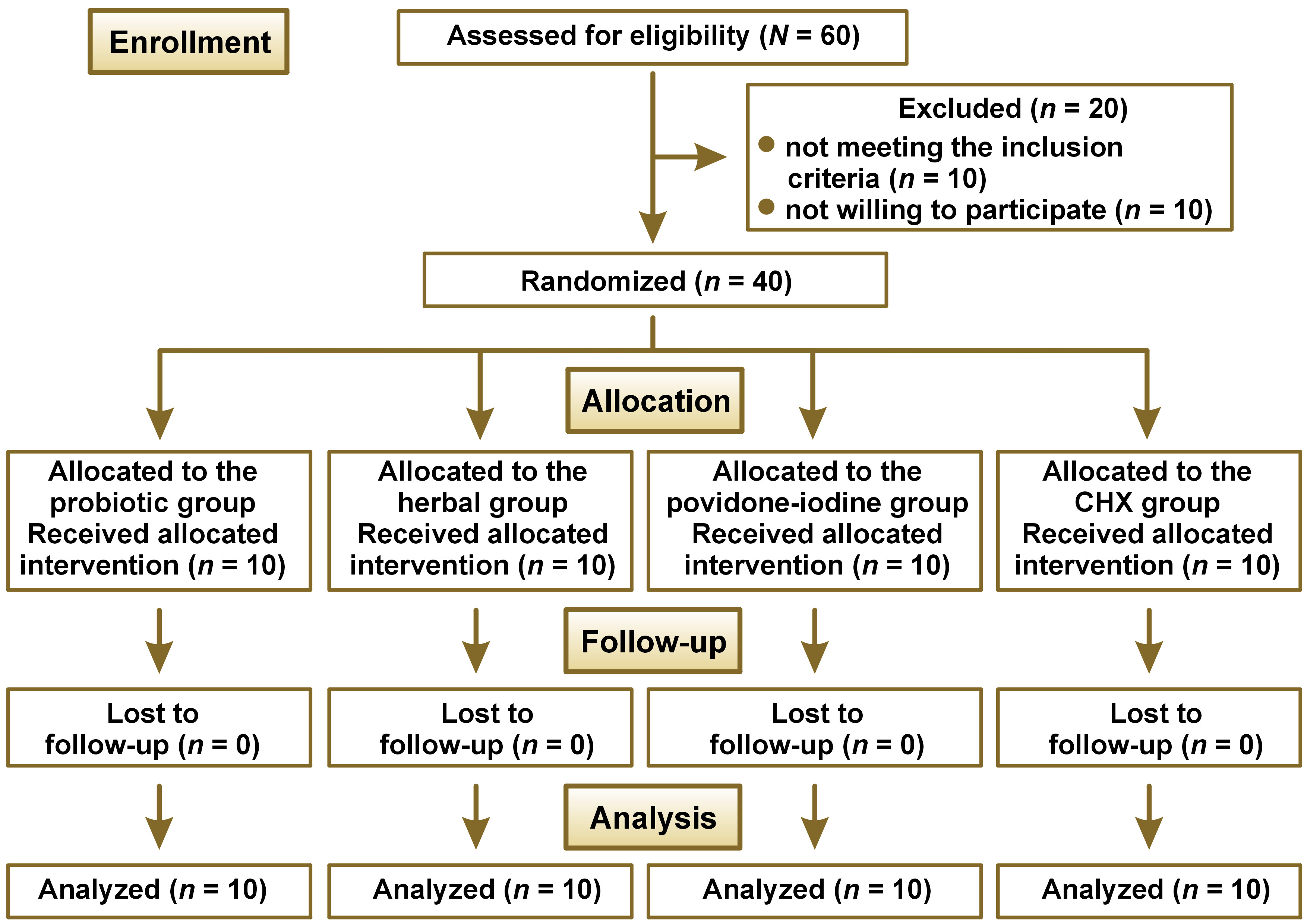
As per the calculation, a minimum of 10 participants was required to achieve a power of 80% and detect differences in the mean plaque and bleeding scores between the 4 study groups by the end of a 28-day period. Therefore, the study recruited a total of 60 patients to account for potential dropouts. The study followed the Consolidated Standards of Reporting Trials (CONSORT) statement (Figure 1).
Eligibility criteria
The trial participants were selected randomly from among individuals who reported for consultation at the Department of Periodontology, Sibar Institute of Dental Sciences, Takkellapadu, India, according to the following eligibility criteria: patients within the age range of 18–45 years; of both genders; with no history of allergies to the components used in the study; and who were systemically healthy. The study excluded patients who had habits such as smoking, tobacco chewing or alcohol consumption, as well as those who used drugs in any form, were systemically compromised, were pregnant or lactating, or were unable to attend follow-up visits.
Randomization and blinding
The selected 40 subjects were randomly assigned to groups, using the Research Randomizer software, v. 2.0 (https://www.randomizer.org).
Blinding of the patients to the intervention was maintained throughout the trial.
Interventions
Forty patients, aged 18–45 years, were randomly divided into 4 groups of 10 patients each: probiotic (Darolac® sachets; Aristo Pharmaceuticals, Vijayawada, India) mouthwash group (group 1); herbal (Aloe vera) mouthwash group (group 2); povidone-iodine mouthwash group (group 3); and CHX mouthwash group (group 4). All participants were instructed to brush their teeth twice daily with the same type of manual toothbrush for effective plaque removal. They were provided with the Pepsodent® toothpaste and a respective mouthwash for twice-daily use until the end of the observation period.8
Estimation of clinical parameters
Following the initial screening and oral prophylaxis, clinical parameters, such as the new marginal plaque index (MPI) and bleeding on interdental brushing (BOIB), were recorded at baseline, and on the 14th and 28th day of the study period.
New marginal plaque index
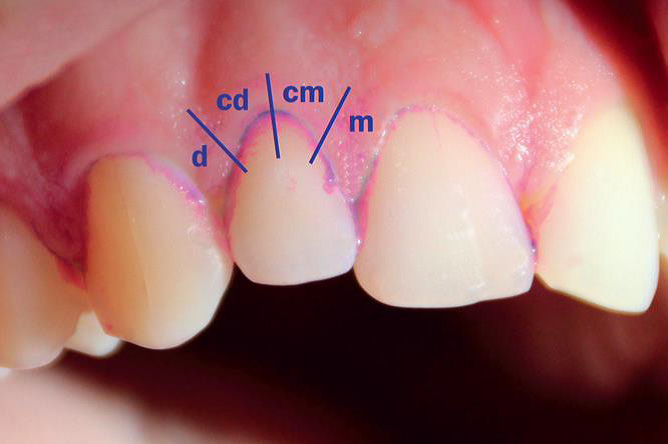
Plaque was assessed at the proximal and cervical sections of the gingival margin. Plaque deposits were identified using a disclosure solution that stains old plaque deposits blue and fresh deposits pink.
The new MPI, proposed by Deinzer et al. in 2014,9 assesses the presence (score 1) or absence (score 0) of plaque within 8 equal areas of a tooth (4 at the vestibular and 4 at the oral gingival margin). The gingival margin of each site (vestibular and oral) is divided into 4 equal areas: 1) distal; 2) cervico-distal; 3) cervico-mesial; and 4) mesial. Eight measures were recorded per tooth: 1) vestibular distal; 2) vestibular cervico-distal; 3) vestibular cervico-mesial; 4) vestibular mesial; 5) oral distal; 6) oral cervico-distal; 7) oral cervico-mesial; and 8) oral mesial. These measures can be combined to obtain all MPI values as the overall mean of all sections scoring 1, the MPI proximal values (i.e., the percentage of distal plus mesial sections scoring 1) and the MPI cervical values (i.e., the percentage of cervico-distal plus cervico-mesial sections scoring 1). The index also enables separate aggregation of recordings at the vestibular and oral sites (Figure 2).
Bleeding on interdental brushing
In 2010, Hofer et al. developed the BOIB index.10 The measurement involves inserting a light interdental brush buccally, just below the contact point, and gliding between the teeth in a jiggling motion without force. Bleeding is scored as either present or absent for each interdental site after 30 s. The number of sites with bleeding on probing is noted.
Outcomes
The mean changes in the plaque and bleeding scores were evaluated among the 4 groups before and after the intervention, from baseline to day 28.
Statistical analysis
Statistical analysis employed IBM SPSS Statistics for Windows, v. 23.0 (IBM Corp., Armonk, USA). The data obtained from the clinical evaluation is presented as mean and standard deviation (M ±SD). The MPI and BOIB parameters were analyzed using the one-way analysis of variance (ANOVA) and the post hoc test for pairwise comparisons (Tukey’s honestly significant difference (HSD) test). For all tests, a p-value <0.05 was considered statistically significant.
Formulation of hypotheses
The null hypothesis (H0) states that there is no difference in clinical parameters with regard to various treatment modalities. The alternate hypothesis (Ha) suggests that there is a difference in clinical parameters with regard to various treatment modalities.
A p-value <0.05 was considered statistically significant. If the obtained p-value is <0.05, the null hypothesis can be rejected and the alternate hypothesis considered.
Results
Pre-treatment equivalence
Forty patients were randomly assigned to one of the 4 groups (n = 10 patients per group), using a 1:1:1:1 allocation ratio, between February 2021 and April 2021. All patients were included in the statistical analysis, and no patients were lost during follow-up (Figure 1). The mean age of the patients in the probiotic, herbal, povidone-iodine, and CHX groups was 28.5 ±7.0, 30.8 ±6.7, 25.7 ±8.1, and 27.4 ±4.2 years, respectively (Table 1).
Clinical parameters were recorded at baseline, and on the 14th and 28th day for all patients. The baseline clinical parameters were not significantly different between the groups, indicating that all groups were evenly matched at the beginning of the study (Table 1). The final differences were not influenced by the initial defect characteristics, allowing the post-treatment results to be compared.
None of the patients in any of the groups exhibited any adverse effects from the agents used.
Clinical parameters
Clinical parameters –MPI and BOIB – were recorded at baseline, on the 14th day and on the 28th day. Any differences in the mean scores of the indices were recorded at baseline and on the 28th day for all groups.
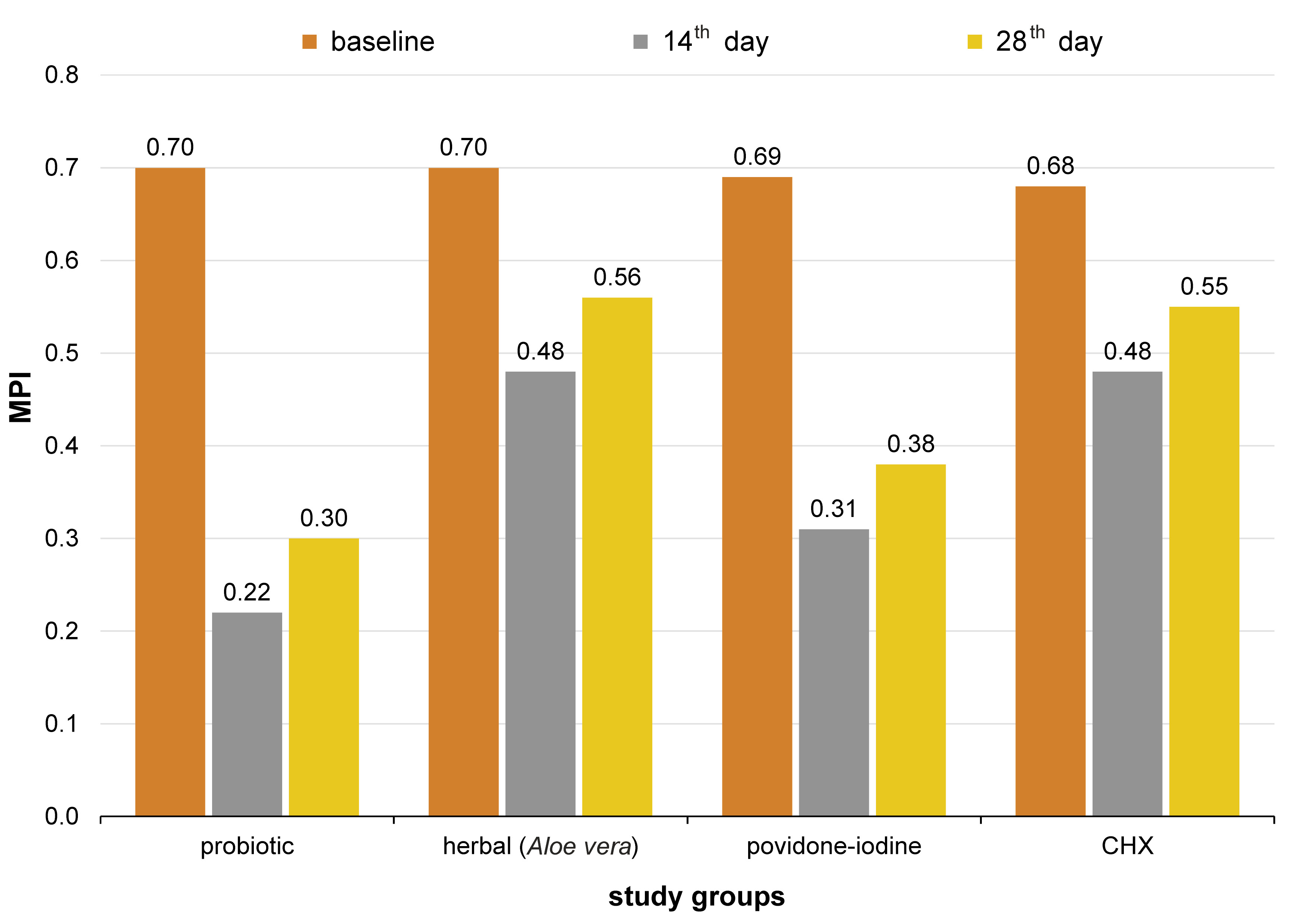
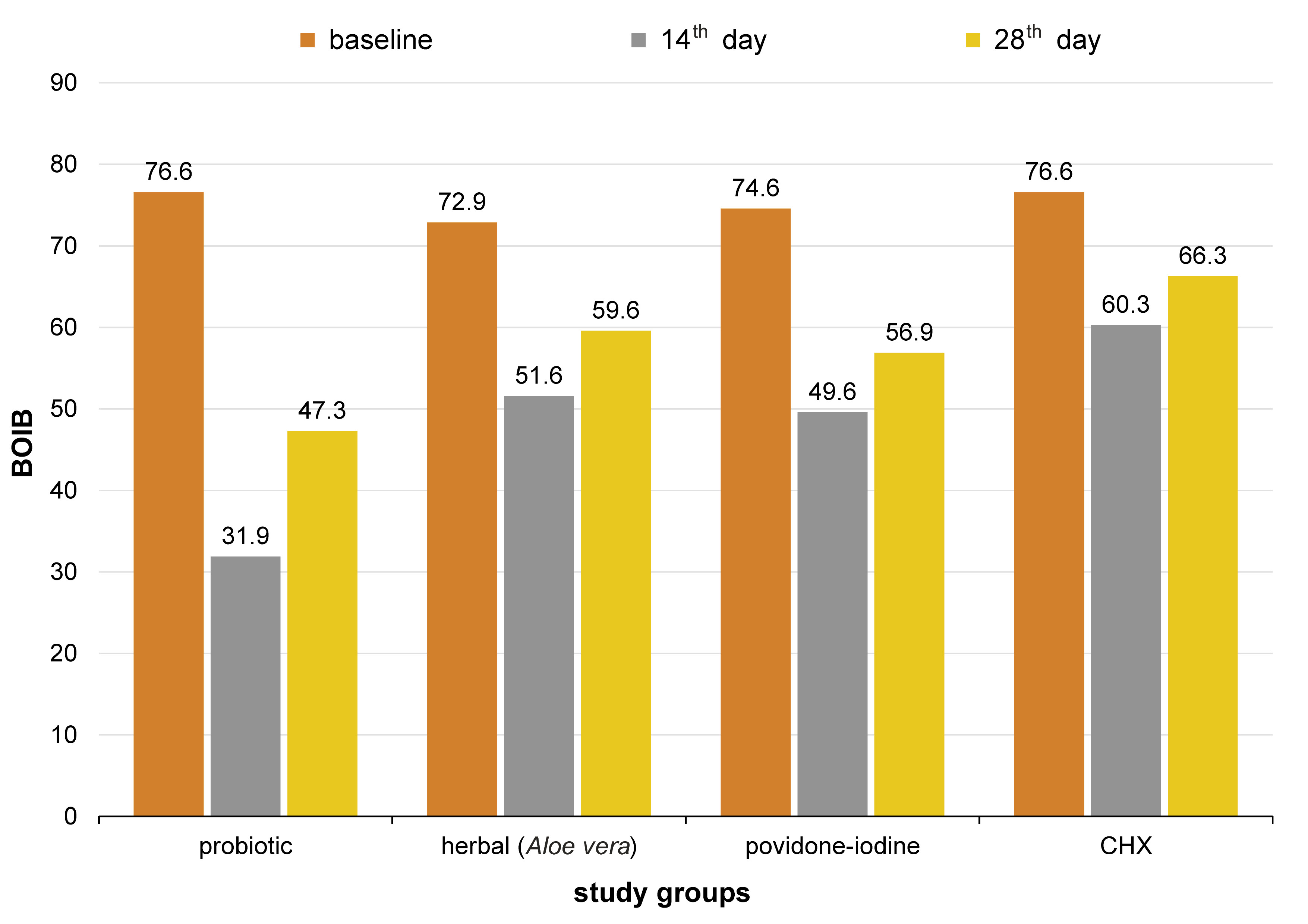
At baseline, the mean MPI score for groups 1, 2, 3, and 4 was 0.70 ±0.06, 0.70 ±0.06, 0.69 ±0.06, and 0.68 ±0.03 (Table 1, Figure 3). On day 14 after intervention, it was 0.22 ±0.01, 0.48 ±0.07, 0.31 ±0.07, and 0.48 ±0.06, and on day 28, it was 0.30 ±0.01, 0.56 ±0.08, 0.38 ±0.06, and 0.55 ±0.05, respectively (Table 2, Figure 3). The mean BOIB score at baseline was 76.6 ±11.1, 72.9 ±14.1, 74.6 ±11.1, and 76.6 ±14.1, respectively (Table 1, Figure 4); on day 14 after intervention, it was 31.9 ±0.9, 51.6 ±2.3, 49.6 ±0.0, and 60.3 ±2.4, and on day 28, it was 47.3 ±4.7, 59.6 ±4.7, 56.9 ±4.7, and 66.3 ±2.4, respectively (Table 2, Figure 4).
The ANOVA revealed significant differences in MPI and BOIB between the groups and within the groups at different time points. Specifically, there were no significant differences in MPI at baseline (p = 0.863), but significant differences were observed on days 14 and 28 (p = 0.000) (Table 3). Similarly, there were no significant differences in BOIB at baseline (p = 0.822), but significant differences were observed on days 14 and 28 (p = 0.000) (Table 4).
The comparative analysis of MPI with the use of the post hoc test was conducted at different time points. At baseline, there were no significant differences between the groups (p = 0.990). On the 14th day, groups 1 and 3 did not demonstrate significant differences as compared to each other, with a p-value of 0.020, while groups 2 and 4 showed no significant changes when compared to each other (p = 1.000). On the 28th day, groups 1 and 3 did not demonstrate significant differences as compared to each other (p = 0.057), and groups 2 and 4 also did not show significant changes when compared to each other (p = 0.997) (Table 5). Therefore, it can be inferred that all groups exhibited a similar reduction in the plaque scores from baseline to the 28th day, and probiotics were found to be more effective and comparable to CHX.
The comparative analysis of BOIB with the use of the post hoc test was conducted at different time points. At baseline, there were no significant differences between the groups (p = 1.000). On the 14th day, group 1 demonstrated significant changes when compared to other groups, with a p-value of 0.000. Similarly, group 4 also demonstrated statistically significant changes when compared to the other 3 groups (p = 0.000, p = 0.032 and p = 0.006, respectively). When groups 2 and 3 were compared to each other, the results were not significant (p = 0.910), but with regard to groups 1 and 4, the differences were significant. On the 28th day, group 1 showed statistically significant differences when compared to other groups, with p < 0.05. In contrast, the comparison of groups 2 and 3 showed non-significant differences (p = 0.826), as well as the comparison of groups 2 and 4 (p = 0.158) (Table 6). It can be inferred that all groups showed a similar reduction in the bleeding scores from baseline to the 28th day, and probiotics were found to be more effective and comparable to CHX.
No adverse effects or harmful events were observed in any of the groups.
Discussion
Maintaining adequate oral hygiene is crucial in preventing dental diseases. Several researchers have proposed using chemical plaque control measures as an adjunct to mechanical plaque control at home. In vitro microbiological research studies have shown that antimicrobial agents can penetrate the bacterial biofilm and exert their bactericidal properties.11, 12 Furthermore, chemical agents can reach interproximal areas that are difficult to clean, and inhibit bacterial growth and the subsequent biofilm formation on soft tissues. The use of these agents is safe and does not appear to increase the resistance of bacterial species.13
Various types of mouthwashes are available on the market and they are commonly used for routine oral hygiene. However, to the best of our knowledge, few studies evaluated the clinical efficacy of different mouthwashes and compared them with CHX. Hence, the present study was conducted to evaluate the clinical efficacy of probiotic, herbal (Aloe vera) and povidone-iodine mouthwashes in the treatment of CP patients in comparison with a positive control using a CHX mouthwash.
In the present study, group 1 participants were advised to use a probiotic mouthwash. On day 28, a significant mean change was demonstrated with regard to MPI and BOIB, with a p-value of 0.000. The present study employed Darolac sachets dissolved in water, using the swish-and-swallow technique, in accordance with a study conducted by Jindal et al.14 Our study showed a statistically significant reduction in bleeding on probing, which is consistent with the findings of studies conducted by Vivekananda et al.,15 Penala et al.,16 İnce et al.,17 Vicario et al.,18 and Della Riccia et al.19 The decrease in the plaque index observed in our study was congruent with the results of studies conducted by Penala et al.,16 İnce et al.,17 Vicario et al.,18 Riccia et al.,19 Krasse et al.,20 and Nadkerny et al.21
The role of probiotics is based on the premise that they produce antibacterial compounds, enhance the epithelial barrier and sequester essential nutrients from pathogens, which prevents their adhesion and growth. Probiotics can adhere to surfaces and balance the replacement of pathogenic microorganisms with non-pathogenic strains.
The results of group 2, in which the participants were instructed to use a herbal mouthwash, demonstrated a significant reduction in the plaque and bleeding scores, as in the probiotic group. Yet, even though a herbal (Aloe vera) mouthwash showed significant results, it was not as effective as probiotic and CHX mouthwashes. The decrease in the plaque and bleeding indices was similar to that observed in studies conducted by Lee et al.,22 Chandrahas et al.23 and Moghaddam et al.24
Aloe vera has various beneficial properties, such as anti-inflammatory (due to the presence of sterols and anthrax quinones) and anti-septic (due to the presence of lupeol, salicylic acid, phenols, and sulfur) activity, and has the capability to enhance wound healing.25 These characteristics make it a good agent for preventing gingivitis. In our study, the clinical efficacy of the Aloe vera mouthwash was found to be good, although not as good as in the case of CHX and probiotic mouthwashes. The clinical improvement attributed to Aloe vera may have been due to its antibacterial, anti-plaque and healing properties.
The results of group 3, in which the participants were instructed to use a povidone-iodine mouthwash, demonstrated a significant decrease from baseline to day 28 in the plaque and bleeding scores. This may be attributed to the antimicrobial activity of povidone-iodine. Yet, the improvement was not comparable to that in the probiotic group.
In a study conducted by Yoneyama et al., using povidone-iodine gargle and mouthwash (benzethonium chloride (BEC) and chlorhexidine gluconate (CHG)) samples from healthy volunteers, povidone-iodine was found to show stronger bactericidal activity against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa than BEC and CHG.26
Limitations
The study did not include a negative control group or a no-treatment group. Future studies presenting microbiological comparisons between groups may provide a better insight while evaluating different mouthwashes in terms of gingival inflammation reduction.
Conclusions
The results of the present study indicate that the use of a mouthwash leads to a significant reduction in the plaque and bleeding indices. Within the study limitations and based on the obtained results, it can be inferred that although CHX is considered the gold standard, a probiotic mouthwash demonstrates comparable results to CHX, and is equally effective in reducing the plaque and bleeding scores. Therefore, conducting additional studies that would employ microbiological analysis, with a negative control, may provide a better insight into the treatment of gingival inflammation and confirm the improved outcomes.
Ethics approval and consent to participate
The study was approved by the institutional research ethics committee (ethical clearance No. Pr.2115/IEC/SIBAR(UG)2021), and was conducted in compliance with the ethical standards established by the World Medical Association (WMA) in the Declaration of Helsinki. Each patient was given a detailed verbal and written description of the study, and provided signed consent to participate in it.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.